Abstract
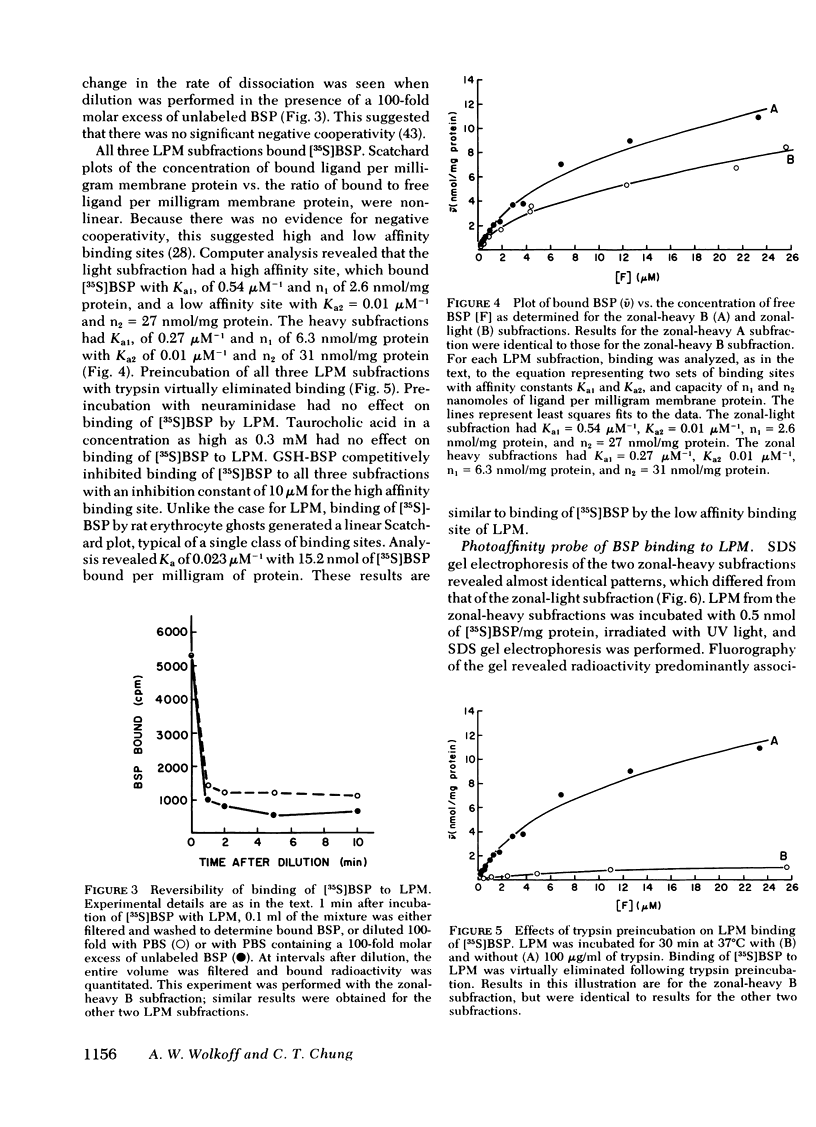
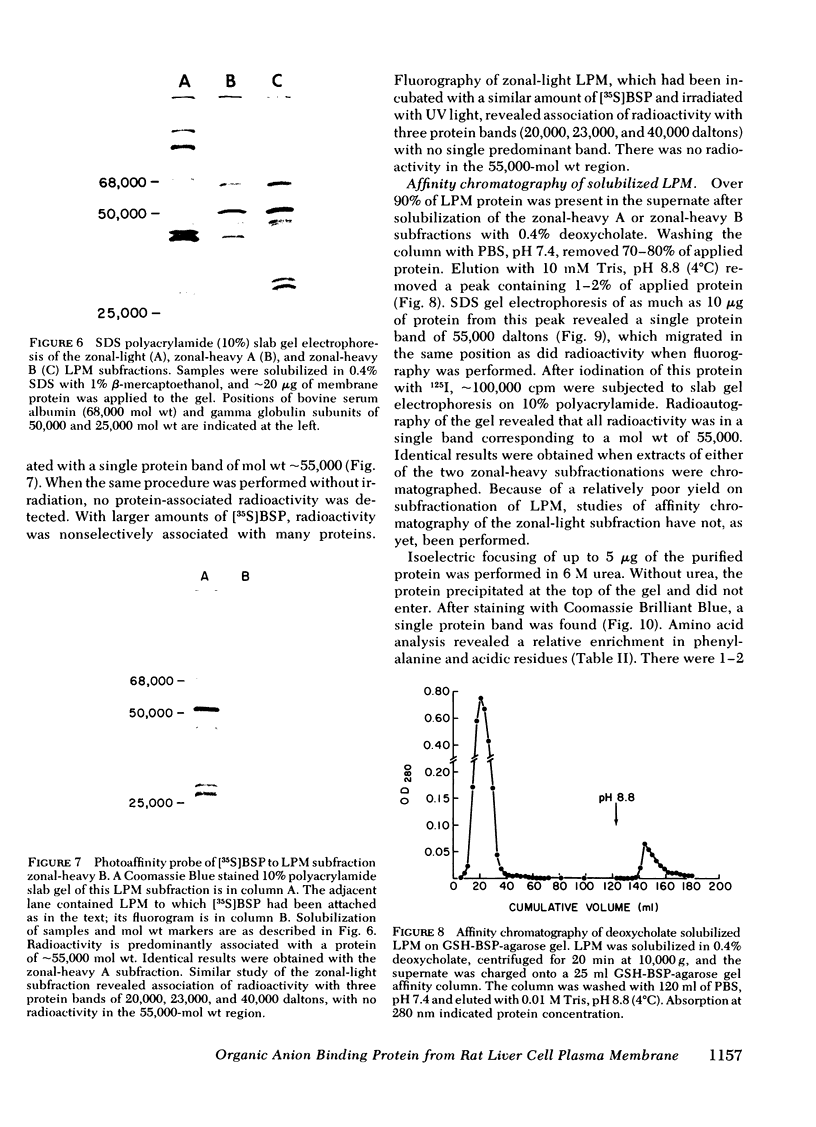
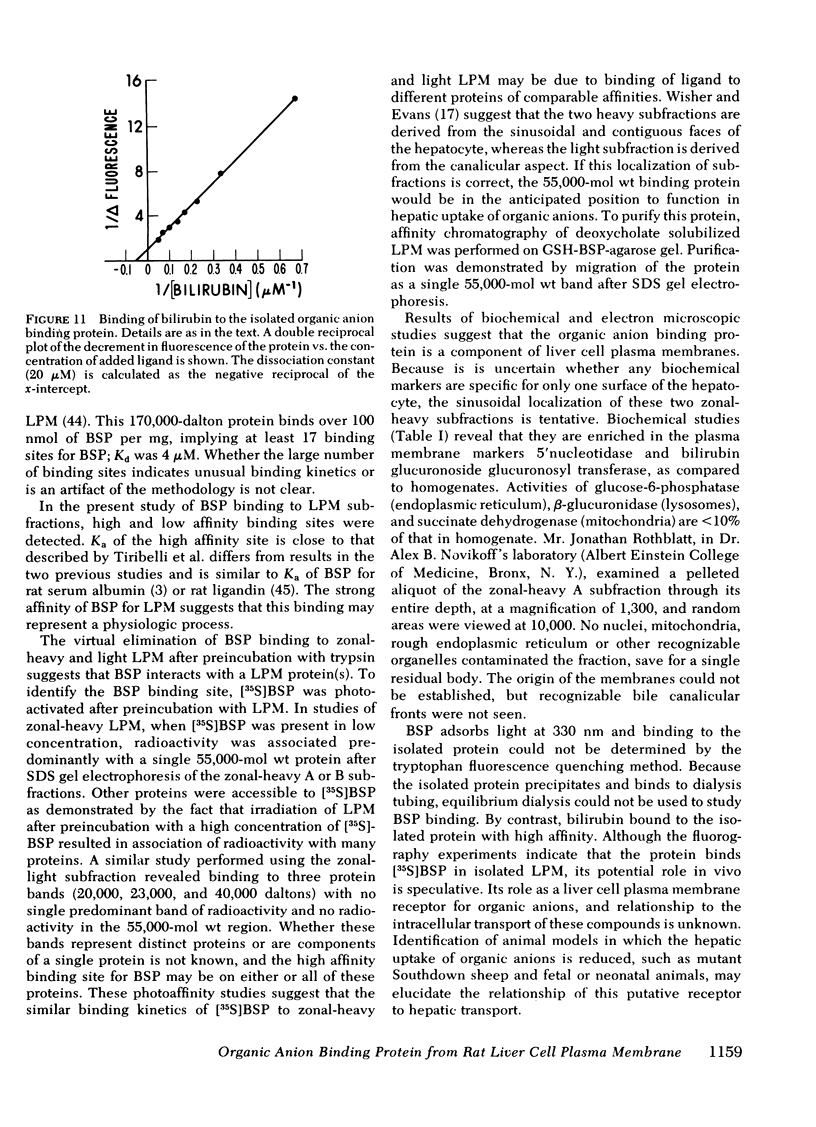
Uptake of bilirubin, sulfobromophthalein (BSP), and other organic anions by the liver is a process with kinetics consistent with carrier mediation. The molecular basis of this transport mechanism is unknown. In the search for the putative organic anion carrier or receptor, the interaction of BSP with rat liver cell plasma membrane (LPM) has been studied. Specific binding of [35S]BSP to LPM was determined using a filtration assay. Results revealed high affinity (Ka = 0.27 μM−1), saturable (6.3 nmol/mg protein) binding, which was eliminated after preincubation with trypsin. Although [35S]BSP was strongly bound to LPM, the binding was rapidly reversible, preventing direct identification and study of a specific binding site(s). To avoid this problem, a photoaffinity probe was devised, in which [35S]BSP is covalently bound to LPM after exposure to ultraviolet light. Subsequent sodium dodecyl sulfate gel electrophoresis and fluorography revealed radioactivity predominantly associated with a single 55,000-mol wt protein. A protein with identical electrophoretic mobility was purified from deoxycholate solubilized LPM after affinity chromatography on glutathione-BSP-agarose gel. This protein migrated as a single band on sodium dodecyl sulfate gel electrophoresis and on urea gel isoelectric focusing. It contained 1-2 residues of sialic acid per 55,000-dalton protein, and was immunologically distinct from rat albumin and ligandin. It bound bilirubin with a Kd of 20 μM, as determined by tryptophan fluorescence quenching. Although the high affinity of this LPM protein for organic anions suggests that it may function as a hepatocellular organic anion receptor, its role in transport of these compounds is unknown.
