Abstract
OBJECTIVE
Impaired parasympathetic and sympathetic nervous system activity have been demonstrated in patients with diabetes mellitus (DM) and correlated with worse prognosis. Few data are available on the effect of DM on cardiac neuropathy in heart failure (HF). The aim of the current study was to assess cardiac sympathetic activity in HF patients with and without DM.
RESEARCH DESIGN AND METHODS
Patients with severe HF (n = 75), with (n = 37) and without DM (n = 38), and 14 diabetic patients with normal cardiac function underwent 123I meta-iodobenzylguanidine scintigraphy from which early and late heart-to-mediastinum (H/M) ratios were calculated. Clinical, echocardiographic, and biochemical data were measured.
RESULTS
DM compared with non-DM patients showed significantly lower early (1.65 ± 0.21 vs. 1.75 ± 0.21; P < 0.05) and late H/M ratios (1.46 ± 0.22 vs. 1.58 ± 0.24; P < 0.03). Early and late H/M were significantly higher in DM patients without HF (2.22 ± 0.35 and 1.99 ± 0.24, respectively) than HF patients with (P < 0.0001) and without (P < 0.0001) DM. In HF patients, an inverse correlation between early or late H/M ratio and hemoglobin A1c (HbA1c) (Pearson = −0.473, P = 0.001; Pearson = −0.382, P = 0.001, respectively) was observed. In DM, in multivariate analysis, HbA1c and ejection fraction remained significant predictors of early H/M; HbA1c remained the only significant predictor of late H/M. No correlation between early or late H/M and HbA1c was found in non-DM patients.
CONCLUSIONS
Diabetic patients with HF show lower cardiac sympathetic activity than HF patients not having DM or than DM patients with a similar degree of autonomic dysfunction not having HF. HbA1c correlated with the degree of reduction in cardiac sympathetic activity.
Heart failure (HF) is a leading cause of morbidity and mortality worldwide and is characterized by sympathetic nervous system hyperactivity that significantly worsens prognosis (1–8). Cardiac adrenergic nerve activity has been assessed by 123I meta-iodobenzylguanidine (123I MIBG) imaging (9) and, as demonstrated by the AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF) study (10), the heart-to-mediastinum (H/M) ratio is an independent predictor of HF progression, arrhythmic cardiac events, and cardiac death. Reduced 123I MIBG uptake, likely due to diabetic neuropathy, has also been demonstrated in patients with diabetes mellitus (DM) without HF and correlated with worse prognosis (11,12). DM is common in HF patients with a prevalence range from 10 to 30% (13) and adversely influences long-term morbidity and mortality of symptomatic and asymptomatic HF patients (14,15). In diabetic HF patients enrolled in the ADMIRE-HF trial, it has been recently demonstrated that the combination of DM and reduced 123I MIBG cardiac uptake is an independent predictor of HF progression (16). Yet, the distinct impact of DM on cardiac 123I MIBG uptake in patients with HF has not been largely investigated, and no previous studies have assessed cardiac innervation in matched HF patients with and without DM. Therefore, the aim of this study was to evaluate 123I MIBG uptake in matched DM and non-DM patients with severe systolic HF.
RESEARCH DESIGN AND METHODS
Population and study protocol
We enrolled 37 consecutive patients with systolic HF and type 2 DM and 38 HF patients without DM referring to the outpatient clinic for HF at the University of Naples Federico II. To be included in the study, patients needed to fulfill the following criteria: left ventricular ejection fraction (LVEF) ≤40% and dilated cardiomyopathy in at least two consecutive echocardiographic evaluations, diagnosis of HF since at least 6 months, stable clinical conditions (New York Heart Association [NYHA] II–III), coronary angiography within 1 year from enrollment, and no acute coronary syndrome or angina in the 6 months before inclusion in the study. Ischemic cardiomyopathy was defined as ventricular dysfunction in myocardial regions subtended by significant (>70% diameter) coronary stenosis, with normal regional contractile function at echocardiography and/or invasive angiography in regions subtended by coronary arteries without significant stenosis. At the time of enrollment, all patients were on optimized medical therapy for HF treatment including the use of angiotensin-converting enzyme inhibitors or AT1 antagonists when not tolerated, β-blockers, loop diuretics, antialdosterone, and digitalis, when necessary, in addition to conventional drugs used for the treatment of cardiovascular risk factors and for secondary prevention of coronary heart disease. Fourteen type 2 DM patients with normal cardiac function were also included in the study. The diagnosis of DM was confirmed by clinical history or through the assessment of at least two determinations of fasting plasma glucose ≥126 mg/dL or a random plasma glucose test ≥200 mg/dL or with blood glucose levels ≥200 mg/dL 120 min after an oral glucose tolerance test performed with 75 g of glucose dissolved in water and confirmed by