Abstract
OBJECTIVE
To evaluate the effects of two bariatric procedures versus intensive medical therapy (IMT) on β-cell function and body composition.
RESEARCH DESIGN AND METHODS
This was a prospective, randomized, controlled trial of 60 subjects with uncontrolled type 2 diabetes (HbA1c 9.7 ± 1%) and moderate obesity (BMI 36 ± 2 kg/m2) randomized to IMT alone, IMT plus Roux-en-Y gastric bypass, or IMT plus sleeve gastrectomy. Assessment of β-cell function (mixed-meal tolerance testing) and body composition was performed at baseline and 12 and 24 months.
RESULTS
Glycemic control improved in all three groups at 24 months (N = 54), with a mean HbA1c of 6.7 ± 1.2% for gastric bypass, 7.1 ± 0.8% for sleeve gastrectomy, and 8.4 ± 2.3% for IMT (P < 0.05 for each surgical group versus IMT). Reduction in body fat was similar for both surgery groups, with greater absolute reduction in truncal fat in gastric bypass versus sleeve gastrectomy (−16 vs. −10%; P = 0.04). Insulin sensitivity increased significantly from baseline in gastric bypass (2.7-fold; P = 0.004) and did not change in sleeve gastrectomy or IMT. β-Cell function (oral disposition index) increased 5.8-fold in gastric bypass from baseline, was markedly greater than IMT (P = 0.001), and was not different between sleeve gastrectomy versus IMT (P = 0.30). At 24 months, β-cell function inversely correlated with truncal fat and prandial free fatty acid levels.
CONCLUSIONS
Bariatric surgery provides durable glycemic control compared with intensive medical therapy at 2 years. Despite similar weight loss as sleeve gastrectomy, gastric bypass uniquely restores pancreatic β-cell function and reduces truncal fat, thus reversing the core defects in diabetes.
Type 2 diabetes mellitus and obesity are closely interrelated chronic conditions growing in incidence worldwide, with diabetes-related deaths projected to double between 2005 and 2030 (1). The development of both insulin resistance and insulin secretory defects is the hallmark of type 2 diabetes, resulting in progressive hyperglycemia, subsequent microvascular complications, and macrovascular complications. Although lifestyle modifications and oral hypoglycemic agents improve glycemic control, the majority of patients do not achieve the optimal glycohemoglobin (HbA1c) levels recommended by current guidelines (≤7.0%). The disease inexorably progresses in the majority of patients, ultimately requiring insulin replacement therapy. Most patients with type 2 diabetes are overweight or obese (BMI ≥30 kg/m2), and abdominal adiposity, particularly, is tightly linked to induction of insulin resistance, metabolic syndrome, and increased cardiovascular risk. Many hypoglycemic agents, especially insulin, exacerbate weight gain and thwart lifestyle efforts, potentially contributing to the underlying pathophysiologic disorder.
Because of the limitations to medical therapy, surgical approaches for the treatment of obesity have increased 10-fold in the past decade. Roux-en-Y gastric bypass surgery is the most commonly performed in the United States, followed closely by the sleeve gastrectomy (2). Recently, two randomized controlled trials (3,4) demonstrated improved glycemic control in patients undergoing bariatric surgery compared with intensive medical therapy, resulting in the ability to withdraw or reduce glucose-lowering medications. The rapid rate of glucose lowering, disproportionate to degree of weight loss, suggests that bariatric surgery reverses the fundamental pathophysiological defects of type 2 diabetes. Animal studies suggest that bariatric surgery increases insulin secretion or improves enteroinsulinar responses, specifically, the main incretin hormones glucagon like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP) (5–7). Previous small-scale studies from matched case-control and observational studies in severely obese diabetic individuals have reported that weight loss improves insulin sensitivity, reduces hyperinsulinemia, and improves pancreatic β-cell function by weight-independent mechanisms related to an incretin effect (8–10). However, there are no data from a randomized controlled trial examining the prolonged metabolic adaptations in conjunction with clinical efficacy outcomes after bariatric surgery relative to the effects of intensive medical therapy in moderately obese subjects with poorly controlled type 2 diabetes.
The STAMPEDE trial evaluated the efficacy and safety of intensive medical therapy (IMT) alone or intensive medical therapy combined with Roux-en-Y gastric bypass or sleeve gastrectomy to achieve a primary end point of HbA1c level of ≤6% (with or without medications) after 1 year of follow-up (11). The current report is a 2-year extension of a metabolic substudy of the STAMPEDE trial designed to thoroughly evaluate the effects of the three treatments on glucose regulation, pancreatic β-cell function (insulin secretion/sensitivity), and body composition in a subset of 60 subjects.
RESEARCH DESIGN AND METHODS
Study design
The STAMPEDE study rationale and design have been previously reported (3,11). The first consecutive 60 subjects randomized in the main trial, with ∼20 randomized to each treatment group, were included in the substudy. STAMPEDE was a single-center study that randomized patients in a 1:1:1 ratio to intensive medical therapy alone or intensive medical therapy combined with either Roux-en-Y gastric bypass or sleeve gastrectomy with stratification by use of insulin at screening. Intensive medical therapy included the use of the latest lifestyle guidelines by the American Diabetes Association, frequent home monitoring and titration strategies, and use of the latest U.S. Food and Drug Administration–approved drug therapy including incretin analogs or mimetics and insulin sensitizers for treatment of hyperglycemia. Patients were examined in the outpatient clinic every 3 months by a diabetes specialist at the Cleveland Clinic (S.R.K.). The bariatric procedures were performed by a single primary surgeon (P.R.S.).
