Abstract
OBJECTIVE
Identify determinants of weight gain in people with type 2 diabetes mellitus (T2DM) allocated to intensive versus standard glycemic control in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.
RESEARCH DESIGN AND METHODS
We studied determinants of weight gain over 2 years in 8,929 participants (4,425 intensive arm and 4,504 standard arm) with T2DM in the ACCORD trial. We used general linear models to examine the association between each baseline characteristic and weight change at the 2-year visit. We fit a linear regression of change in weight and A1C and used general linear models to examine the association between each medication at baseline and weight change at the 2-year visit, stratified by glycemia allocation.
RESULTS
There was significantly more weight gain in the intensive glycemia arm of the trial compared with the standard arm (3.0 ± 7.0 vs. 0.3 ± 6.3 kg). On multivariate analysis, younger age, male sex, Asian race, no smoking history, high A1C, baseline BMI of 25–35, high waist circumference, baseline insulin use, and baseline metformin use were independently associated with weight gain over 2 years. Reduction of A1C from baseline was consistently associated with weight gain only when baseline A1C was elevated. Medication usage accounted for <15% of the variability of weight change, with initiation of thiazolidinedione (TZD) use the most prominent factor. Intensive participants who never took insulin or a TZD had an average weight loss of 2.9 kg during the first 2 years of the trial. In contrast, intensive participants who had never previously used insulin or TZD but began this combination after enrolling in the ACCORD trial had a weight gain of 4.6–5.3 kg at 2 years.
CONCLUSIONS
Weight gain in ACCORD was greater with intensive than with standard treatment and generally associated with reduction of A1C from elevated baseline values. Initiation of TZD and/or insulin therapy was the most important medication-related factor associated with weight gain.
Weight gain is a well-known consequence of the intensive treatment of type 2 diabetes mellitus (T2DM) (1). However, the definition of intensive therapy varies, and no studies have attempted near-normal glycemia, as in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Furthermore, some currently available therapies have a greater effect on weight, although the key determinants of weight gain in relation to intensive therapy remain unclear. Therefore, data from this trial could give us insight into the determinants of weight gain with intensive therapy.
The ACCORD trial randomized 10,251 people with type 2 diabetes and other cardiovascular risk factors to one of two glycemic targets: 1) an intensive A1C target of <6.0%; or 2) a standard target of between 7 and 7.9% (2). Participants were followed for a mean of 3.5 years until the intervention was stopped due to increased mortality in the intensive group. During this follow-up period, weight was measured regularly, and participants in the intensive group experienced greater weight gain than participants in the standard group. The data collected in the ACCORD trial provide an opportunity to identify determinants of weight gain in people with T2DM allocated to intensive versus standard glycemic control and to assess the relationship between changes in glycemic control and changes in weight.
The main outcomes of the ACCORD trial were previously reported (2). We present results on 8,929 participants (4,425 randomized to the intensive arm and 4,504 to the standard arm) with valid data at baseline and at least 2 years of follow-up. Participants who were not included did not have weights or withdrew or died during the first 2 years. In this analysis, we focus on weight gain as the dependent variable and describe the time course of weight change, its relationship to baseline characteristics and allocated treatment arm (intensive and standard), and its relationship to the postrandomization change in glycemic control (A1C) and use of glucose-lowering medications.
We posed several questions regarding potential causes of weight gain during the first 2 years of the trial, and the differences in weight gain experienced by the two allocated groups. First, was weight gain explained by the baseline characteristics (including prior medications)? Second, was weight gain explained by the change in A1C? Third, was weight gain explained by postrandomization medication use? Finally, were the factors that led to the change in weight the same in the intensive and standard groups?
RESEARCH DESIGN AND METHODS
Study design and participants
The ACCORD trial design, inclusion criteria, subject characteristics, and main results have been previously published (2). The ACCORD trial was designed to determine whether cardiovascular event rates in T2DM could be reduced through intensive glycemic control, intensive blood pressure control, and lipid management targeting triglycerides and HDL cholesterol in addition to LDL cholesterol. Each participant was randomized to either intensive (A1C goal <6.0%) or standard (A1C goal 7.0–7.9%) glycemic control and was assigned to either the blood pressure trial (intensive vs. standard arm) or the lipid trial (statin alone vs. statin plus fenofibrate).