repeating the test on another day (17). On the same day, patients underwent clinical examination, venous blood sample collection, transthoracic echocardiography, and 123I MIBG imaging. Demographic data, including age, sex, height, body weight, BMI, HF medications, NYHA class, tobacco use, hypertension, dyslipidemia, family history of coronary events, duration of DM, presence of comorbidities, and ischemic versus nonischemic HF etiology were also collected. A venous blood sample was collected in all patients to assess biochemical data, including hemoglobin A1c (HbA1c) and N-terminal pro-brain natriuretic peptide (NT-proBNP); serum creatinine levels were obtained to estimate glomerular filtration rate (GFR) and assess renal impairment, as previously described (18). Diabetic patients were also screened for the presence of diabetic neuropathy using the Michigan Neuropathy Screening Instrument examination (19,20). A standard transthoracic echocardiography was performed in all patients using a VIVID E9 ultrasound system (GE Healthcare) with second-harmonic capability and a 3.5-MHz probe. All measurements were performed according to the European Society of Cardiology Recommendations for Chamber Quantification (21). Left ventricular (LV) diameters were obtained in the M-mode view. Global and regional LV function was evaluated and LVEF was calculated from apical four- and two-chamber views using the Simpson biplane method (21). Wall motion score index (WMSI) was calculated to assess the extent of regional wall motion abnormalities. At the end of this initial evaluation, synaptic noradrenaline reuptake was assessed by 123I MIBG scintigraphy. All patients gave written informed consent, and the local ethic committee approved the protocol.
123I MIBG imaging procedures
After blockage of the thyroid gland with 300 mg perchlorate, an activity of 111 MBq 123IMIBG (Mallinckrodt, Covidien) was administered over 1–2 min, and a 10-min planar anterior chest image was performed at 15 min (“early” image) and again at 3 h and 50 min (“late” image). Imaging was performed with low-energy/high-resolution collimators, and the camera peaked at 159 keV with a symmetrical 20% energy window. The images were acquired and stored in a 128 × 128 matrix (22). Two observers, blinded about patient status (i.e., diabetic or nondiabetic), analyzed 123I MIBG studies (10,23). H/M ratios were calculated from the early and late images after drawing regions of interest over the entire heart and upper mediastinum (7 × 7 pixels). Care was taken to exclude lung or liver from the myocardial and large vessels and lung from the mediastinum region of interest. 123I MIBG washout rate was calculated using the following formula: [(early heart counts/pixel – early mediastinum counts/pixel) – (late heart counts/pixel decay-corrected – late mediastinum counts/pixel decay-corrected)]/(early heart counts/pixel – early mediastinum counts/pixel).
Assessment of cardiac autonomic neuropathy
Evaluation of autonomic neuropathy was performed as previously described (11,24). In particular, five tests were used: 1) blood pressure change during standing up and 2) during sustained handgrip and 3) heart rate response to Valsalva maneuver, 4) to standing up, and 5) to deep breathing. Blood pressure response to standing up was evaluated through the difference of systolic blood pressure measured after 2 min of lying down and systolic blood pressure after standing up, whereas blood pressure response to 5 min of sustained handgrip at 30% of maximum voluntary contraction was evaluated through the difference of diastolic blood pressure assessed just before release of the handgrip and diastolic blood pressure measured before starting the maneuver. For heart rate responses, Valsalva maneuver was continued for 15 min at 40 mmHg, and then the ratio between the longest R wave–to–R wave (RR) interval soon after the release and the shortest RR during the maneuver was evaluated. Heart rate response to standing up was assessed as the ratio between the longest RR interval around the 30th beat and the shortest RR around the 15th beat (30:15 ratio), and finally heart rate changes to deep breathing were calculated through the mean of the differences of maximum and minimum heart rate of three consecutive deep breathings (six breaths per minute). A mean autonomic score was then calculated, referring to previously described normal, borderline, or abnormal values (24), and the presence of autonomic impairment was defined as an abnormal response to two or more of the five tests (11).
Statistical analysis
Data are expressed as mean ± SD. The Student t test was used for continuous variables. Correlation between variables was assessed by linear regression analysis, and variables that revealed a statistical significance in the univariate model were then included in a multivariate analysis. Categorical variables such as NYHA classification were analyzed by χ2 test. All data were collected in an Excel database and analyzed by SPSS 20.0. Statistical significance was accepted at P ≤ 0.05.