During the screening period, all patients received nutritional counseling by a certified diabetes educator. Subjects were encouraged to participate in Weight Watchers for additional nutritional counseling. Patients underwent a psychological evaluation before randomization to assess qualification for bariatric surgery. Subjects randomized to bariatric surgery had periodic evaluation by nutrition, psychology, bariatricians, and the surgery team as clinically indicated. The Data and Safety Monitoring Board convened yearly to review progress and safety of the trial. The protocol was developed with the assistance of the Cleveland Clinic Coordinating Center for Clinical Research and was approved by the Cleveland Clinic Institutional Review Board. All participants provided written informed consent.
After randomization at the baseline visit and at 12 and 24 months after randomization, subjects underwent metabolic assessment with a liquid mixed-meal tolerance test and body composition measurements with dual-energy X-ray absorptiometry (iDXA; Lunar Prodigy, Madison, WI) scan.
Metabolic studies
The mixed-meal tolerance test was performed to assess glucose tolerance and metabolic measures of insulin sensitivity and secretion in response to a physiological stimulus. The liquid mixed meal consisted of a commercial product (Boost; 8 ounces, 350 kcal, 55% carbohydrate, 25% protein, 20% fat) and was consumed over 5 min after a 12- to 14-h overnight fast in a similar manner per protocol at baseline and 12 and 24 months. Fasting blood samples were obtained for glucose, C-peptide, insulin, lipids, HbA1c, adipokines, and a complete metabolic panel. Blood was drawn every 30 min for 120 min during the mixed meal tolerance testing for determination of glucose, insulin, C-peptide, and free fatty acid responses. Additional blood was drawn at fasting and at 60 min after ingestion for determination of GLP-1 and GIP responses. Glucagon levels were determined at fasting and at 120 min. Diabetes medications were withheld for 24 h before study, including insulin administration.
Analytic determinations
Blood glucose was measured using the glucose oxidase method (YSI 2300 STAT Plus; YSI, Yellow Springs, OH). Plasma insulin was assayed by a double-antibody radioimmunoassay (RIA; Linco Research, St. Charles, MO). The intra-assay and interassay coefficients of variations were 2.6% and 3.0%, respectively. C-peptide was assayed using a chemiluminescence immunoassay (Linco Research). The intra-assay and interassay coefficients of variations were 3.5% and 7.2%, respectively. Blood collected for GLP-1 (active) and GIP (total) analyses was treated immediately with a DPP4 and protease cocktail inhibitor (Sigma) and assayed using ELISA kits (ALPCO Diagnostics, Salem, NH). The intra-assay and interassay coefficients of variations were 3.6% and 9.3%, respectively. To correct for interassay variability, all premeasurements and postmeasurements for each individual were run on the same plate. Free fatty acid levels were determined by standard colorimetric methods (Wako Chemicals, Richmond, VA). The intra-assay and interassay coefficients of variation were 3.0% and 4.6%, respectively. Leptin was assayed using ELISA kits (R&D Systems, Minneapolis, MN). The intra-assay and interassay coefficients of variations were 3.6% and 5.3%, respectively.
Calculations
Insulin secretion rate (ISR) in vivo was reconstructed by deconvolution of plasma concentrations of C-peptide, a peptide with linear kinetics that is cosecreted with insulin but is not extracted by the liver as previously described (8,12). ISRs over each sampling period were derived by using a well-accepted two-compartment model described by Van Cauter et al. (12) of C-peptide distribution and degradation and standard parameters for C-peptide clearance estimated for each subject, taking into account body surface area, sex, and age. ISR was related to the glucose stimulus by dividing the incremental area under curve (AUC) for ISR by the incremental AUC for plasma glucose. Pancreatic β-cell function measured by the insulin secretion/insulin resistance (disposition) index was determined by dividing the ΔISR/Δglucose by the severity of insulin resistance (ΔISR [AUC] / ΔG [AUC] ÷ IR), as measured by the inverse of the Matsuda index (13). The Matsuda index incorporates both hepatic and muscle components of insulin resistance, correlates well with euglycemic insulin clamp, and was calculated as follows:
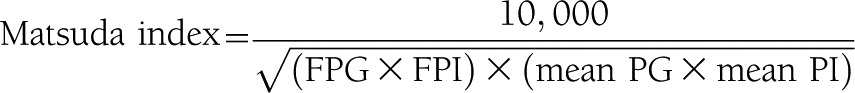 |
Of note, the Matsuda index was performed in those subjects not using exogenous insulin. The incremental AUC for ISR (ΔISR [AUC]) and the incremental AUC for plasma glucose (ΔG[AUC]) were calculated according to the trapezoid rule. The incretin response during meal testing subtracted the fasting value from the meal value at 60 min.