This article represents a post hoc analysis of data available for ACCORD participants in both glycemia arms who completed at least 2 years of follow-up in the trial and had weight and A1C data available at the end of 2 years. Since most of weight gain occurred in the first 2 years, we focused our evaluation on this period. Furthermore, we have presented results from >90% of participants in the trial. At the time of discontinuation of the glycemic arm of the trial (and stopping intensive treatment), a large proportion of participants had not completed 3 years in the trial.
Weight measurements were obtained at every scheduled clinic visit (every 2 months in the intensive arm and every 4 months in the standard arm). Weight measurement of participants in the ACCORD trial was standardized by the use of high-quality scales in the clinics with a firm, flat surface and a zeroing system, weighing participants with minimal clothing and without shoes, and with weight distributed over both feet as evenly as possible. Weight was recorded in the ACCORD database only at 4-month intervals. Our analyses are constrained to data registered in the database. Information on baseline demographics, clinical characteristics, treatment, and treatment changes was obtained from the ACCORD trial database.
Statistical analysis
Baseline characteristics.
We used general linear models to examine the association between each baseline characteristic and weight change at the 2-year visit. For continuous variables, we report the estimated slope and for categorical variables the least square means. We then added the glycemia randomization arm and included an interaction term between the baseline characteristic and glycemia arm.
Change in A1C.
To examine the relationship between the 1- and 2-year change in weight and change in A1C, we first fit a linear regression of these changes from baseline to year 1 by each arm. We chose to do this analysis at the end of the first year because most of the changes in A1C occurred in the first year. We examined this relationship by tertiles of baseline A1C.
Medications.
To examine the effect of medications on weight change, we considered both baseline and postrandomization medication use. For all analyses, each medication except insulin was represented as an indicator regardless of dosing. Insulin was represented as units per kilogram of body weight.
We used general linear models to examine the association between each medication at baseline and weight change at the 1- and 2-year visits. Due to significant interactions between the effects of medication by arm, all further analyses were performed separately by glycemia arm. We limited our analyses of medications to those that had a significant effect (P < 0.05) on weight change both at baseline and postrandomization.
We then fit a modified R2 model described by Edwards et al. (3), which allows the direct comparison of the predictive effects of time-varying variables on an outcome measured at repeated time points. We used separate models for intensive and standard glycemia with the following predictors: baseline medications, baseline A1C, on-study medication, change in A1C and time interactions at 4-month intervals modeling the change in weight, at 4-month intervals. To determine whether the effects of the medication were different during the first and second years, we further stratified the analysis by first and second year. We considered all data available regardless of length of participation within the study.
To estimate the on-trial effects of medication, we fit separate repeated-measures linear models for standard and intensive arms with time-dependent covariates in which the dependent variable is change in weight measured at 4-month intervals through year 2. All models began with indicators for medication use at baseline. Since there were significant levels of interaction between the effects of on-study medication and use prior to study entry, we created combinations of patterns of each medication the baseline and on-study medications for each arm. This approach facilitated analysis of the effect of medications that were not used at baseline but started during the trial. For this analysis, insulin was categorized as on or off, regardless of dose or type. We report the least square means for each medication combination.
RESULTS
As previously described (2), there was significantly more weight gain in the intensive glycemia arm of the trial compared with the standard arm. The first 2 years accounted for approximately two-thirds of average weight gain. At the end of the second year, the average (± SD) weight gain was 3.0 (± 7.0) kg in the intensive arm. The pace of weight gain slowed thereafter. Weight gain in the standard arm was not only more modest but also did not continue beyond 24 months. Figure 1 illustrates the time course of the weight gain over the duration of the trial in both the intensive and standard groups.
Figure 1.
Time course of weight gain in the ACCORD trial by treatment allocation.
