Abstract
OBJECTIVE
Pulse pressure (PP), a strong predictor of cardiovascular events in type 2 diabetes, is a composite measure affected by several hemodynamic factors. Little is known about the hemodynamic determinants of central PP in type 2 diabetes or whether abnormalities in central pulsatile hemodynamics are already present in individuals with impaired fasting glucose (IFG). In a population-based study, we aimed to compare central PP and its hemodynamic determinants among adults with normal fasting glucose (n = 1654), IFG (n = 240), and type 2 diabetes (n = 33).
RESEARCH DESIGN AND METHODS
We measured carotid pressure, left ventricular outflow, aortic root diameter, carotid artery flow, and distension in order to measure various structural and hemodynamic arterial parameters.
RESULTS
IFG was associated with a greater mean arterial pressure (MAP) but was not associated with intrinsic aortic stiffening or abnormal aortic pulsatile indices after adjustment for MAP. After adjustment for age, sex, and MAP, type 2 diabetes was associated with a higher aortic root characteristic impedance (Zc), aortic root elastance-thickness product (Eh), and aortic root pulse wave velocity (but not aortic root diameter), a greater carotid-femoral pulse wave velocity, and lower total arterial compliance and wave reflection magnitude. Carotid size, Zc, distensibility, or Eh did not significantly differ between the groups.
CONCLUSIONS
Type 2 diabetes, but not IFG, is associated with greater large artery stiffness, without abnormalities in aortic root diameter or carotid stiffness. Subjects with type 2 diabetes demonstrate a decreased reflection magnitude, which may indicate an increased penetration of pulsatile energy to distal vascular beds.
Cardiovascular disease is a leading cause of death in adults with type 2 diabetes. Pulse pressure (PP) is a strong predictor of cardiovascular events (1–3) and independently accounts for an important proportion of such events in patients with type 2 diabetes (3). Increased PP has also been associated with microalbuminuria in type 2 diabetes (1), consistent with a role for pressure pulsatility in microvascular target organ damage, as suggested by hemodynamic principles (4,5). Whether nondiabetic subjects with impaired fasting glucose (IFG) demonstrate pulsatile hemodynamic abnormalities is unknown. IFG is far more prevalent than type 2 diabetes, is associated with an increased risk of cardiovascular mortality, and contributes to a large number of cardiovascular deaths in the general population (6). A small study demonstrated that subjects with IFG demonstrated greater aortic PP measured in the catheterization laboratory than those with normal fasting glucose (FG) (7), but it is currently unclear whether an elevated PP (or abnormalities in its hemodynamic determinants) occur in association with IFG in unselected samples from the general population.
PP is a composite hemodynamic measure affected by several factors. Early in systole, the aortic root offers an impedance to blood flow that results in an increase in pulsatile pressure. The aortic property that determines the amount of pressure increase for any given left ventricular flow output during early systole is the proximal aortic characteristic impedance (Zc). Provided a given proximal aortic relative geometry (wall thickness/lumen ratio), aortic Zc is linearly related to the inverse of aortic luminal cross-sectional area and to the square root of the elastic modulus (stiffness) of the proximal aortic wall material. Aortic PP is also affected by wave reflections arising from the periphery, which generally arrive to the aorta in midsystole and augment mid- to late systolic pressure. The magnitude of wave reflections is influenced by the impedance mismatch between the central and peripheral arteries, whereas for any given distance to the reflection sites, the timing of arrival of wave reflections to the proximal aorta is influenced by aortic pulse wave velocity (PWV). Aortic PWV, in turn, is affected by wall stiffness and, provided any given relative geometry (lumen diameter to wall thickness ratio), is not affected by absolute aortic dimension. In addition to the factors mentioned above, PP is influenced by the total compliance of the arterial tree (TAC), which is influenced by arterial size, geometry and wall stiffness. Given the multiple determinants of PP, it follows that multiple potential abnormalities in arterial structure and hemodynamic function may lead to an increased PP in type 2 diabetes. Given the impact of PP on cardiovascular risk in type 2 diabetes, a better understanding of such determinants is important. A previous study demonstrated a greater proximal aortic Zc among 17 patients with type 1 diabetes mellitus compared with nondiabetic control subjects (8), but to our knowledge, the hemodynamic determinants of PP in type 2 diabetes have not been systematically investigated. Clearly, there is a need to: 1) understand the determinants of increased PP in type 2 diabetes and its potential impact on the pulsatility transmission to target organs and 2) assess whether abnormalities in pulsatile hemodynamic function are already present in subjects with IFG.
In this study, we aimed to comprehensively assess and compare parameters of arterial structure and pulsatile hemodynamic function among middle-aged adults with type 2 diabetes, IFG, and normal FG in the general population.