RESULTS
Patient characteristics
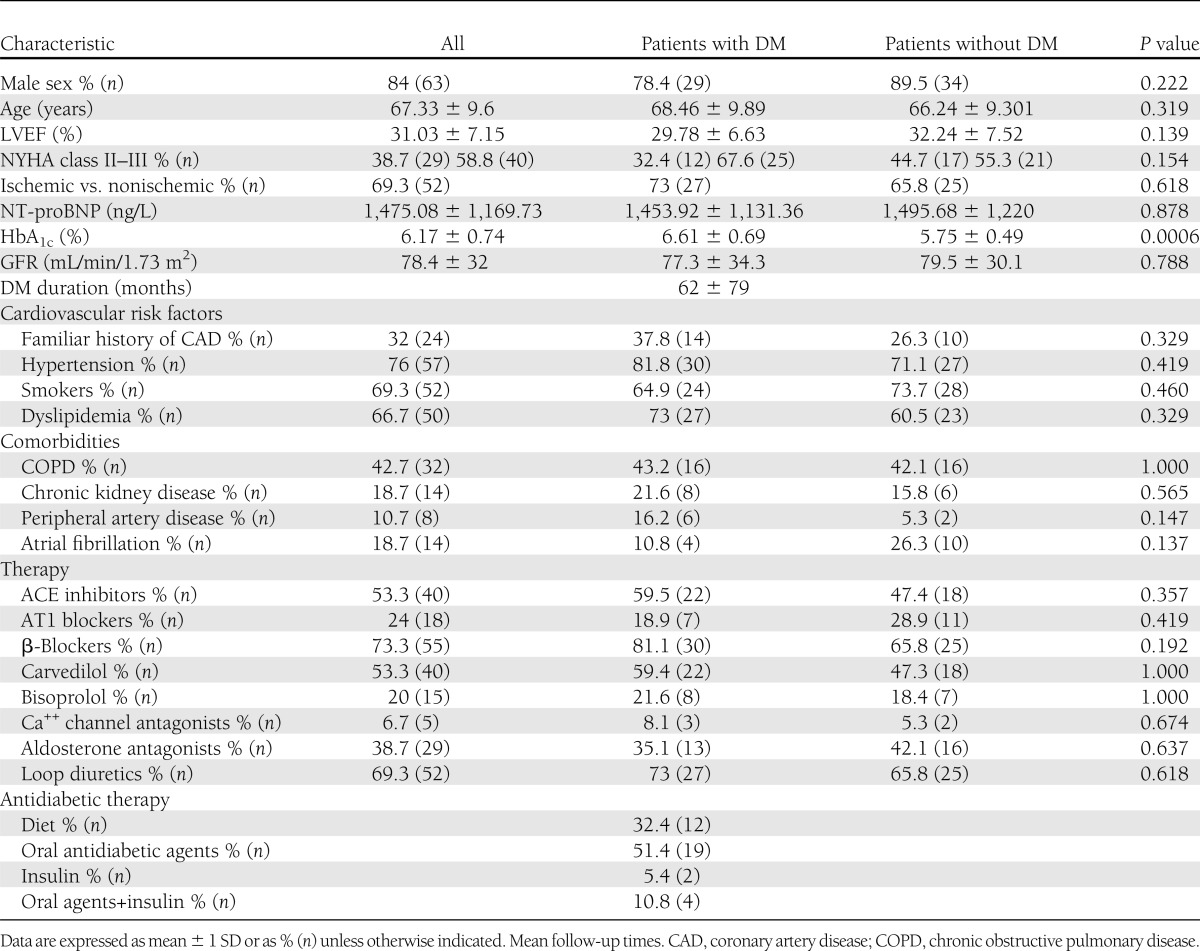
Mean age of the 75 patients with HF was 67.33 ± 9.6 years (84% male patients) with mean LVEF of 31.03 ± 7.15%. In 52 subjects (69.3%), HF was of ischemic etiology, and in 23 (30.7%), the etiology was an idiopathic dilated cardiomyopathy. All diabetic patients had a diagnosis of type 2 DM. No statistically significant differences between HF patients with and without DM were found for cardiovascular risk factors, demographic variables, comorbidities, LV systolic function, NYHA functional class, serum NT-proBNP levels, and HF therapy, as shown in Table 1. Medical treatment of DM was as follows: 19 patients (51.4%) were on oral antidiabetic agents alone, 2 (5.4%) on insulin alone, 4 (10.8%) on oral drugs + insulin, and 12 (32.4%) on diet only. Mean HbA1c was 6.61 ± 0.69% (49 mmol/mol), and 22 patients (59.5%) had an HbA1c measurement ≥6.5%, whereas 11 subjects (29.7%) had an HbA1c value >7%. Mean duration of DM was 62 ± 79 months. In 14 patients with DM without HF, mean age was 65.9 ± 8.8 years, mean DM duration was 58 ± 36 months, and mean HbA1c was 7.5 ± 1.5% (58 mmol/mol).