Statistical analysis
This is a preplanned substudy with prespecified analysis. However, because of the lack of published data in the literature regarding specified metabolic outcome measures at the time of trial design (2004–2005), and because of the exploratory nature of the substudy, no power calculations were performed for the substudy measures. Continuous variables with a normal distribution are reported as means and SDs. Variables with a non-normal distribution are reported as medians and interquartile ranges. Categorical variables were summarized using frequencies and were tested with the χ2 statistic or Fisher exact test (two-tailed), as appropriate. One-way ANOVA was used to analyze continuous laboratory parameters, and comparisons between treatment groups were performed with either the Student t test or the Wilcoxon test. Glucose and insulin measures collected during the mixed-meal tolerance test were plotted graphically.
RESULTS
Patients
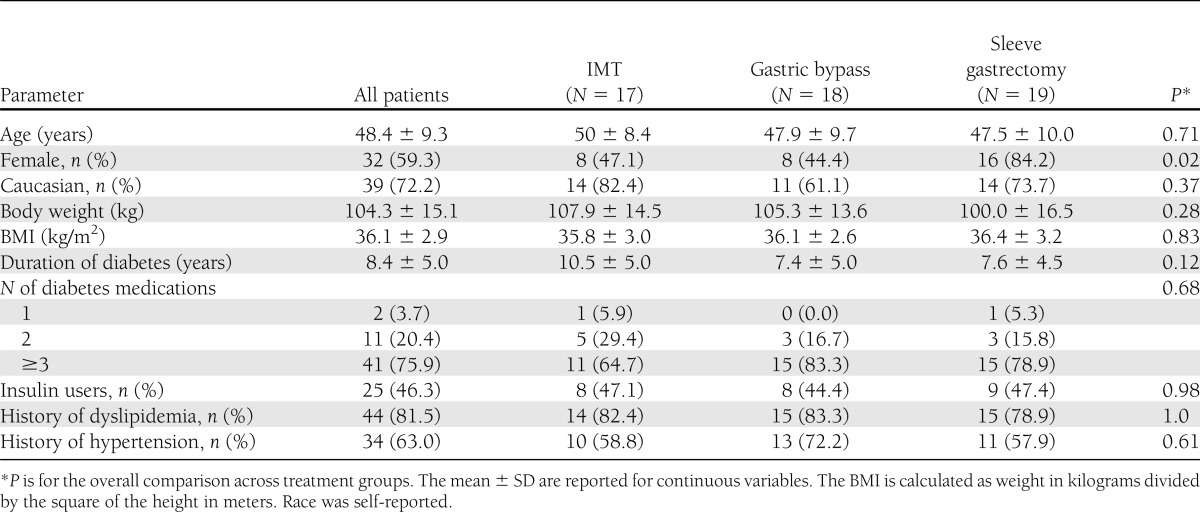
Sixty subjects enrolled in the substudy after randomization. At 24 months, 10% were lost to follow-up, with 17 subjects remaining in intensive medical therapy and 18 and 19 subjects remaining in the gastric bypass and sleeve gastrectomy groups, respectively. Baseline characteristics for the three study groups that were followed up for 24 months are shown in Table 1. The subjects were middle-aged, with a predominance of females, particularly in the sleeve gastrectomy group. The average BMI was 36 kg/m2 with prolonged diabetes duration with mean baseline HbA1c levels of ∼9%, indicating poor glycemic control despite using multiple glucose-lowering agents. Nearly half were insulin users, and a majority had hypertension and hyperlipidemia.
Table 1.
Baseline characteristics
Glycemic and cardiovascular risk control
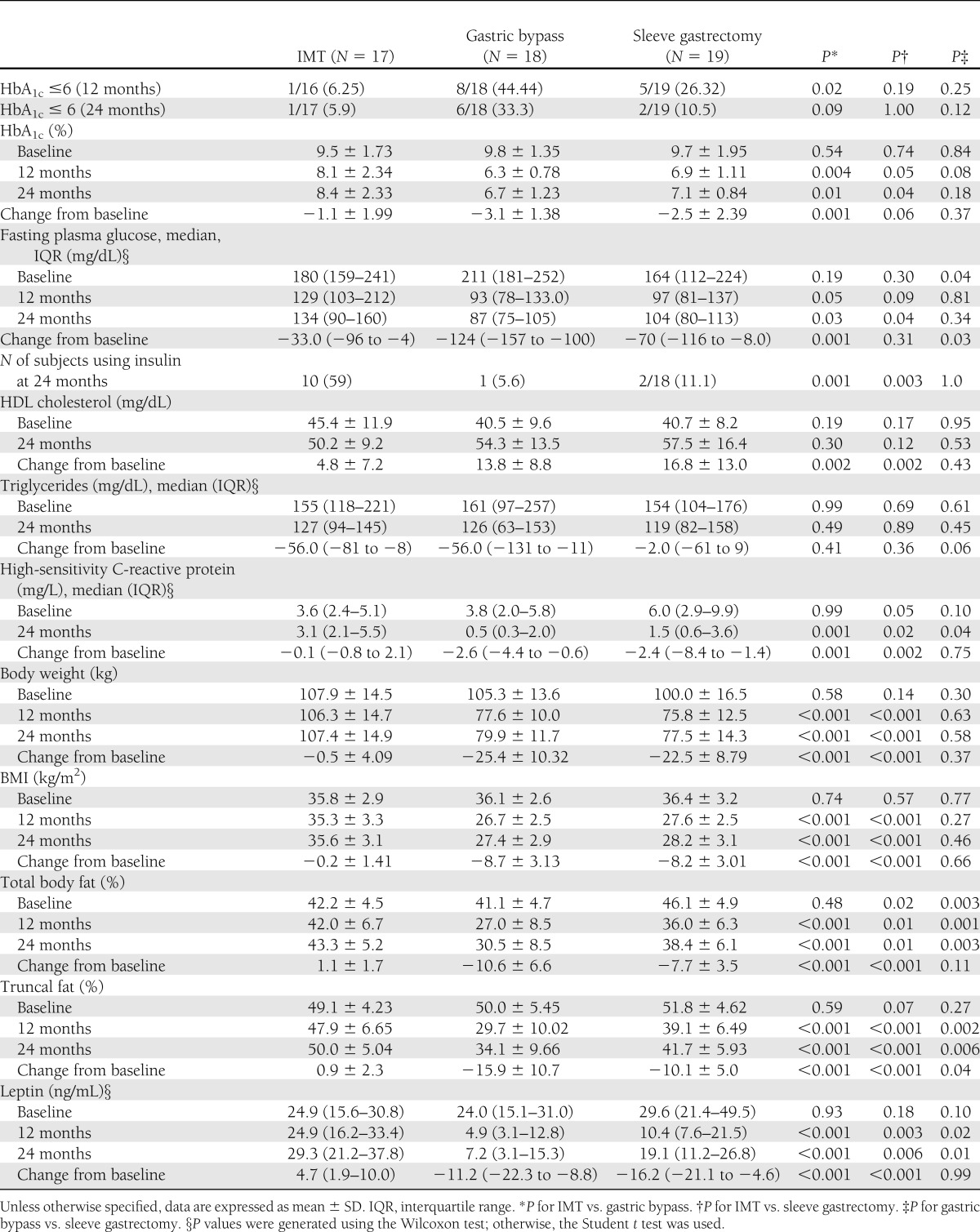