RESEARCH DESIGN AND METHODS
Study population
The Asklepios study recruited a representative cohort of apparently healthy, community-dwelling male and female volunteers aged 35–55 years, sampled from the twinned Belgian communities of Erpe-Mere and Nieuwerkerken as previously described (9). Subjects with clinically evident atherosclerosis/atherothrombosis, malignant tumors, cardiac disease, atrial fibrillation, renal disease, hepatic insufficiency, previous organ transplant, life expectancy <5 years, or pregnancy in the preceding 6 months were excluded. All analyses are based on data acquired during the baseline study visit, which occurred between October 2002 and October 2004. Central hemodynamic data derived from aortic pressure-flow analyses were available from 2,368 subjects (1,223 women and 1,145 men), which constituted the population for this study. None of the participants had a history of cardiovascular disease or stroke. All subjects provided informed consent. This study was approved by the ethical committee of the Ghent University Hospital and the University of Pennsylvania Institutional Review Board. IFG and diabetes were defined according to current American Diabetes Association criteria (10).
Hemodynamic assessments
Brachial blood pressure was measured with a validated oscillometric device (Omron HEM-907 device; Omron, Matsuka, Japan). Hypertension was defined as a brachial systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or antihypertensive medication use. Echocardiographic examinations were performed using a Vivid-7 ultrasound platform (GE Healthcare Vingmed Ultrasound, Trondheim, Norway) (9). Pulsed-wave Doppler measurements of flow velocities in the left ventricular outflow tract were performed and recorded, placing the Doppler sample immediately proximal to the aortic valve leaflets within the centerline of the left ventricular outflow tract. We computed left ventricular outflow tract cross-sectional area from its radius measured in the parasternal long axis view (area = pir2). Flow recordings were performed along with carotid artery applanation tonometry recordings using a Millar pen-type high-fidelity tonometer (SPT 301; Millar Instruments, Houston, TX) and dedicated hardware and software for acquisition of the arterial pulse (11). Carotid (central) pressure waveforms were calibrated according to brachial mean and diastolic pressure (11) because in contrast to systolic pressure, mean and diastolic pressure exhibit little variation in the arterial tree (12,13).
Pressure-flow analyses
Pressure and Doppler flow velocity recordings were processed offline using custom-designed software written in Matlab (The Mathworks, Natick, MA) as previously described (11). Proximal aortic Zc was computed in the time domain as the slope of the early systolic pressure-flow relation using the method described by Mitchell and colleagues (11,14). Reflection magnitude was computed using wave-separation analysis (11,12). In this method, after separation of the pressure waveform into its forward and backward components, reflection magnitude is computed as the ratio of the amplitudes of the backward/forward components (11,12). Reflected wave transit time was also computed from wave-separation analysis (15). TAC was calculated with the pulse pressure method (11). Cardiac output was computed as the product of stroke volume and heart rate. Systemic vascular resistance (SVR) was computed as mean arterial pressure/cardiac output.
Assessment of local proximal aortic stiffness
We measured aortic diameter as the distance between the internal borders (intima) of the aortic walls and in the proximal ascending aorta 1 to 2 cm distal to the sinotubular junction. Aortic cross-sectional area was computed as pir2. Aortic root PWV was calculated from the water hammer equation: PWV = (Zc × A)/ρ, where Zc is aortic characteristic impedance, A is ascending aortic cross-sectional area, and ρ is blood density, assumed to be 1.06 g/cm3. The product of aortic elastic modulus and wall thickness (Eh) was then computed using a rearranged Moens-Korteweg equation as follows: Eh = PWV2 × ρ × D, where D is measured ascending aortic diameter. Proximal aortic diameter could be reliably measured in 1,927 subjects (1,654 subjects with normal FG, 240 subjects with IFG, and 33 subjects with type 2 diabetes).
Assessment of carotid Zc, PWV, and Eh and distensibility
Carotid Zc was measured as the slope of the early systolic carotid pressure-flow relation in a carotid pressure-flow loop as previously described (16). Carotid PWV and Eh were computed using carotid Zc and diameter as described above for the ascending aorta. Carotid PWV was computed using the Moens-Korteweg equation as described above for the ascending aorta.
Comparisons of aortic root dimension between men and women after accounting for body size
Aortic root dimension was compared between the groups after allometric normalization for body surface area (BSA). To assess the normal allometric relation between aortic root area and BSA, we first selected a healthy reference sample as previously described (17). We used the following general allometric equation: y = axb + ε, where x is BSA, a and b are parameters, and ε is a random additive error term. The allometric model also included a sex term to satisfy the group-difference principle (17,18). The allometric power to normalize aortic area for BSA was estimated using nonlinear regression, in which an iterative technique is applied to estimate model parameters while maximizing data fit (17).
Supplementary Table 1 summarizes the definitions of key hemodynamic indices and measures of arterial stiffness used in this study.