Table 1.
Baseline characteristics of HF patients with and without DM
123I MIBG imaging
Early and late H/M ratios were significantly lower in patients with HF and DM compared with patients with HF without DM. In particular, DM patients showed a mean early H/M ratio of 1.65 ± 0.21 vs. 1.75 ± 0.21 in non-DM subjects (P < 0.05) and a mean late H/M ratio of 1.46 ± 0.22 vs. 1.58 ± 0.24 in non-DM subjects (P < 0.03). 123I MIBG washout rate did not significantly differ between the two groups (38 ± 22 vs. 34 ± 22%; P = 0.44). Both early and late H/M were significantly higher in DM patients without HF (2.22 ± 0.35 and 1.99 ± 0.24, respectively) when compared with HF patients with (P < 0.0001) and without (P < 0.0001) DM.
Cardiac autonomic neuropathy
Mean autonomic score was 2.85 ± 0.80 in 20 patients with HF and DM and 3.06 ± 0.62 in 14 patients with DM without HF (P = NS). Autonomic impairment was found in all but 1 diabetic patient with HF and in 12 (86%) diabetic patients without HF. H/M ratios in 12 DM patients without HF with autonomic dysfunction were significantly higher compared with 19 DM patients with HF and with autonomic dysfunction (early H/M 2.24 ± 0.37 vs. 1.62 ± 0.16, respectively, P < 0.0001; late H/M 1.96 ± 0.24 vs. 1.42 ± 0.16, respectively, P < 0.0001). In the whole group of 34 DM patients evaluated for autonomic impairment, no correlation was found between autonomic score and both early and late H/M ratios (r = −0.70, P = 0.723 for early H/M; r = −0.787, P = 0.340 for late H/M). In addition, no correlation was found between mean autonomic score and HbA1c (r = −0.006, P = 0.977).