Association of weight changes with baseline characteristics
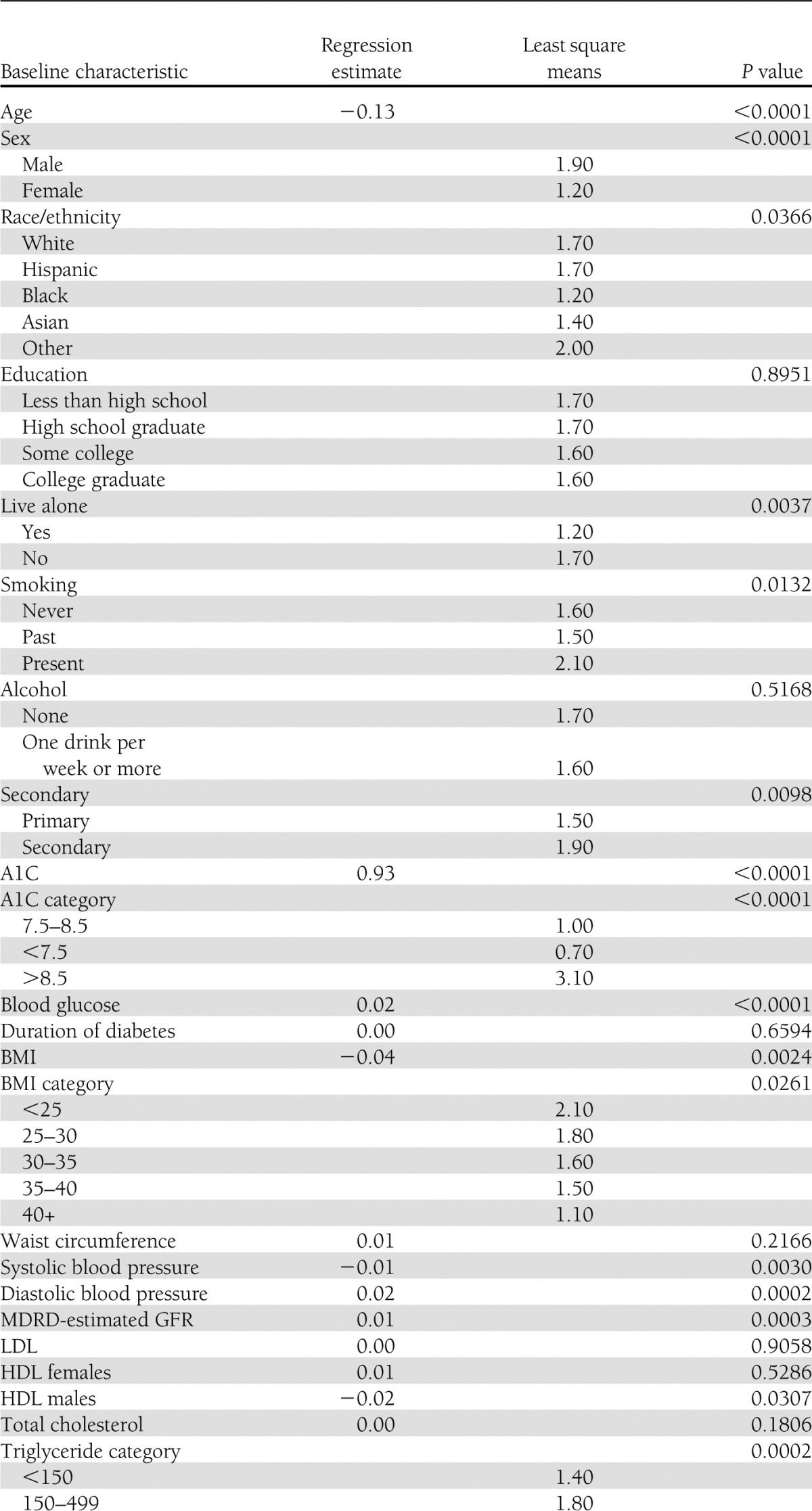
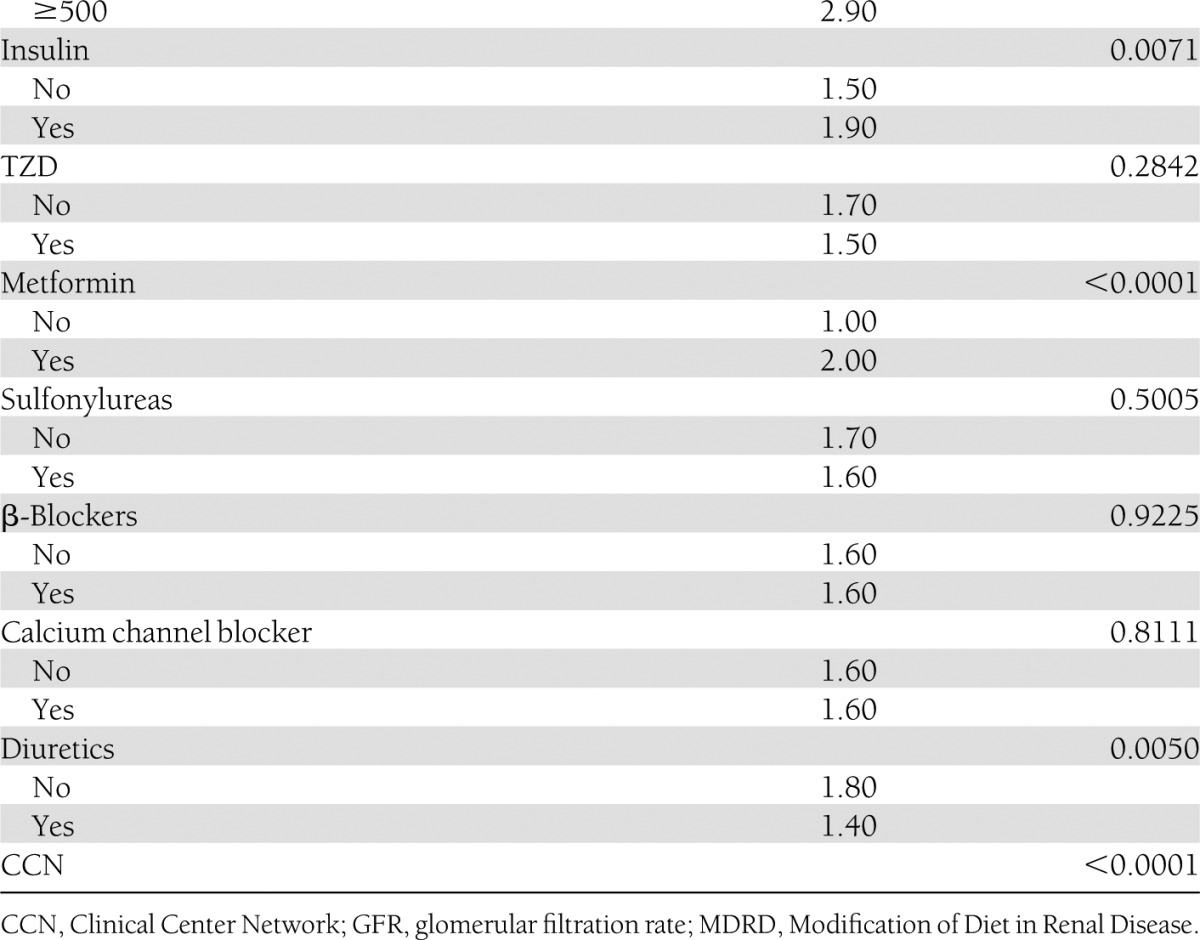
By univariate analysis (Table 1), the baseline characteristics of participants associated with increase in weight at 2 years in both glycemia arms were decreasing age, male sex, living with another person, smoking history, baseline A1C >8.5, high baseline BMI, race, high blood pressure, impaired renal function, low HDL cholesterol in males, high triglycerides, baseline insulin use, baseline metformin use, and not using diuretics. On multivariate analysis, younger age, male sex, Asian race, no smoking history, high A1C, BMI of 25–35, high waist circumference, insulin use, and metformin use were independently associated with weight gain over 2 years.
Table 1.
Baseline characteristics associated with weight gain at 2 years in both glycemia arms
While most of the weight change occurred during the first 2 years, univariate analysis (Supplementary Table 1) demonstrates that the baseline characteristics of participants associated with increase in weight from years 2–6 were younger age, diastolic blood pressure, HDL in females, metformin, and sulfonylurea use.
Change of A1C.
The relationship between the baseline and postrandomization A1C and change in weight differed by glycemia arm (Supplementary Table 2). Overall, the relationship between change in A1C and change in weight for those allocated to intensive glucose control was statistically significant (P < 0.001) with a fall in A1C associated with weight gain. In the intensive treatment arm, decreased A1C (when defined as a decrease of 0.5% in A1C from baseline) and weight gain occurred in 59.9% of participants, whereas weight loss and decreased A1C occurred in 28.8% of participants. Few intensive participants experienced weight gain and increased A1C. In only 6% of intensive participants did weight gain occur without any change in A1C.