Statistical analysis
Hemodynamic indices were compared between subjects with normal FG, IFG, and type 2 diabetes using ANOVA. Eh was log-transformed to improve normality for statistical models. Age- and sex-adjusted comparisons were performed with ANCOVA. For comparisons of pulsatile hemodynamic indices, further adjustments for MAP were performed, because MAP determines the operating wall stiffness (and arterial compliance) for any given wall-material properties (12). Because the relationship between MAP and stiffness is nonlinear, we included both a MAP and a MAP-squared term in these models. MAP and MAP-squared were mean-centered to minimize colinearity. Similarly, all hemodynamic indices that depend on volume flow (stroke volume, cardiac output, SVR, Zc, and TAC) are markedly influenced by body size and were therefore normalized for BSA, as estimated with the Gehan method (19) according to previous analyses that derived appropriate allometric powers that linearize the normal relationship between BSA and hemodynamic indices in this population (17). Therefore, for stroke volume, cardiac output, SVR, and TAC, a BSA term was introduced to all linear statistical models as a covariate, whereas for aortic Zc, BSA was raised to the power of −0.64 and introduced in the linear models (17). All probability values are two-tailed. Statistical significance was defined as α < 0.05. Statistical analyses were performed using SPSS for Mac OS v19 (SPSS Inc., Chicago, IL).
RESULTS
Baseline characteristics of study subjects with normal FG, IFG, and type 2 diabetes are shown in Table 1. Age, BMI, waist circumference, serum fasting triglycerides, serum high-sensitivity C-reactive protein, the prevalence of hypertension, and antihypertensive medication use were highest in subjects with type 2 diabetes and lowest in those with normal FG, whereas HDL cholesterol was highest is subjects with normal FG and lowest in subjects with type 2 diabetes. Men constituted a higher proportion of the IFG or type 2 diabetes group compared with the normal FG group. Heart rate was significantly higher among subjects with type 2 diabetes compared with either those with IFG or those with normal FG. There were no significant differences in cardiac index or stroke volume index between the groups. Subjects with IFG demonstrated significantly greater SVR compared with subjects with normal FG.
Table 1.
General characteristics of subjects with normal FG, IFG, and type 2 diabetes
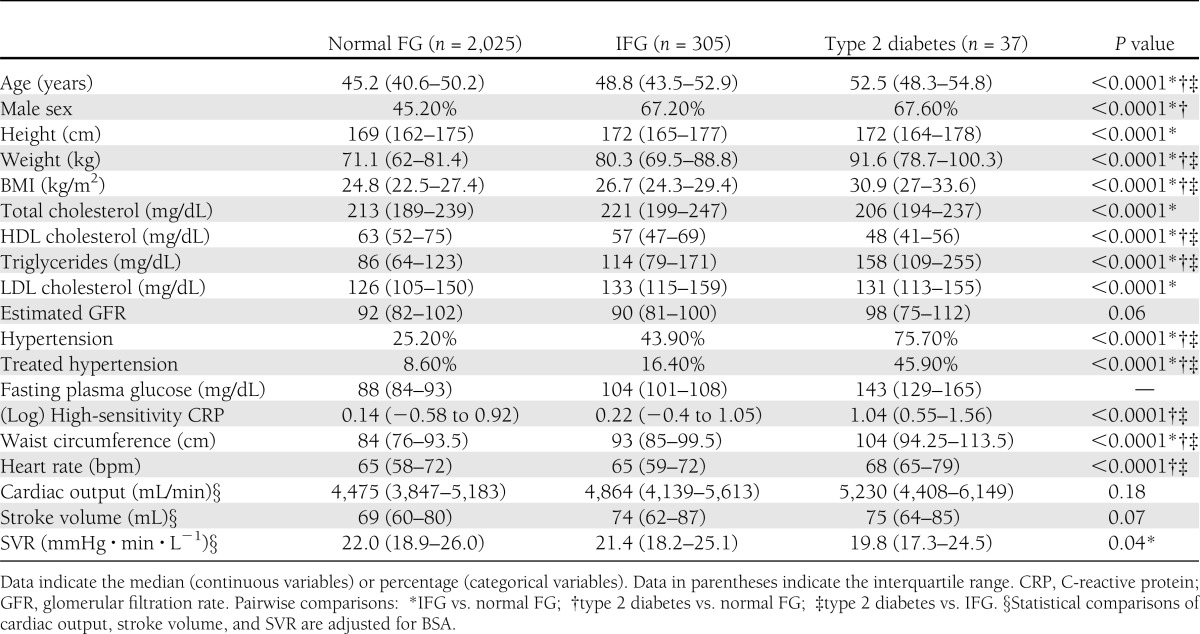
Thirteen of the 37 subjects with type 2 diabetes had a previous diagnosis (i.e., known type 2 diabetes). Among these subjects, mean diabetes duration was 4.8 years (interquartile range 2.3–6.3 years), 11.8% were treated with diet only, 52.9% were treated with a sulfonylurea, 70.6% were treated with metformin, and 5.9% were treated with insulin. The proportions of subjects treated with one, two, or three or more drugs were 52.9, 29.4, and 5.9%, respectively, and median HbA1c was 6.7% (interquartile range 5.15–8.1%).