Table 2 shows glycemic and lipid outcomes at 12 and 24 months. Although glycemic control improved in all three arms at 24 months as compared with baseline, the gastric bypass group had significantly greater reduction in fasting glucose and HbA1c levels compared with IMT (P < 0.05) (Table 2). At 24 months, the proportion of patients with HbA1c ≤6% attenuated in the sleeve gastrectomy group from 26% to 11% but persisted in the gastric bypass group. The percent of patients using insulin at 24 months was markedly lower in gastric bypass and sleeve gastrectomy groups as compared with IMT. Large increases in HDL cholesterol and reductions in levels of triglycerides and high-sensitivity C-reactive protein were noted in both surgery groups as compared with the IMT group. Other laboratory parameters and medication usage are available in Supplementary Table 1.
Table 2.
Clinical changes at 12 and 24 months: glycemic and lipid control
Three subjects randomized to bariatric surgery required reoperation, including laparoscopy to assess nausea and vomiting, for cholecystectomy and for jejunostomy for feeding access to treat a gastric leak after sleeve gastrectomy. There were no deaths or episodes of serious hypoglycemia requiring intervention, malnutrition, or excessive weight loss among the three groups.
Body weight, body composition, and adipokines
Greater total body weight loss occurred after bariatric procedures compared with IMT at 12 months and was maintained at 24-month follow-up. A similar reduction in body weight, BMI, and absolute change in total body fat percent was observed between the sleeve gastrectomy and gastric bypass group at 24 months. However, despite similar weight loss, the absolute reduction in percent truncal fat was greater in the gastric bypass versus sleeve gastrectomy group (−16% vs. −10%; P = 0.04). Leptin levels reduced markedly after surgical weight loss, especially gastric bypass, compared with IMT. Suppression of free fatty acid concentration during mixed-meal testing was evident after both surgical procedures versus IMT (Supplementary Table 1).