Determinants of 123I MIBG uptake in HF patients with and without DM
In the group of 75 patients with HF, in univariate analysis, early H/M ratio significantly correlated with age, LVEF, NYHA class, HF etiology, NT-proBNP, presence of DM, and HbA1c (Table 2). In multivariate analysis, only HbA1c remained a significant predictor of early H/M ratio (Table 2).
Table 2.
Determinants of 123I MIBG in all patients and in DM and non-DM patients
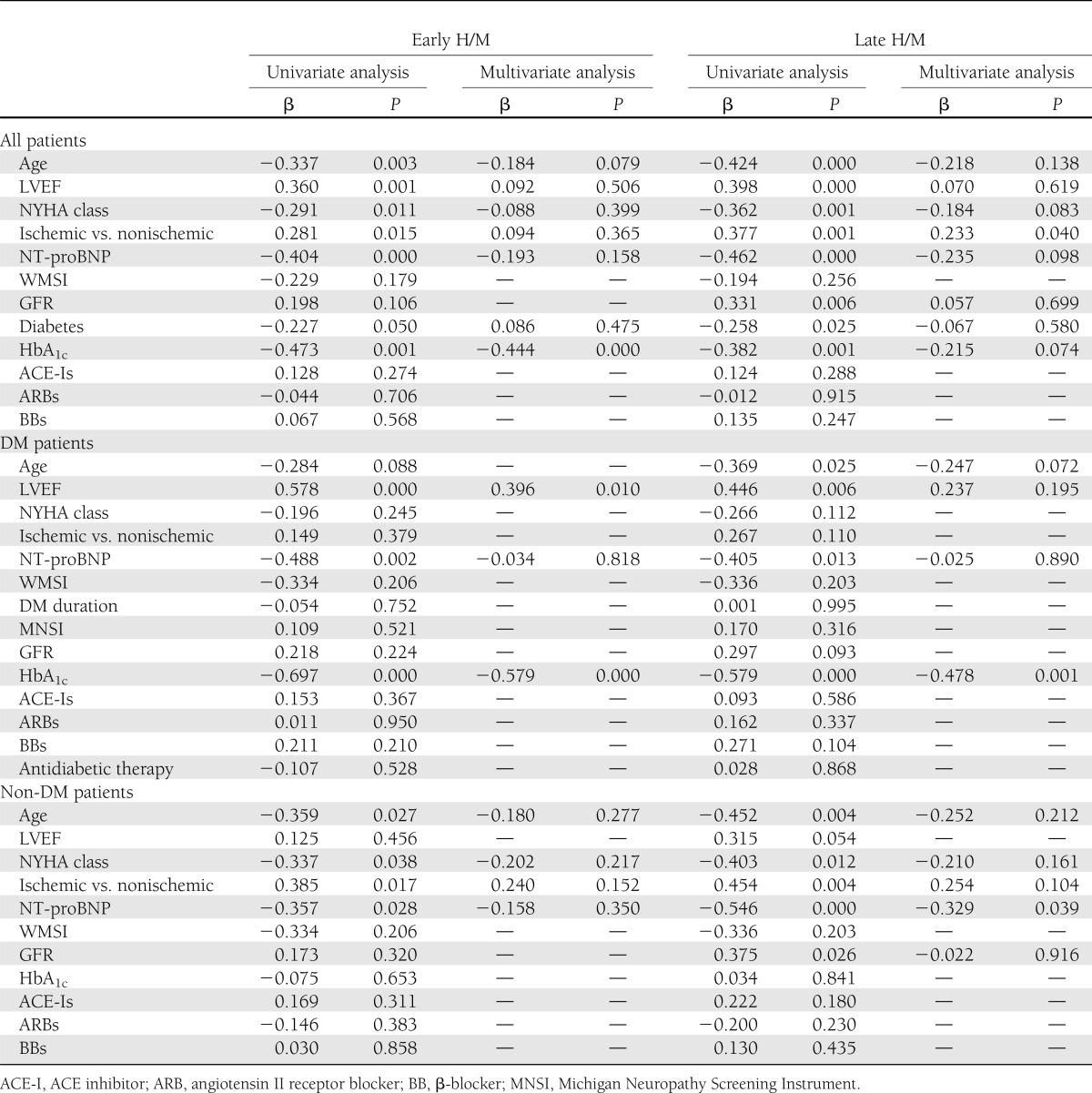
In univariate analysis, late H/M ratio significantly correlated with the same variables associated with early H/M and, in addition, with GFR (Table 2). In multivariate analysis, only etiology of HF remained significantly associated with late H/M. Interestingly, when “presence of DM” was eliminated from the multivariate analysis, HbA1c, in addition to HF etiology, remained significantly correlated with H/M ratio, surely because HbA1c acted as a surrogate for DM.
Determinants of 123I MIBG uptake in HF patients with DM
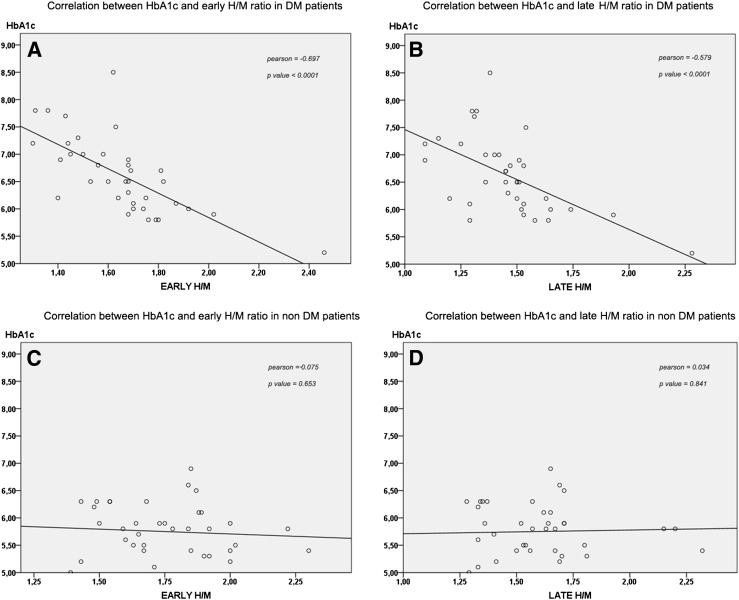
In the group of HF patients with DM, in univariate analysis, early H/M ratio significantly correlated with LVEF, NT-proBNP, and HbA1c (Table 2 and Fig. 1A). In multivariate analysis, LVEF and HbA1c remained significantly associated with H/M (Table 2).
Figure 1.
Correlation between HbA1c and early and late H/M ratio in HF patients with and without DM. A: Correlation between HbA1c and early H/M ratio in DM patients. B: Correlation between HbA1c and late H/M ratio in DM patients. C: Correlation between HbA1c and early H/M ratio in non-DM patients. D: Correlation between HbA1c and late H/M ratio in non-DM patients.