Blood pressure differences between the groups
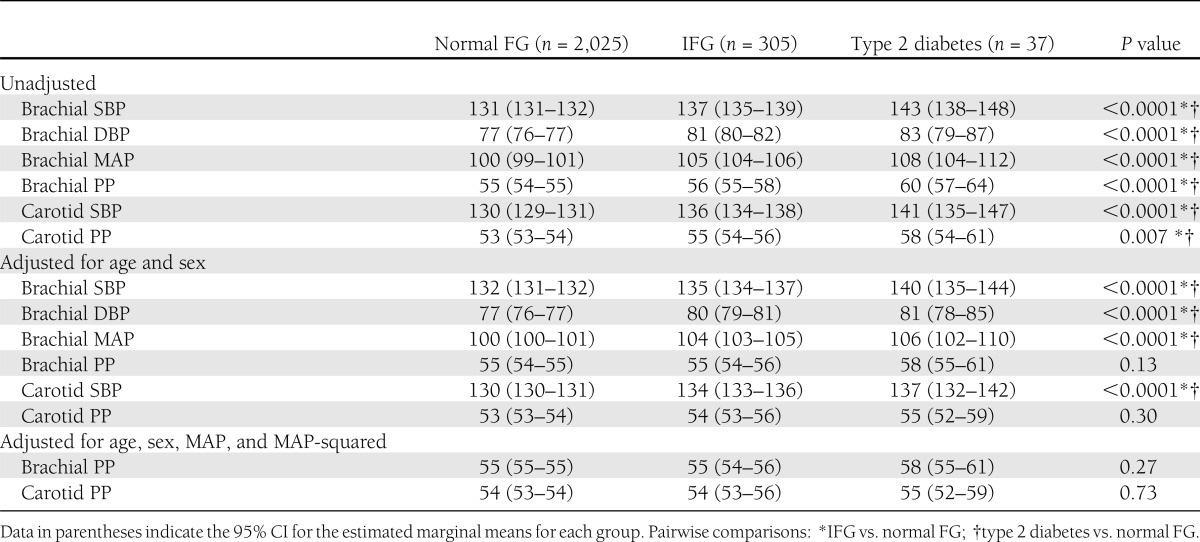
Brachial and central SBP, PP, DBP, and MAP were significantly lower in subjects with normal FG compared with subjects with IFG and type 2 diabetes, without significant differences between the latter two groups (Table 2). After adjustment for age and sex, the differences in brachial or carotid PP disappeared, whereas the differences in SBP, MAP, and DBP persisted. Similarly, after further adjustment for MAP, no differences in brachial or central PP were observed between the groups.
Table 2.
Comparison of peripheral and central blood pressure in subjects with normal FG, IFG, and type 2 diabetes
Aortic root dimensions
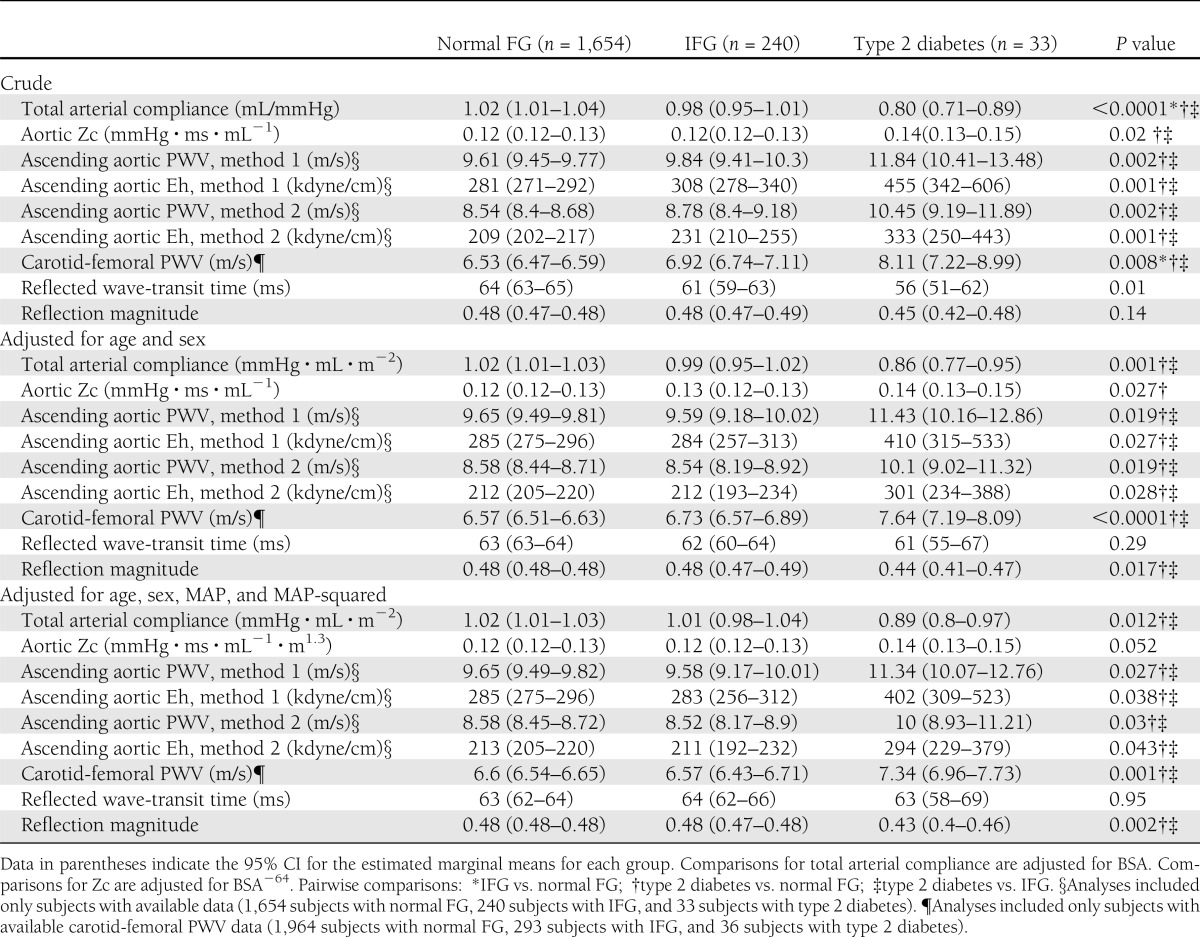
Cross-sectional proximal ascending aortic areas were significantly greater in subjects with IFG or type 2 diabetes compared with those with normal FG in unadjusted analyses (Supplementary Table 2). The allometric power to normalize aortic cross-sectional area for BSA was 0.50 (95% CI 0.14–0.86). Aortic area was directly associated with increasing age (P < 0.0001) and male sex (P < 0.0001). After adjustment for age and sex, allometrically normalized aortic areas were not significantly different between subjects with normal FG, IFG, and type 2 diabetes.
Indices of pulsatile load
In unadjusted analyses, subjects with type 2 diabetes demonstrated a greater carotid-femoral PWV, aortic root PWV, and aortic root Eh and a lower TAC index compared with subjects with normal FG (Table 3). After adjustment for age and sex, subjects with type 2 diabetes demonstrated a higher aortic root Zc index, aortic root PWV, aortic root Eh, and carotid-femoral PWV and a lower TAC index and reflection magnitude compared with the other groups, without significant differences between subjects with IFG and normal FG in any of these hemodynamic indices. Similar differences were observed after further adjustment for MAP. There were no significant differences in reflected wave-transit time between the groups. These trends were not appreciably changed with further adjustment for BMI or for serum HDL cholesterol, LDL cholesterol, or triglycerides (data not shown).
Table 3.
Pulsatile load and arterial stiffness measures in subjects with normal FG, IFG, and type 2 diabetes
Carotid pulsatile hemodynamics
After adjustment for age, sex, and MAP, no significant differences were found between the groups in carotid Zc, carotid diameter, carotid PWV, or carotid Eh (Supplementary Table 3). Similarly, reflection magnitude measured at the carotid site was not significantly different between the groups.
CONCLUSIONS