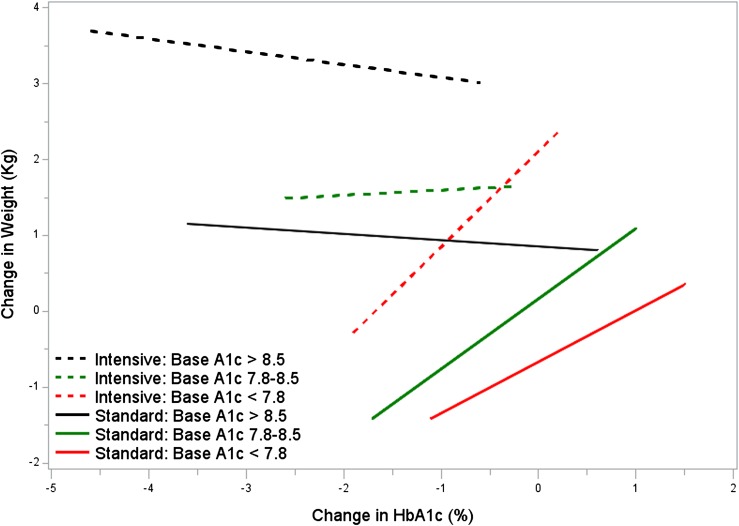
Figure 2 plots the relationship of change in weight with change in A1C over the first year by glycemia arm stratified by the baseline A1C categorized into thirds. In both treatment arms, participants with the highest baseline A1C on average gained weight with improvement in A1C. In contrast, when A1C was <7.8% at baseline, a drop in A1C during treatment was associated with a decrease in weight in both arms.
Figure 2.
Plots of change in A1C and change in weight by baseline A1C. Lines represent the estimated equation from the 5th to 95th percentile for each combination of baseline A1C and glycemia arm. Solid lines represent intensive arm, and interrupted lines represent standard treatment. Each treatment group has been divided into tertiles of baseline A1C.
Medications and weight gain during treatment.
Supplementary Table 3 summarizes the relationships between various medication-related factors and weight gain. The overall change in weight due to medication use accounted for <15% of the variability in any of the models. The glucose-lowering medications that had the most effect on weight gain were insulin, TZDs (mainly rosiglitazone), and metformin (Tables 2 and 3). The medications taken at baseline and follow-up and change in A1C explain 6 and 14% of the variability in the weight change from baseline during the first year 1, in the standard and intensive arms, respectively, and 10 and 12% of the variability in weight change during the second year, respectively.
Table 2.
Weight gain by metformin use
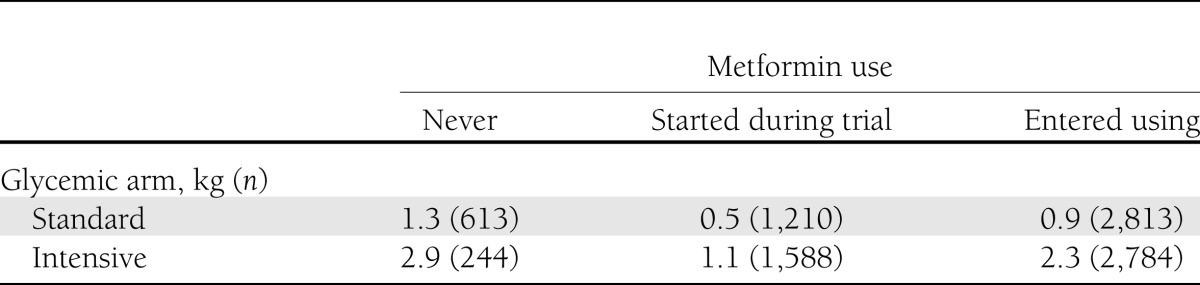
Table 3.
Weight gain by insulin and TZD use

The effects of insulin and TZD were not the same when considered in a linear mixed model. Therefore, we redefined TZD and insulin use in the following classification for both models in the standard and intensive arms. Table 3 summarizes the weight changes during use of various TZD/insulin combinations and metformin in the intensive group in the trial. There was a significant increase in weight (1.4 to 6.3 kg). These changes in weight with this combination were more marked in participants in the intensive arm of the trial.
Intensive participants who never took insulin or a TZD (n = 95) had an average weight loss of 2.9 kg during the first 2 years of the trial. In contrast, intensive participants who had never previously used insulin or TZD but began this combination at some time after enrolling in the ACCORD trial had a weight gain of 4.6 to 5.3 kg at 2 years. For participants who entered the ACCORD trial on metformin, were enrolled in the intensive arm, and began taking both a TZD and insulin over the first 2 years of the trial, the mean weight gain at 2 years was 4.9 kg.