Mixed-meal tolerance
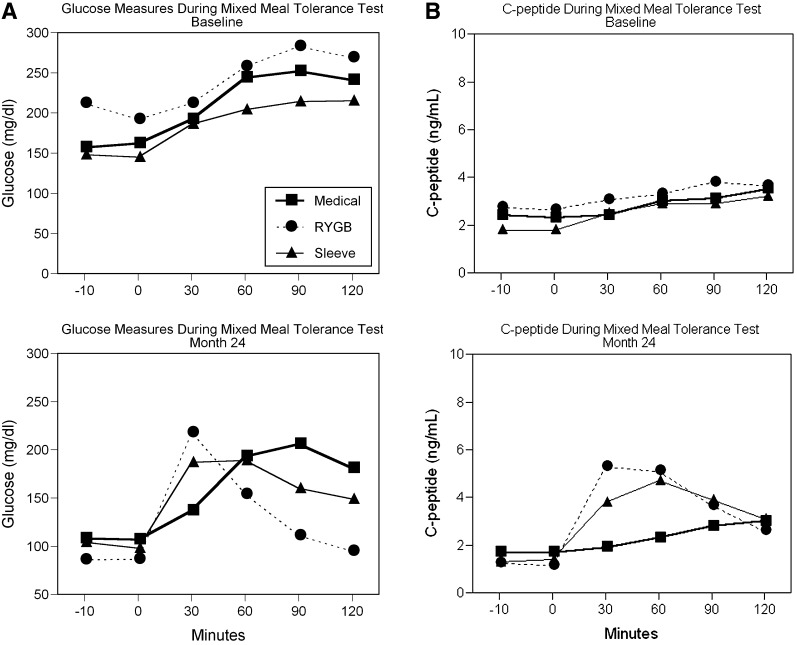
Figure 1 demonstrates the median glucose and C-peptide levels during the mixed-meal tolerance test at baseline and 24 months. At 24 months, the shape of the glucose tolerance curve for gastric bypass normalized with a marked reduction in fasting and postprandial glucose levels. Intermediate effects on postprandial glucose lowering were noted in sleeve gastrectomy, and IMT showed the least change. Large reductions in fasting C-peptide levels were observed in sleeve gastrectomy and gastric bypass at 24 months but did not change in the IMT group. Postprandial C-peptide levels and insulin secretion rate at 30 and 60 min increased by more than two-fold in gastric bypass and sleeve gastrectomy, with greater increases noted with gastric bypass. The average AUC for insulin secretion rate at 24 months was significantly greater with both gastric bypass (4.4 ± 4 pmol/min) and sleeve gastrectomy (3.3 ± 2.5 pmol/min) than IMT (1.7 ± 2.4 pmol/min; both P < 0.01).
Figure 1.
Glucose (A) and C-peptide (B) during the mixed-meal tolerance test performed at time of randomization (baseline) and at 24 months after randomization for IMT, sleeve gastrectomy, and gastric bypass. Mixed meal consisted of Boost (8 ounces) with 30-min interval blood sampling for glucose and C-peptide values. Data are shown in median values. RYGB, Roux-en-Y gastric bypass.
Insulin sensitivity
Median values for the insulin sensitivity (Matsuda index) in noninsulin-using subjects increased at 24 months after gastric bypass (N = 9) by 2.7-fold (3.8 vs. 1.4; P < 0.001) and 1.2-fold after sleeve gastrectomy (N = 10; 5.8 vs. 5.3) and did not change with IMT (2.6 vs. 2.4; P = not significant). The absolute change in median insulin sensitivity (Matsuda index) at 24 months tended to be higher in gastric bypass compared with sleeve gastrectomy (2.3 [quartile 1: 0.9; quartile 3: 3.1] vs. 0.9 [quartile 1: −1.5; quartile 3: 4.6]), despite equivalent weight loss.
Pancreatic hormonal function
The absolute change in median values for pancreatic β-cell function (oral disposition index) at 24 months was markedly greater in gastric bypass than IMT (0.196 [quartile 1: 0.14; quartile 3: 0.29) vs. 0.027 [quartile 1: −0.011; quartile 3: 0.074]; P = 0.001) but not different between sleeve gastrectomy and medical therapy (0.058 [quartile 1: −0.009; quartile 3: 0.416] vs. 0.027 [quartile 1: −0.011; quartile 3: 0.074]; P = 0.30). A median 5.8-fold (quartile 1: −7.00; quartile Q3: 11.29) increase in β-cell function from baseline was noted in gastric bypass, with negligible increases in sleeve gastrectomy and IMT.
The change in β-cell function over 24 months for the substudy cohort correlated with the change in percentage of truncal fat (r = 0.43; P = 0.0013) and change in body weight (r = 0.49; P < 0.001). At 24 months, both percentage of truncal fat (r = −0.32; P = 0.02) and prandial free fatty acid levels (r = −0.48; P = 0.0003) were inversely correlated with β-cell function. In a multivariable analysis including both factors, prandial free fatty acid levels remained significant (P = 0.004), whereas percentage of truncal fat was no longer significant (P = 0.41).
Fasting glucagon concentrations were similar among the three groups at baseline. At 12 months, median glucagon levels tended to be lower in gastric bypass versus IMT (46 vs. 77 pg/mL; P = 0.07) and were reduced in sleeve gastrectomy versus IMT (38 vs. 77 pg/mL; P < 0.02). However, at 24 months fasting glucagon levels were not different among the three groups (∼60 pg/mL).
Postprandial glucagon levels reduced in all three groups at 24 months from baseline values, but postprandial glucagon levels in gastric bypass were higher (77 pg/mL) than IMT (64 pg/mL; P < 0.05) and not different between sleeve gastrectomy (65 pg/mL) and IMT.