We comprehensively characterized, for the first time, central PP and its hemodynamic determinants in subjects with IFG and type 2 diabetes. We demonstrate that IFG and type 2 diabetes are associated with an increase in MAP and central PP. However, in contrast to type 2 diabetes, after adjustment for age, sex, and MAP, IFG was not associated with intrinsic arterial stiffening or abnormalities in pulsatile arterial hemodynamic function. Subjects with type 2 diabetes, in contrast, demonstrated an increase in proximal aortic Zc (which resulted from an increase in the elastance-thickness product, rather than an abnormal aortic diameter), an increase in carotid-femoral PWV, and a decrease in TAC. In contrast, carotid size, Zc, distensibility, and elastance-thickness product were not abnormal in type 2 diabetes or IFG. Subjects with type 2 diabetes also demonstrated paradoxically lower wave reflections. Our findings are important because they demonstrate that 1) large artery stiffening, rather than muscular artery stiffness, wave reflections, or aortic geometric remodeling, is the key abnormality underlying the higher central pulsatile load type 2 diabetes; 2) large artery stiffening is only apparent in established type 2 diabetes rather than IFG, although subjects with IFG had greater operant PP due to their higher MAP; and 3) type 2 diabetes is associated with reduced reflection magnitude, which suggests increased penetration of pulsatility to the microvasculature, which may promote target organ damage.
Type 2 diabetes was associated with an increased aortic Zc, due to an increased aortic root elastance-thickness product, but not a reduced ascending aortic cross-sectional area. Aortic root stiffness and hemodynamic function have not been previously examined in type 2 diabetes. This segment exerts an important influence on central PP and is not assessed with usual measurements of carotid-femoral PWV. Our findings are consistent with those of a previous study that reported greater aortic Zc among 17 individuals with type 1 diabetes, compared with nondiabetic control subjects (8). Also, consistent with our results, most (20–23), but not all (24,25), previous studies failed to show a significant association between type 2 diabetes and aortic dimension. We note that this is the first study to perform allometric normalization of aortic dimension for body size, an important methodologic aspect. We found that ascending aortic area relates to the square root of BSA (allometric power 0.5), statistically rejecting a linear relationship, thus precluding linear ratiometric adjustments. It follows that linear adjustment for BSA will overcorrect ascending aortic area in subjects with high BSA and undercorrect it in those with low BSA. Since type 2 diabetes is associated with obesity and thus a larger BSA, this may explain the slightly lower linearly adjusted aortic size values in type 2 diabetes in a previous study (25).