Metformin use was significantly associated with weight change in participants in the intensive arm of the trial only. Participants in the intensive arm of the trial who never used metformin (n = 244) had a mean weight gain of 2.7 kg. Participants who entered the trial on metformin and continued (n = 2,784) gained an average of 1.9 kg, and those who had metformin added during the trial (n = 1,588) gained an average of 0.7 kg (P < 0.001 for effect). There was no interaction effect between the TZD/insulin combinations and metformin use.
CONCLUSIONS
Weight gain with intensive glycemia treatment was reported in the main ACCORD trial results paper (2). We report in this paper a description of the time course of weight changes in the ACCORD trial, along with baseline characteristics and medications and changes in A1C that were associated with changes in body weight following institution of intensive glycemic therapy in participants with type 2 diabetes. No single factor was strongly associated with, and potentially responsible for, the change in weight in each group. The models we tested explain only about 15% of the variability in body weight gain over the first 2 years of the trial.
In both treatment arms, participants with the highest baseline A1C gained weight with improvement in A1C. In contrast, when A1C was lower than 7.8% at baseline, a drop in A1C during treatment was associated with a decrease in weight in both arms. However, the degree of both A1C decline and weight loss in these participants was relatively small. Thus, in clinical practice, an attempt to intensify treatment in patients with a very high A1C is likely to lead to a significant weight gain. It is unclear whether the weight gain in such patients relates just to improvement in glycemia itself or greater use of insulin and TZD in patients who have higher baseline A1Cs.
We have presented results from >90% of participants in the trial. At the time of discontinuation of the glycemic arm of the trial (and stopping intensive treatment), a large proportion of participants had not completed 3 years in the trial. Thus, although the data suggest some continued weight gain beyond 2 years, the number of participants decreases rapidly over the years. Due to the lack of power, none of the statistical tests used are appropriate for use beyond the 2-year time point.
Some of the results are not surprising, particularly the associations of weight gain with high baseline A1C and insulin use subsequent to randomization. Similarly, the greatest gain in weight occurred in participants using combination of insulin and TZD during the trial. This combination was used more extensively in the intensive treatment arm of the trial. Those participants who used insulin at entry into the trial tended to gain more weight, perhaps due to substantial increases in their insulin dose and/or the addition of a TZD.
Change in A1C during the trial did not predict weight gain uniformly, suggesting that it is possible to improve glycemic control without weight gain in some individuals, perhaps using appropriate strategies to change lifestyle (4,5). Without adjustment for baseline A1C, a weight gain of 0.65 kg was associated with each 1% (absolute) reduction of A1C. However, assessment within treatment groups and by stratification according to the A1C at baseline showed in some circumstances either little change or a decrease in weight accompanying reductions of A1C. This may reflect the success of strategies to change lifestyle in some participants in the intensive arm, some of whom successfully lost weight while improving glycemic control.
The medications taken at baseline and follow-up explain 6 and 14% of the variability in the weight change from baseline during the first year in the standard and intensive arms, respectively, and 10 and 12% of the variability in weight change during the second year, respectively. Of interest, during the first year of treatment, variability in the subjects' baseline medications and baseline A1C account for almost all (88%) of the change in weight in the intensive arm but less than half of the weight change in the standard arm. Overall, TZD use accounted for a much higher percent of variability in weight in the intensive than the standard arm. This difference may relate to a greater use of TZDs in the intensive compared with the standard arm. It should also be noted that the overall amount of variability explained by medication use after randomization was relatively modest, never accounting for >15% of the variability in any of the models. However, in clinical practice, what matters most is the net effect on weight. Our data suggest that intensive therapy will probably cause weight gain (unless appropriate lifestyle interventions are instituted), and weight gain is likely to be maximal when the intensive therapy consists of a TZD-plus-insulin combination.
Metformin use at baseline was associated with weight gain during the trial compared with those who did not enter the trial on metformin. This may relate to the known effect of metformin to decrease body weight, which may have already occurred with maximal effect prior to entry into the study. In contrast, we were unable to observe an effect of metformin on attenuation of weight gain when used in conjunction with insulin, as has been seen in other studies (6,7). Lack of attenuation of insulin-induced weight gain by metformin has been observed in other studies (8), although these analyses may have been confounded by differences in doses of insulin used between those using or not using metformin. Furthermore, the A1C goals in these other trials may have been higher than in ACCORD, leading to less uptitration of insulin.