Incretin responses
Median levels of GLP-1 60 minutes after mixed-meal ingestion (taking into account fasting levels) increased dramatically 24 months after gastric bypass (12.5 vs. 2 pmol/L; P < 0.001) and sleeve gastrectomy (7.3 vs. 2.4 pmol/L; P < 0.01) and did not change with IMT (1.5 vs. 1.4 pmol/L; P = not significant). In contrast, median levels of GIP in response to mixed meal were reduced 24 months after gastric bypass (13.5 vs. 30.7 pmol/L; P < 0.01) and were significantly different (P < 0.01) from sleeve gastrectomy (36.9 pmol/L) and IMT (29.7 pmol/L). Both surgical groups had an overall increase in the incremental change in median GLP-1 response to mixed meal as compared with IMT at 24 months (gastric bypass 10.0 pmol/L [quartile 1: 5.2; quartile 3: 15.2] vs. sleeve gastrectomy 4.5 pmol/L [quartile 1: 2.9; quartile 3: 8.2]; P = 0.07; vs. IMT −0.4 pmol/L [quartile 1: −1.8; quartile 3: 1.1]; P < 0.001 for both). However, a reduction in GIP response was noted in gastric bypass only at 24 months (gastric bypass, −20 pmol/L [quartile 1: −44.2; quartile 3: −10.2] vs. IMT 5.4 pmol/L [quartile 1: −6.3; quartile 3: 30.4]; P < 0.001 vs. sleeve gastrectomy 0.6 pmol/L [quartile 1: −18.6; quartile 3: 33.2]; P = NS).
Metabolic determinants of HbA1c ≤6% at 24 months
A multivariate logistic model of metabolic parameters that were associated with HbA1c ≤6% at 24 months in the substudy cohort demonstrated that the fold increase in the oral disposition index (β-cell function) was associated with an increased odds ratio of 1.67 (CI 1.012–1.124; P = 0.016), and the increase in truncal fat was associated with a lower odds ratio of 0.878 (CI 0.777–0.991; P = 0.036) to achieve glycemic control at 24 months.
CONCLUSIONS
Two recent bariatric surgery studies have shown markedly improved glycemic control in surgically treated patients with obesity and type 2 diabetes compared with medical therapy (3,4). In one of those studies, the STAMPEDE trial, 1 year after randomization, patients assigned to bariatric surgery were significantly more likely to achieve an HbA1c level of 6% compared with patients treated using IMT alone (3). In the current metabolic substudy, we extended follow-up of the STAMPEDE trial patients to 2 years and sought to determine the durability of the initial results and to examine the metabolic adaptations responsible for the improved glycemic control observed with bariatric surgery. We measured a wide range of metabolic parameters at three time points, at baseline, after the original 1-year follow-up, and repeated measurements 2 years after initial randomization.
After 2 years, gastric bypass provided more durable glycemic control with little or no need for glucose-lowering agents in patients randomized to this strategy. Despite comparable weight loss compared with sleeve gastrectomy, more durable glycemic control was achieved in patients randomized to gastric bypass, with a substantially greater percentage of patients attaining the target HbA1c levels of ≤6%. Attenuation of improvement in diabetes control was noted in the sleeve gastrectomy treatment group despite persistent weight loss. Other long-term observational studies have documented greater relapse rates for glycemic control after gastric restrictive procedures such as sleeve gastrectomy, suggesting that surgical weight loss from enforced caloric restriction itself is insufficient to halt the disease (14,15). Our results extend the findings from our initial 12-month report and suggest factors beyond weight loss that are specific to intestinal bypass patients help regulate glucose levels and restore pancreatic β-cell function.
Striking metabolic changes were observed in patients randomized to bariatric surgery compared with intensive medical therapy, particularly in the gastric bypass treatment group. At baseline, all randomized patients exhibited poor pancreatic secretory function. After both 1 and 2 years of follow-up, gastric bypass patients achieved near-normal glucose tolerance after a physiological liquid mixed meal. These effects were associated with a remarkable 5.8-fold increase in overall pancreatic β-cell function. In gastric bypass patients, both insulin sensitivity and secretion components increased, but despite comparable weight loss in sleeve gastrectomy, insulin sensitivity was only partially restored and pancreatic β-cell function did not improve. Both bariatric surgery procedures stimulated incretins with markedly increased postprandial GLP-1 levels as noted in previous observational studies in obese patients with type 2 diabetes (8,9,16). However, divergence in postprandial GIP levels was noted, with a reduction seen only in gastric bypass that may be related to anatomical exclusion of the duodenum (which produces GIP) or may be reflective of improved GIP action that is noted to be defective in type 2 diabetes (17).
The metabolic changes observed in these bariatric surgical patients are markedly different from previous studies performed in medically treated patients with type 2 diabetes and highlight the clinical value of improving β-cell function to achieve glycemic control. The UK Prospective Diabetes Study (UKPDS) demonstrated that pancreatic β-cell function continues to deteriorate over time despite diet, exercise, and administration of hypoglycemic agents (18). In UKPDS, the natural history of type 2 diabetes was characterized by an average increase in HbA1c level of 1% over 2 years despite the use of medications. In patients with well-controlled diabetes prescribed a single oral hypoglycemic agent, the mean time of deterioration of glycemic control was 33–60 months (19). For patients using metformin and additionally prescribed a sulfonylurea, median HbA1c levels deteriorated as early as 6 months at a rate similar to that observed with metformin alone (20). These findings suggest that with continuing decline in β-cell function, single or even multiple agents are no longer sufficient to control blood glucose levels, which is why many patients ultimately require insulin therapy. For those who initiate insulin therapy, less than half achieve a desired HbA1c of ≤7% (21).