We also found a decrease in TAC among patients with diabetes. Arterial compliance is directly proportional to arterial size and distensibility. In turn, for any given relative geometry (wall-to-thickness ratio), distensibility is directly related to the elastic modulus of the wall material. The TAC is provided mainly by large arteries, although smaller arteries do contribute (12,13,26). We were unable to detect abnormalities in aortic diameter or carotid stiffness or diameter, suggesting that the decrease in TAC seen in type 2 diabetes is purely the result of aortic wall stiffening. Although we only assessed the aortic root, other studies have similarly failed to show smaller distal aortic segments in type 2 diabetes (21,27,28).
Potential mechanisms of arterial wall stiffening in type 2 diabetes include excessive extracellular matrix deposition of collagen or enhanced collagen cross-linking by advanced glycation end products (29,30), aortic wall calcification (31,32), endothelial dysfunction, chronic low-grade inflammation, increased oxidative stress, and increased sympathetic tone (33). Given the prognostic value of arterial stiffness in type 2 diabetes (34), further studies are required to better understand its mechanistic determinants in order to design therapies to reduce it.
The role of large artery stiffness in prediabetic states is also a subject of great interest. Glycemic spikes have been associated with a higher PP in patients with impaired glucose tolerance and essential hypertension (35), whereas PP and arterial stiffness have been associated with insulin resistance (36). In a recent study among high-risk hypertensive subjects (37), PP measured at baseline was a significant predictor of new-onset diabetes. Therefore, it was suggested that the relationship between large artery stiffness and type 2 diabetes is bidirectional. Microvascular alterations in association with increased arterial stiffness leading to impaired tissue perfusion and impaired insulin-mediated changes in muscle perfusion and glucose metabolism have been suggested as potential mechanisms by which increased arterial stiffness may promote the development of type 2 diabetes (33). Our findings indicate that greater large artery stiffness is not yet present in subjects with IFG, whereas prominent stiffening leading to a wide range of hemodynamic abnormalities are present in those with type 2 diabetes. This may indicate that aortic stiffening occurs only after type 2 diabetes has established or that subjects with IFG who demonstrate stiff large arteries preferentially develop to type 2 diabetes. Indeed, both mechanisms may be at play, and longitudinal studies are required to assess this issue.
Abnormal interactions between large and small vessels may also be important in the progression of microvascular complications in established type 2 diabetes. Retinopathy and nephropathy have been shown to be associated with carotid-femoral PWV (but not with carotid-radial PWV, a measure of muscular artery stiffness) in type 2 diabetes (38). In contrast to the clearly higher aortic wall stiffness in type 2 diabetes, we did not find greater carotid Zc, abnormal carotid diameter, or increased elastance-thickness product in type 2 diabetes. Our data regarding carotid diameter are consistent with a previous study (39). This selective stiffening of the aorta in type 2 diabetes without stiffening of more distal arteries may promote more penetration of pulsatile energy into the microcirculation of the brain and kidneys and reduce partial reflections in proximal arterial bifurcations. This may explain the apparently paradoxical observation of a lower reflection magnitude in subjects with type 2 diabetes. In a previous study, we showed that type 2 diabetes is associated with a lower augmentation index, a rough index of wave reflections, in a large multiethnic sample (40). Our finding is novel and contrasts with the current thinking that links diabetes with increased wave reflections (33), although it should be taken conservatively, because antihypertensive medication use was more common in type 2 diabetes and may have impacted the properties of muscular arteries, leading to decreased wave reflections.
Our study has limitations. Oral glucose tolerance tests were not performed, and our definition of IFG was based on a single measurement of FPG rather than repeated measurements. It is possible that individuals with normal FG in our study may include some individuals with impaired glucose tolerance. Our findings are restricted to a middle-aged population without established cardiovascular disease, which, however, was suitable to assess early vascular changes in diabetes and IFG. We used carotid pressure as a surrogate for aortic pressure, a limitation inherent to the noninvasive nature of our study in a large general population sample. Our comparisons were also limited by the fact that more subjects in the IFG and type 2 diabetes groups were receiving antihypertensive medication. We note, however, that this therapy would bias the comparisons toward the null and that we found greater (not lower) levels of MAP and intrinsic arterial stiffness in type 2 diabetes. Antihypertensive therapy, however, may have affected our comparisons of reflection magnitude (which was lower in type 2 diabetes), and therefore, findings regarding reflection magnitude should be taken conservatively, as discussed above. Our study aimed to characterize prevalent abnormalities in pulsatile load in diabetes and IFG, but specific data regarding retinal or renal microvascular complications were not available.