Full text
PDF





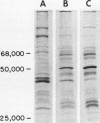
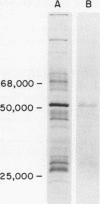
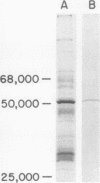
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN W. R., GRODSKY G. M., CARBONE J. V. INTRACELLULAR DISTRIBUTION OF TRITIATED BILIRUBIN DURING HEPATIC UPTAKE AND EXCRETION. Am J Physiol. 1964 Dec;207:1237–1241. doi: 10.1152/ajplegacy.1964.207.6.1237. [DOI] [PubMed] [Google Scholar]
- Berk P. D., Wolkoff A. W., Berlin N. I. Inborn errors of bilirubin metabolism. Med Clin North Am. 1975 Jul;59(4):803–816. doi: 10.1016/s0025-7125(16)31976-9. [DOI] [PubMed] [Google Scholar]
- Bernstein L. H., Ezzer J. B., Gartner L., Arias I. M. Hepatic intracellular distribution of tritium-labeled unconjugated and conjugated bilirubin in normal and Gunn rats. J Clin Invest. 1966 Jul;45(7):1194–1201. doi: 10.1172/JCI105425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Jansen P. L., Fischberg E. B., Daniller A., Arias I. M. Hepatic conversion of bilirubin monoglucuronide to diglucuronide in uridine diphosphate-glucuronyl transferase-deficient man and rat by bilirubin glucuronoside glucuronosyltransferase. J Clin Invest. 1978 Jul;62(1):191–196. doi: 10.1172/JCI109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C. E., Ben-Ezzer J., Arias I. M. Binding of sulfobromophthalein sodium (BSP) and other organic anions by isolated hepatic cell plasma membranes in vitro. Proc Soc Exp Biol Med. 1967 Feb;124(2):665–667. doi: 10.3181/00379727-124-31819. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin receptor of liver and fat cell membranes. Fed Proc. 1973 Aug;32(8):1838–1846. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dhumeaux D., Berthelot P. Chronic hyperbilirubinemia associated with hepatic uptake and storage impairment. A new syndrome resembling that of the mutant Southdown sheep. Gastroenterology. 1975 Oct;69(4):988–993. [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertingshausen G., Adler H. J. A new accelerated fully automated system for amino acid analysis by ion-exchange chromatography. J Chromatogr. 1969 Nov 11;44(3):620–623. doi: 10.1016/s0021-9673(01)92589-0. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Bergeron J. J., Geschwind I. I. Distribution of insulin receptor sites among liver plasma membrane subfractions. FEBS Lett. 1973 Aug 15;34(2):259–262. doi: 10.1016/0014-5793(73)80807-5. [DOI] [PubMed] [Google Scholar]
- Fleischner G., Robbins J., Arias I. M. Immunological studies of Y protein. A major cytoplasmic organic anion-binding protein in rat liver. J Clin Invest. 1972 Mar;51(3):677–684. doi: 10.1172/JCI106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORESKY C. A. INITIAL DISTRIBUTION AND RATE OF UPTAKE OF SULFOBROMOPHTHALEIN IN THE LIVER. Am J Physiol. 1964 Jul;207:13–26. doi: 10.1152/ajplegacy.1964.207.1.13. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Jansen P. L., Chowdhury J. R., Fischberg E. B., Arias I. M. Enzymatic conversion of bilirubin monoglucuronide to diglucuronide by rat liver plasma membranes. J Biol Chem. 1977 Apr 25;252(8):2710–2716. [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Betheil J. J., Arias I. M. Competitive binding of bilirubin, sulfobromophthalein, indocyanine green and other organic anions to human and bovine serum albumin. Biochim Biophys Acta. 1974 Sep 13;365(1):169–180. doi: 10.1016/0005-2795(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Gatmaitan Z., Arias I. M. Interactions of bilirubin and other ligands with ligandin. Biochemistry. 