Although weight loss associated with hypocaloric diet has been shown to reduce insulin resistance, reduce hyperinsulinemia, and improve β-cell function (22–24), both bariatric surgery procedures displayed much larger postprandial insulinotropic effects compared with IMT. Greater insulin secretory effects of gastric bypass coupled with a nearly threefold improvement in insulin sensitivity likely account for complete normalization of postprandial glucose tolerance seen uniquely with gastric bypass patients. Lack of suppression of glucagon is known to contribute to postprandial hyperglycemia in diabetes (25), and, contrary to our expectation, bariatric surgery did not restore this defect. A slight increase in postprandial glucagon level was noted in gastric bypass, a finding consistent with a previous study (26).
Massive weight loss and reduction of adipose tissue mass clearly contribute to the major improvements in insulin sensitivity and cardiovascular risk profile observed after bariatric surgery. However, the marked improvements in insulin sensitivity and glycemic control observed in the gastric bypass group suggest factors specifically linked to the presence of abdominal (truncal) fat. Ectopic abdominal fat presence has long been recognized to induce insulin resistance, subclinical inflammation, and cardiovascular risk specific to type 2 diabetes (27,28). Previous studies also have demonstrated greater improvements in insulin sensitivity with intestinal bypass procedures (i.e., gastric bypass and biliopancreatic diversion) compared with BMI-matched nonsurgical patients, presumably because these procedures produce greater nutrient and fat malabsorption (29,30). In the current study, adipogenic inflammation was significantly reduced after both bariatric procedures, especially gastric bypass, mediated by factors such as free fatty acids, leptin, and C-reactive protein, which impair glucose uptake by insulin-dependent tissues (muscle and liver).
Although modest improvement in glycemic control, glucose metabolism, and clinical parameters was noted with IMT in our trial, more vigorous and behavioral/lifestyle modification strategies as used in the Look AHEAD trial (31) that aggressively target weight loss are clearly needed. Future randomized control trials are needed to compare such strategies results with bariatric surgery. However, maintaining weight loss in patients with diabetes who require insulin and other hypoglycemic agents is difficult in the “real world” clinical setting because conventional drug therapy generally results in weight gain. In addition, fear of hypoglycemia and patient burdens related to administration of multiple drugs for diabetes and cardiovascular risk control presents significant barriers to implementing and adhering to IMT.
A limitation of this study is the validity of the incretin hormone responses that were obtained after the assigned interventions. Concentrations of GLP-1 and GIP were obtained at fasting and at 60 min after meal ingestion, and this likely underestimates the incretin surge that normally occurs rapidly (within 15 min) after meal ingestion. Nevertheless, large incremental changes in prandial GLP-1 levels and corresponding C-peptide levels were noted 2 years after both bariatric procedure types that were not observed with IMT. Further studies are warranted to thoroughly investigate the long-term effects of bariatric surgery on incretin responses and action to modulate insulin secretion. Additionally, insulin sensitivity determined by the Matsuda index was performed only in a subset of subjects not using exogenous insulin at baseline and followed trends similar to the whole cohort because insulin administration was withheld 24 h before meal testing.
In summary, bariatric surgery induces powerful metabolic effects in moderately obese patients with advanced type 2 diabetes inadequately controlled with currently available drug therapy. Bariatric surgery, particularly gastric bypass surgery, uniquely restores normal glucose tolerance and pancreatic β-cell function, presumably by targeting the truncal fat that represents the core metabolic defect involved in diabetes pathogenesis. Longer-term multicenter studies with safety outcomes are warranted to test the durability of these metabolic benefits.
Acknowledgments
Primary funding for the STAMPEDE trial is from Ethicon Endo-Surgery EES IIS 19900 (to P.R.S.). The American Diabetes Association clinical translational award (1-11-26 CT) to S.R.K. provided ancillary funding for STAMPEDE. A grant from the National Institutes of Health (R01-DK089547) to S.R.K., P.R.S., and J.P.K. was provided.
S.R.K. obtained research grants from Ethicon Endo-Surgery, National Institutes of Health, and American Diabetes Association. D.L.B. is a member of the Advisory Board for Medscape Cardiology, is on the Board of Directors for Boston VA Research Institute and Society of Chest Pain Centers, is the Chair of American Heart Association Get With The Guidelines Science Subcommittee, received honoraria from American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), and WebMD (CME steering committees), is the Senior Associate Editor of Journal of Invasive Cardiology, received research grants from Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi, The Medicines Company, and FlowCo, and performed unfunded research for PLx Pharma and Takeda. D.L.B. receives honoraria from Ethicon Endo-Surgery as scientific advisory board member, consultant, and speaker, and has received honoraria from Covidien for speaking. J.P.K. receives grant funding from National Institutes of Health, Nestle Inc., and ScottCare. P.R.S. obtained research grants from Ethicon Endo-surgery, National Institutes of Health, and Bard-Davol; received educational grants from Stryker Endoscopy, Gore, Baxter, Covidien, and Allergan; and received honoraria from Ethicon Endo-surgery as a scientific advisory board member, consultant, and speaker. P.R.S. has been a consultant/advisory board member for RemedyMD, StrykerEndoscopy, Bard-Davol, Gore, Barosense, Surgiquest, and Carefusion. S.N. has consulted with Orexigen and Vivus. S.R.K., P.R.S., and the Cleveland Clinic Coordinating Center for Clinical Research had full and independent access to all of the data in the study. The sponsor participated in discussions regarding study design and protocol development and provided logistical support during the trial. The database, statistical analysis, and monitoring were all performed by the Cleveland Clinic Coordinating Center for Clinical Research. The manuscript was prepared by the corresponding author and modified after consultation with co-authors. The sponsor was permitted to review the manuscript and suggest changes, but the final decision on content and submission was exclusively retained by the academic authors. J. Michael Henderson, MD (Chair), James B. Young, MD, and Venu Menon, MD, Cleveland Clinic, Cleveland, Ohio, are members of the Data and Safety Monitoring Board. No other potential conflicts of interest relevant to this article were reported.