In summary, we comprehensively characterized, for the first time, pulsatile arterial hemodynamic abnormalities in IFG and type 2 diabetes in a large community-based sample of middle-aged adults. We found that IFG is associated with a greater MAP but not with intrinsic large artery stiffening, whereas type 2 diabetes is associated with higher aortic root Zc, elastance-thickness product, PWV, carotid-femoral PWV and lower TAC, without significant differences in aortic root or carotid diameter, Zc, or Eh. Our findings, therefore, identify aortic wall stiffening, rather than muscular arterial abnormalities, aortic root geometric remodeling, or wave reflections, as the key abnormality that increases pulsatile load type 2 diabetes. Future longitudinal studies are required to assess the role of the hemodynamic macrovascular–microvascular cross-talk in the development of microvascular complications in type 2 diabetes and in the risk of progression of earlier stages of the metabolic disease process to frank type 2 diabetes.
Acknowledgments
This study was supported by Fonds voor Wetenschappelijk Onderzoek Vlaanderen Grant G.0.838.10 and the American Heart Association Research Award 0885031N. J.A.C. received significant (>$10,000) grants from the National Institutes of Health and the American Heart Association for research studies related to arterial hemodynamics and has received minor support (equipment loans) from Atcor Medical, Cardiodynamics, and APC Cardiovascular Ltd. No other potential conflicts of interest relevant to this article were reported.
J.A.C. was responsible for study design, data analysis, and manuscript preparation. P.S., T.C.G., M.L.D.B., C.M.V.d., D.D.B., and E.R.R. were responsible for parent cohort study design and study design and reviewed and edited the manuscript. Z.A.K. and U.K. were responsible for data analysis and reviewed and edited the manuscript. J.A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data from this study were presented at the Annual Scientific Session of the American College of Cardiology, San Francisco, California, 9–11 March 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1463/-/DC1.
References
- 1.Schram MT, Kostense PJ, Van Dijk RA, et al. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002;20:1743–1751 [DOI] [PubMed] [Google Scholar]
- 2.Cockcroft JR, Wilkinson IB, Evans M, et al. Pulse pressure predicts cardiovascular risk in patients with type 2 diabetes mellitus. Am J Hypertens 2005;18:1463–1467; discussion 1468–1469 [DOI] [PubMed] [Google Scholar]
- 3.Nilsson PM, Cederholm J, Eeg-Olofsson K, Eliasson B, Zethelius B, Gudbjörnsdóttir S, Swedish National Diabetes Register (NDR) Pulse pressure strongly predicts cardiovascular disease risk in patients with type 2 diabetes from the Swedish National Diabetes Register (NDR). Diabetes Metab 2009;35:439–446 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 2008;105:1652–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–204 [DOI] [PubMed] [Google Scholar]
- 6.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–157 [DOI] [PubMed] [Google Scholar]
- 7.Cay S, Ozturk S, Funda Biyikoglu S, Atak R, Balbay Y, Aydogdu S. Association of aortic pressures with fasting plasma glucose in patients with and without impaired fasting glucose. Blood Press 2008;17:164–169 [DOI] [PubMed] [Google Scholar]
- 8.Sweitzer NK, Shenoy M, Stein JH, et al. Increases in central aortic impedance precede alterations in arterial stiffness measures in type 1 diabetes. Diabetes Care 2007;30:2886–2891 [DOI] [PubMed] [Google Scholar]
- 9.Rietzschel ER, De Buyzere ML, Bekaert S, et al. Asklepios Investigators Rationale, design, methods and baseline characteristics of the Asklepios Study. Eur J Cardiovasc Prev Rehabil 2007;14:179–191 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2005;28(Suppl. 1):S37–S42 [DOI] [PubMed] [Google Scholar]
- 11.Segers P, Rietzschel ER, De Buyzere ML, et al. Asklepios investigators Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension 2007;49:1248–1255 [DOI] [PubMed] [Google Scholar]
- 12.Nichols WW, O'Rourke MF, Vlachopolous C. McDonald’s Blood Flow in Arteries. Theoretical, Experimental and Clinical Principles. London, Hodder Arnold, 2011 [Google Scholar]
- 13.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension 2010;56:563–570 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Tardif JC, Arnold JM, et al. Pulsatile hemodynamics in congestive heart failure. Hypertension 2001;38:1433–1439 [DOI] [PubMed] [Google Scholar]
- 15.Segers P, Rietzschel ER, De Buyzere ML, et al. Assessment of pressure wave reflection: getting the timing right! Physiol Meas 2007;28:1045–1056 [DOI] [PubMed] [Google Scholar]