1975 May 20;14(10):2175–2180. doi: 10.1021/bi00681a021. [DOI] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- LEVVY G. A., MARSH C. A. Preparation and properties of beta-glucuronidase. Adv Carbohydr Chem. 1959;14:381–428. doi: 10.1016/s0096-5332(08)60227-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Regoeczi E., Debanne M. T., Hatton M. C., Koj A. Elimination of asialofetuin and asialoorosomucoid by the intact rat. Quantitative aspects of the hepatic clearance mechanism. Biochim Biophys Acta. 1978 Jul 3;541(3):372–384. doi: 10.1016/0304-4165(78)90196-4. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Waggoner J. G., Berk P. D. Hepatic organic anion uptake in the rat. J Clin Invest. 1975 Nov;56(5):1280–1292. doi: 10.1172/JCI108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiribelli C., Lunazzi G., Luciani M., Panfili E., Gazzin B., Liut G., Sandri G., Sottocasa G. Isolation of a sulfobromophthalein-binding protein from hepatocyte plasma membrane. Biochim Biophys Acta. 1978 Jan 25;532(1):105–112. doi: 10.1016/0005-2795(78)90453-1. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]
- Wolkoff A. W., Goresky C. A., Sellin J., Gatmaitan Z., Arias I. M. Role of ligandin in transfer of bilirubin from plasma into liver. Am J Physiol. 1979 Jun;236(6):E638–E648. doi: 10.1152/ajpendo.1979.236.6.E638. [DOI] [PubMed] [Google Scholar]
- Wolkoff A. W., Ketley J. N., Waggoner J. G., Berk P. D., Jakoby W. B. Hepatic accumulation and intracellular binding of conjugated bilirubin. J Clin Invest. 1978 Jan;61(1):142–149. doi: 10.1172/JCI108912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]