S.R.K. was responsible for the study concept and design, acquired data, provided administrative technical or material support, performed study supervision, analyzed and interpreted data, drafted the manuscript, and obtained funding. D.L.B. was responsible for the study concept and design and analysis, interpreted data, critically revised the manuscript for important intellectual content, provided administrative technical or material support, performed study supervision, and drafted the manuscript. K.W. analyzed and interpreted data, performed statistical analysis, and drafted the manuscript. R.M.W. analyzed and interpreted data. M.A-.G. analyzed and interpreted data. B.A. was responsible for subject recruitment and for carrying out the mixed-meal testing and DXA scan procedures. C.E.P. was responsible for the study concept and design, drafted the manuscript, and provided administrative technical or material support. S.B. was responsible for the study concept and design. S.N. was responsible for the study concept and design, analyzed and interpreted data, and critically revised manuscript for important intellectual content. M.G. acquired data. J.P.K. analyzed and interpreted data and critically revised the manuscript for important intellectual content. P.R.S. was responsible for study concept and design, acquired data, analyzed and interpreted data, critically revised the manuscript for important intellectual content, obtained funding, provided administrative technical or material support, and performed study supervision. S.R.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was presented as a late-breaking poster at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors are grateful for the skilled nursing care provided by Sharon O’Keefe and to Chytaine Hall, both affiliated with the Endocrinology Institute at Cleveland Clinic, for performing the metabolic studies. The authors appreciate the skilled technical expertise of Sarah Neal (Lerner Institute, Cleveland Clinic) and the laboratory staff of Preventive Research Laboratory, a core reference laboratory under the guidance of Stanley Hazen, MD, PhD (Heart and Vascular Institute and Lerner Institute, Cleveland Clinic), for performing all gut and adipose cytokines. The authors thank Rose Lounsbury, BS, Clinical Endocrinology Laboratory (Endocrinology Institute, Cleveland Clinic), for performing the assays related to fatty acids and glucagon.
Footnotes
Clinical trial reg. no. NCT00432809, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1596/-/DC1.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. ; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg 2009;19:1605–1611 [DOI] [PubMed] [Google Scholar]
- 3.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 5.Gatmaitan P, Huang H, Talarico J, et al. Pancreatic islet isolation after gastric bypass in a rat model: technique and initial results for a promising research tool. Surg Obes Relat Dis 2010;6:532–537 [DOI] [PubMed] [Google Scholar]
- 6.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004;240:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007;3:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap SR, Bhatt DL, Schauer PR; STAMPEDE Investigators Bariatric surgery vs. advanced practice medical management in the treatment of type 2 diabetes mellitus: rationale and design of the Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently trial (STAMPEDE). Diabetes Obes Metab 2010;12:452–454 [DOI] [PubMed] [Google Scholar]
- 12.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 13.Kanat M, Mari A, Norton L, et al. Distinct β-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes 2012;61:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256, e5 [DOI] [PubMed] [Google Scholar]
- 15.Pournaras DJ, Aasheim ET, Søvik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg 2012;99:100–103 [DOI] [PubMed] [Google Scholar]
- 16.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.K. Prospective Diabetes Study Group U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 19.Kahn SE, Haffner SM, Heise MA, et al. ; ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 20.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care 2005;28:995–1000 [DOI] [PubMed] [Google Scholar]
- 21.Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004;27:17–20 [DOI] [PubMed] [Google Scholar]
- 22.Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991;14:802–823 [DOI] [PubMed] [Google Scholar]
- 23.Hofsø D, Jenssen T, Bollerslev J, et al. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol 2011;164:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol 1999;277:E283–E290 [DOI] [PubMed] [Google Scholar]
- 26.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azrad M, Gower BA, Hunter GR, Nagy TR. Intra-abdominal adipose tissue is independently associated with sex-hormone binding globulin in premenopausal women. Obesity (Silver Spring) 2012;20:1012–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heshka S, Ruggiero A, Bray GA, et al. ; Look AHEAD Research Group Altered body composition in type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bikman BT, Zheng D, Pories WJ, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab 2008;93:4656–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 2006;55:2025–2031 [DOI] [PubMed] [Google Scholar]
- 31.Wadden TA, West DS, Delahanty L, et al. ; Look AHEAD Research Group The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]