It is important to recognize that while we examined the relationship between the insulin dose (units per kilogram) and body-weight change, we did not do such an analysis for other medications. This is due to the wide range of doses used in insulin titration, whereas dose titration with oral medications were usually two to three steps only and included both increases and decreases in dose.
The physiologic mechanisms underlying weight gain in this study are not clarified by our analyses. Other studies have suggested that several factors are involved, including a reduction in glycosuria and thus retention of calories otherwise lost, changes in food intake, or changes in energy expenditure (9–13). In addition, insulin is known to be an anabolic hormone that also has some effects on weight regulation via the central nervous system, and TZDs have effects on adipogenesis. Drugs that have a lower propensity to cause weight gain or even weight loss, such as those affecting the incretin system or insulin detemir, were used only in a small proportion of participants in the ACCORD trial and for a relatively short duration, as they were not available at the start of the trial.
There are several limitations of our analysis. First, it was retrospective and not prespecified. Second, we could only include data on participants who had weight data available at 2 years and excluded those whose weight was not recorded or who had died or withdrawn from the study. Unfortunately, lifestyle changes were not quantified in the trial. All patients were advised regarding lifestyle changes according to American Diabetes Association guidelines, with varying degrees of compliance. We also did not assess factors associated with weight gain beyond the first 2 years in the trial, due to the fact that a large proportion of patients had not completed ≥3 years in the trial at the time of discontinuation of intensive treatment. We have included the baseline factors associated with weight gain during years 2–6 (Supplementary Table 1), although because of the diminishing number of patients, these data should be interpreted cautiously.
In summary, we have identified and characterized many features associated with weight gain with intensification of glycemic control. On multivariate analysis, younger age, male sex, Asian race, no smoking history, high A1C, BMI of 25–35, high waist circumference, insulin use, and metformin use at baseline were independently associated with weight gain over 2 years. Following randomization, the intensive group participants with the greatest reduction in A1C gained the most weight. Insulin and TZD use was associated with the greatest weight gain. However, all of these factors explain only a small proportion of the weight gain. Nevertheless, appreciation of these characteristics may help develop strategies to prevent weight gain when initiating intensive glycemic control in the future.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
V.F., R.M., J.C., R.M.C., M.F., H.C.G., F.I.-B., R.P.-B., and M.C.R. participated in the conduct of the trial and in the preparation of the manuscript. P.F. and T.M.M. carried out the statistical analysis. V.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00000620, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1391/-/DC1.
A complete list of members of the ACCORD Study Group can be found in the Supplementary Data online.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 statistic for fixed effects in the linear mixed model. Stat Med 2008;27:6137–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asnani S, Richard BC, Desouza C, Fonseca V. Is weight loss possible in patients treated with thiazolidinediones? Experience with a low-calorie diet. Curr Med Res Opin 2003;19:609–613 [DOI] [PubMed] [Google Scholar]
- 5.Unick JL, Beavers D, Jakicic JM, et al. Look AHEAD Research Group Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care 2011;34:2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yki-Järvinen H, Nikkilä K, Mäkimattila S. Metformin prevents weight gain by reducing dietary intake during insulin therapy in patients with type 2 diabetes mellitus. Drugs 1999;58(Suppl. 1):53–54; discussion 75–82 [DOI] [PubMed] [Google Scholar]
- 7.Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–625 [DOI] [PubMed] [Google Scholar]
- 8.Biesenbach G, Raml A, Alsaraji N. Weight gain and insulin requirement in type 2 diabetic patients during the first year after initiating insulin therapy dependent on baseline BMI. Diabetes Obes Metab 2006;8:669–673 [DOI] [PubMed] [Google Scholar]
- 9.Jacob AN, Salinas K, Adams-Huet B, Raskin P. Weight gain in type 2 diabetes mellitus. Diabetes Obes Metab 2007;9:386–393 [DOI] [PubMed] [Google Scholar]
- 10.Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2004;65(Suppl. 1):S23–S27 [DOI] [PubMed] [Google Scholar]
- 11.Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in IDDM. Diabetes 1993;42:1700–1707 [DOI] [PubMed] [Google Scholar]
- 12.McMinn JE, Baskin DG, Schwartz MW. Neuroendocrine mechanisms regulating food intake and body weight. Obes Rev 2000;1:37–46 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MW. Biomedicine. Staying slim with insulin in mind. Science 2000;289:2066–2067 [DOI] [PubMed] [Google Scholar]