Late H/M ratio significantly correlated with age, LVEF, NT-proBNP, and HbA1c (Table 2 and Fig. 1B). In multivariate analysis, only HbA1c remained significantly associated with late H/M (Table 2).
Determinants of 123I MIBG uptake in HF patients without DM
In the group of HF patients without DM, in univariate analysis, early H/M ratio significantly correlated with age, NYHA class, HF etiology, and NT-proBNP (Table 2 and Fig. 1C). In multivariate analysis, none of these parameters remained a significant predictor of early H/M ratio (Table 2).
Late H/M ratio significantly correlated with the same variables associated with early H/M and, in addition, with GFR (Table 2 and Fig. 1D). In multivariate analysis, only NT-proBNP remained significantly associated with late H/M (Table 2).
CONCLUSIONS
The current study demonstrates that in DM patients with severe systolic HF, 123I MIBG cardiac uptake is significantly impaired compared with matched HF patients without DM and with DM patients without HF. In addition, in HF patients, 123I MIBG uptake significantly correlates with metabolic control of DM over the last 1–2 months, as indicated by the inverse association between H/M ratios and HbA1c levels.
Previous studies
Impaired 123I MIBG cardiac uptake was previously reported in DM patients without structural heart disease (12) and in DM patients with silent myocardial ischemia (11). In particular, Yufu et al. (12) recently demonstrated, in 108 subjects with type 2 DM but no cardiac diseases, that the 123I MIBG washout rate predicts major adverse cardiac and cerebrovascular events. Moreover, Scholte et al. (25) reported that 123I MIBG imaging was able to detect cardiac neuropathy in DM patients before the development of signs of cardiac autonomic imbalance, such as heart rate variability, and proposed that 123I MIBG imaging may provide early prognostic information in these patients. Mechanisms of reduced cardiac 123I MIBG uptake in DM patients without structural heart diseases are not completely understood and are presumably different from the mechanisms of reduced 123I MIBG uptake in HF patients with DM. Hyperinsulinemia exerts a sympathoexcitatory effect (26) that may lead to enhanced sympathetic tone and reduced 123I MIBG uptake in early stages of DM, whereas cardiac sympathetic denervation, demonstrated at postmortem studies, would be responsible for reduced 123I MIBG uptake in long-term diabetic patients with structural heart disease. However, few clinical data are available on the impact of DM on cardiac sympathetic activity in patients with HF. In fact, the only available data come from a recent retrospective analysis of the ADMIRE-HF trial (16). In this analysis, Gerson et al. (16) compared 343 DM patients with 618 non-DM patients enrolled in the ADMIRE-HF study (10) and reported that HF patients with DM and 123I MIBG H/M ratio <1.68 had about threefold increased risk of HF progression compared with HF patients without DM and with H/M ratio <1.68. It was also observed that DM patients in the ADMIRE-HF population showed significantly lower 123I MIBG H/M ratios (either early or late H/M) compared with non-DM patients. At variance with our study, 123I MIBG washout rate was also significantly higher in DM compared with non-DM patients. However, due to the retrospective design of that analysis, DM and non-DM patients were not matched for relevant characteristics that may have influenced the differences observed in 123I MIBG parameters. In particular, DM patients had significantly worse clinical conditions and significantly less use of β-blockers and were significantly older than non-DM patients. Since it has been reported that β-blocker therapy restores 123I MIBG uptake (27) and 123I MIBG uptake impairment correlates with the degree of clinical deterioration, it is difficult to dissect from the data of the ADMIRE-HF trial the distinct influence of DM on cardiac 123I MIBG uptake in HF patients.
Apart from the ADMIRE-HF data, no previous studies evaluated the impact of DM on cardiac 123I MIBG uptake in patients with overt HF, whereas an influence of DM on 123I MIBG uptake and an association with subclinical HF were previously observed (28,29). In fact, it was reported that DM patients with normal LV function at rest who developed contractile dysfunction during stress show more impaired 123I MIBG uptake compared with patients with a normal response to stress (28,29).