- 16.Zambanini A, Cunningham SL, Parker KH, Khir AW, McG Thom SA, Hughes AD. Wave-energy patterns in carotid, brachial, and radial arteries: a noninvasive approach using wave-intensity analysis. Am J Physiol Heart Circ Physiol 2005;289:H270–H276 [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA, Rietzschel ER, De Buyzere ML, et al. Asklepios investigators Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension 2009;54:558–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderburgh P. Two important cautions in the use of allometric scaling: the common exponent and group difference principles. Meas Phys Educ Exerc Sci 1998;2:153–164 [Google Scholar]
- 19.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep 1970;54:225–235 [PubMed] [Google Scholar]
- 20.Palmieri V, Bella JN, Arnett DK, et al. Aortic root dilatation at sinuses of valsalva and aortic regurgitation in hypertensive and normotensive subjects: The Hypertension Genetic Epidemiology Network Study. Hypertension 2001;37:1229–1235 [DOI] [PubMed] [Google Scholar]
- 21.Agmon Y, Khandheria BK, Meissner I, et al. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J Am Coll Cardiol 2003;42:1076–1083 [DOI] [PubMed] [Google Scholar]
- 22.Cuspidi C, Negri F, Salvetti M, et al. Working Group on Heart and Hypertension of the Italian Society of Hypertension Aortic root dilatation in hypertensive patients: a multicenter survey in echocardiographic practice. Blood Press 2011;20:267–273 [DOI] [PubMed] [Google Scholar]
- 23.Cuspidi C, Meani S, Fusi V, Valerio C, Sala C, Zanchetti A. Prevalence and correlates of aortic root dilatation in patients with essential hypertension: relationship with cardiac and extracardiac target organ damage. J Hypertens 2006;24:573–580 [DOI] [PubMed] [Google Scholar]
- 24.Chen XF, Wang JA, Lin XF, et al. Diabetes mellitus: is it protective against aortic root dilatation? Cardiology 2009;112:138–143 [DOI] [PubMed] [Google Scholar]
- 25.Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008;1:200–209 [DOI] [PubMed] [Google Scholar]
- 26.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension 2010;56:555–562 [DOI] [PubMed] [Google Scholar]
- 27.Hartley MC, Langan EM, 3rd, Cull DL, Taylor SM, Carsten CG, 3rd, Blackhurst DW. Evaluation of the diameter of the proximal descending thoracic aorta with age: implications for thoracic aortic stent grafting. Ann Vasc Surg 2009;23:639–644 [DOI] [PubMed] [Google Scholar]
- 28.Amer MS, Abdel Rahman TT, Omar OH, Reda RA, Ali DR. Influence of duration of diabetes mellitus, glycemic control, and traditional cardiovascular risk factors on abdominal aortic diameter in older adults with type 2 diabetes mellitus. J Am Geriatr Soc 2011;59:1362–1363 [DOI] [PubMed] [Google Scholar]
- 29.Soldatos G, Cooper ME. Advanced glycation end products and vascular structure and function. Curr Hypertens Rep 2006;8:472–478 [DOI] [PubMed] [Google Scholar]
- 30.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005;25:932–943 [DOI] [PubMed] [Google Scholar]
- 31.Takasu J, Katz R, Nasir K, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2008;155:765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odink AE, van der Lugt A, Hofman A, et al. Risk factors for coronary, aortic arch and carotid calcification; The Rotterdam Study. J Hum Hypertens 2010;24:86–92 [DOI] [PubMed] [Google Scholar]
- 33.Weber T. Arterial stiffness, wave reflections, and diabetes: a bidirectional relationship? Am J Hypertens 2010;23:1047–1048 [DOI] [PubMed] [Google Scholar]
- 34.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–2090 [DOI] [PubMed] [Google Scholar]
- 35.Anan F, Masaki T, Eto T, et al. Postchallenge plasma glucose and glycemic spikes are associated with pulse pressure in patients with impaired glucose tolerance and essential hypertension. Hypertens Res 2008;31:1565–1571 [DOI] [PubMed] [Google Scholar]
- 36.Sengstock DM, Vaitkevicius PV, Supiano MA. Arterial stiffness is related to insulin resistance in nondiabetic hypertensive older adults. J Clin Endocrinol Metab 2005;90:2823–2827 [DOI] [PubMed] [Google Scholar]
- 37.Yasuno S, Ueshima K, Oba K, et al. Is pulse pressure a predictor of new-onset diabetes in high-risk hypertensive patients?: a subanalysis of the Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trial. Diabetes Care 2010;33:1122–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardoso CR, Ferreira MT, Leite NC, Barros PN, Conte PH, Salles GF. Microvascular degenerative complications are associated with increased aortic stiffness in type 2 diabetic patients. Atherosclerosis 2009;205:472–476 [DOI] [PubMed] [Google Scholar]
- 39.Henry RM, Kostense PJ, Dekker JM, et al. Carotid arterial remodeling: a maladaptive phenomenon in type 2 diabetes but not in impaired glucose metabolism: the Hoorn study. Stroke 2004;35:671–676 [DOI] [PubMed] [Google Scholar]
- 40.Chirinos JA, Kips JG, Roman MJ, et al. Ethnic differences in arterial wave reflections and normative equations for augmentation index. Hypertension 2011;57:1108–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]