A provocative observation of the current study is the inverse correlation between HbA1c and either early or late H/M, observed in the whole population and in the subgroup of DM patients but not in non-DM patients. The strength of this association was supported by multivariate analysis that identified HbA1c as the only significant predictor of late H/M ratio and as an independent predictor of early H/M ratio in the subgroup of DM patients. To our knowledge, this observation is novel and, indeed, no such correlation was found in the ADMIRE-HF population (10). However, consistent with our findings, in a previous study, Ziegler et al. (30) observed in a small series of 12 type 1 DM subjects followed up for 4 years that poor glycemic control, assessed by HbA1c, represents a determinant of cardiac 123I MIBG uptake impairment that might be prevented by normoglycemia. In our study, HbA1c assessment and 123I MIBG imaging were obtained in the same day, which may explain the lack of correlation observed in the ADMIRE-HF populations. Notably, MIBG uptake in diabetic and nondiabetic HF patients was significantly lower than that observed in diabetic patients with autonomic dysfunction and normal LV function, suggesting that autonomic dysfunction does not explain the impairment of MIBG uptake in HF diabetic patients. These previous observations and the findings of the current study suggest a potential working hypothesis for future mechanistic studies aimed at assessing whether glycation directly affects the process of noradrenaline reuptake at synaptic level.
Limitations
There are limitations of the study that need to be acknowledged. The first is the relatively small number of patients, which makes our findings preliminary and warranting further confirmation. However, the dispersion of data observed in the current study was of the same magnitude as that observed in the larger ADMIRE-HF population (16), as indicated by the similar coefficient of variations of H/M ratios in the two studies (data not shown). The small number of patients may have prevented differences in the use of β-blockers and ACE inhibitors between DM and non-DM patients with HF to reach statistical significance. However, both classes of drugs demonstrated to improve 123I MIBG uptake (31). Thus, the higher percent of patients taking β-blockers and ACE inhibitors observed in the group of DM patients with HF may only have undermined the differences in H/M ratios observed in the current study. In addition, no influence of type of drug on MIBG uptake was found in univariate analysis. In the current study, 123I MIBG uptake was evaluated from planar images, and, therefore, the value of single-photon emission computed tomography (SPECT) 123I MIBG imaging, reported in previous studies (32), remains to be investigated. Likewise, although our findings were not influenced by wall motion score, lack of SPECT perfusion rest/stress data does not enable us to exclude the impact of myocardial necrosis or myocardial-inducible ischemia on our observations.
In patients affected by chronic, severe systolic HF, DM is associated with reduced cardiac 123I MIBG uptake compared with non-DM patients and with DM patients without HF, and 123I MIBG uptake independently correlates with glycemic control over the last 1–2 months. Additional pathophysiological studies are warranted to assess the biological relevance of these findings and their potential clinical implications for the management of diabetic HF patients.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.P. and G.R. conceived, designed, and executed the research project and wrote the first draft of the manuscript. G.P. designed and executed the research project and analyzed and interpreted data. T.P., M.T., and A.B. executed the research project and acquired scintigraphy data. G.S. analyzed data and performed statistical analysis. G.D.F. executed the research project and analyzed data. E.A. executed the research project and acquired data. R.F. executed the research project and performed autonomic neuropathy tests. L.P. executed the research project and performed autonomic neuropathy tests. F.S. executed the research project and performed laboratory analysis. G.G. executed the research project and reviewed and critiqued the manuscript. D.L., B.T., and A.C. reviewed and critiqued the manuscript. P.P.-F. conceived and designed the research project and reviewed and approved the final content of the manuscript. P.P.-F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Rengo G, Lymperopoulos A, Leosco D, Koch WJ. GRK2 as a novel gene therapy target in heart failure. J Mol Cell Cardiol 2011;50:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: fine-tuning cardiac stimulation. Trends Mol Med 2007;13:503–511 [DOI] [PubMed] [Google Scholar]
- 3.Rengo G, Perrone-Filardi P, Femminella GD, et al. Targeting the β-adrenergic receptor system through G-protein-coupled receptor kinase 2: a new paradigm for therapy and prognostic evaluation in heart failure: from bench to bedside. Circ Heart Fail 2012;5:385–391 [DOI] [PubMed] [Google Scholar]
- 4.Pilotto A, Addante F, Franceschi M, et al. Multidimensional prognostic index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail 2010;3:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Koch WJ. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J Am Coll Cardiol 2011;57:356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW, 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem 2010;285:16378–16386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lymperopoulos A, Rengo G, Zincarelli C, Soltys S, Koch WJ. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Mol Ther 2008;16:302–307 [DOI] [PubMed] [Google Scholar]
- 8.Rengo G, Zincarelli C, Femminella GD, et al. Myocardial β(2) -adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure. Br J Pharmacol 2012;166:2348–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrone-Filardi P, Paolillo S, Dellegrottaglie S, et al. Assessment of cardiac sympathetic activity by MIBG imaging in patients with heart failure: a clinical appraisal. Heart 2011;97:1828–1833 [DOI] [PubMed] [Google Scholar]
- 10.Jacobson AF, Senior R, Cerqueira MD, et al. ADMIRE-HF Investigators Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 2010;55:2212–2221 [DOI] [PubMed] [Google Scholar]
- 11.Langer A, Freeman MR, Josse RG, Armstrong PW. Metaiodobenzylguanidine imaging in diabetes mellitus: assessment of cardiac sympathetic denervation and its relation to autonomic dysfunction and silent myocardial ischemia. J Am Coll Cardiol 1995;25:610–618 [DOI] [PubMed] [Google Scholar]
- 12.Yufu K, Takahashi N, Okada N, et al. Cardiac iodine-123 metaiodobenzylguanidine (123I-MIBG) scintigraphy parameter predicts cardiac and cerebrovascular events in type 2 diabetic patients without structural heart disease. Circ J 2012;76:399–404 [DOI] [PubMed] [Google Scholar]
- 13.Soläng L, Malmberg K, Rydén L. Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur Heart J 1999;20:789–795 [DOI] [PubMed] [Google Scholar]
- 14.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–115 [DOI] [PubMed] [Google Scholar]
- 15.Shindler DM, Kostis JB, Yusuf S, et al. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol 1996;77:1017–1020 [DOI] [PubMed] [Google Scholar]
- 16.Gerson MC, Caldwell JH, Ananthasubramaniam K, et al. Influence of diabetes mellitus on prognostic utility of imaging of myocardial sympathetic innervation in heart failure patients. Circ Cardiovasc Imaging 2011;4:87–93 [DOI] [PubMed] [Google Scholar]
- 17.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg 2006;108:477–481 [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, et al. American Society of Echocardiography’s Nomenclature and Standards Committee. Task Force on Chamber Quantification. American College of Cardiology Echocardiography Committee. American Heart Association. European Association of Echocardiography, European Society of Cardiology Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108 [DOI] [PubMed] [Google Scholar]
- 22.Pace L, Perrone-Filardi P, Mainenti P, et al. Identification of viable myocardium in patients with chronic coronary artery disease using rest-redistribution thallium-201 tomography: optimal image analysis. J Nucl Med 1998;39:1869–1874 [PubMed] [Google Scholar]
- 23.Flotats A, Carrió I, Agostini D, et al. EANM Cardiovascular Committee. European Council of Nuclear Cardiology Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging 2010;37:1802–1812 [DOI] [PubMed] [Google Scholar]
- 24.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985;8:491–498 [DOI] [PubMed] [Google Scholar]
- 25.Scholte AJ, Schuijf JD, Delgado V, et al. Cardiac autonomic neuropathy in patients with diabetes and no symptoms of coronary artery disease: comparison of 123I-metaiodobenzylguanidine myocardial scintigraphy and heart rate variability. Eur J Nucl Med Mol Imaging 2010;37:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation 1997;96:4104–4113 [DOI] [PubMed] [Google Scholar]
- 27.Rengo G, Lymperopoulos A, Zincarelli C, et al. Blockade of β-adrenoceptors restores the GRK2-mediated adrenal α(2)-adrenoceptor-catecholamine production axis in heart failure. Br J Pharmacol 2012;166:2430–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreiner G, Wolzt M, Fasching P, et al. Myocardial m-[123I]iodobenzylguanidine scintigraphy for the assessment of adrenergic cardiac innervation in patients with IDDM. Comparison with cardiovascular reflex tests and relationship to left ventricular function. Diabetes 1995;44:543–549 [DOI] [PubMed] [Google Scholar]
- 29.Scognamiglio R, Avogaro A, Casara D, et al. Myocardial dysfunction and adrenergic cardiac innervation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 1998;31:404–412 [DOI] [PubMed] [Google Scholar]
- 30.Ziegler D, Weise F, Langen KJ, et al. Effect of glycaemic control on myocardial sympathetic innervation assessed by [123I]metaiodobenzylguanidine scintigraphy: a 4-year prospective study in IDDM patients. Diabetologia 1998;41:443–451 [DOI] [PubMed] [Google Scholar]
- 31.Toyama T, Aihara Y, Iwasaki T, et al. Cardiac sympathetic activity estimated by 123I-MIBG myocardial imaging in patients with dilated cardiomyopathy after beta-blocker or angiotensin-converting enzyme inhibitor therapy. J Nucl Med 1999;40:217–223 [PubMed] [Google Scholar]
- 32.Boogers MJ, Borleffs CJ, Henneman MM, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 2010;55:2769–2777 [DOI] [PubMed] [Google Scholar]