Abstract
OBJECTIVE
Hyperglycemia and hypoglycemia currently are considered risk factors for cardiovascular disease in type 1diabetes. Both acute hyperglycemia and hypoglycemia induce endothelial dysfunction and inflammation, raising the oxidative stress. Glucagon-like peptide 1 (GLP-1) has antioxidant properties, and evidence suggests that it protects endothelial function.
RESEARCH DESIGN AND METHODS
The effect of both acute hyperglycemia and acute hypoglycemia in type 1 diabetes, with or without the simultaneous infusion of GLP-1, on oxidative stress (plasma nitrotyrosine and plasma 8-iso prostaglandin F2alpha), inflammation (soluble intercellular adhesion molecule-1 and interleukin-6), and endothelial dysfunction has been evaluated.
RESULTS
Both hyperglycemia and hypoglycemia acutely induced oxidative stress, inflammation, and endothelial dysfunction. GLP-1 significantly counterbalanced these effects.
CONCLUSIONS
These results suggest a protective effect of GLP-1 during both hypoglycemia and hyperglycemia in type 1 diabetes.
Recent evidence suggests that hypoglycemia also may play an important role in favoring diabetic vascular complications (1). Hypoglycemia causes oxidative stress (2), inflammation (3,4), and endothelial dysfunction (5). Oxidative stress is considered the key player in the pathogenesis of diabetes complications (6). It is of interest that during hyperglycemia, oxidative stress is mainly produced at the mitochondrial level (6), similar to what happens in hypoglycemia (2). Therefore, oxidative stress might be considered the common factor linking hyperglycemia, hypoglycemia, and vascular complications of diabetes. Consistent with this hypothesis is the evidence that both hyperglycemia (7) and hypoglycemia produce endothelial dysfunction and inflammation through oxidative stress generation (5,8). Both endothelial dysfunction and inflammation are well-recognized pathogenic factors for vascular disease, particularly in diabetes (9).
Glucagon-like peptide 1 (GLP-1) and its analogs are now being used in clinics to enhance insulin secretion and to reduce body weight in patients with type 2 diabetes (10) in whom a defect of GLP-1 secretion or action in response to the meal often has been reported (11). GLP-1 has been shown to lower postprandial and fasting glucose and HbA1c, to suppress the elevated glucagon level, and to stimulate glucose-dependent insulin synthesis and secretion (10). Recently, a possible beneficial effect of GLP-1 analogs in the management of type 1 diabetes has been suggested (12). GLP-1, in addition to its insulin-tropic action in alleviating hyperglycemia, has beneficial effects in protecting progressive impairment of pancreatic β-cell function, preservation of β-cell mass, and suppression of glucagon secretion, gastric emptying, and appetite, which are all characteristics that could be beneficial for the management of type 1 diabetes (12).
Apart from the well-documented incretin effect of GLP-1, its role in the cardiovascular system also arouses interest. GLP-1 effects on the cardiovascular system may include a direct action on the endothelium in which the presence of specific receptors for GLP-1 has been demonstrated (13). Consistently, GLP-1 has been demonstrated to improve endothelial function in diabetes (14,15). This protective effect should be exerted to improve the antioxidant defenses of the endothelium (16) and to decrease oxidative stress generation (15).
The aim of this study is to test whether GLP-1 can protect endothelial function and reduce the generation of oxidative stress and inflammation during acute hyperglycemia and hypoglycemia in type 1 diabetes.
RESEARCH DESIGN AND METHODS
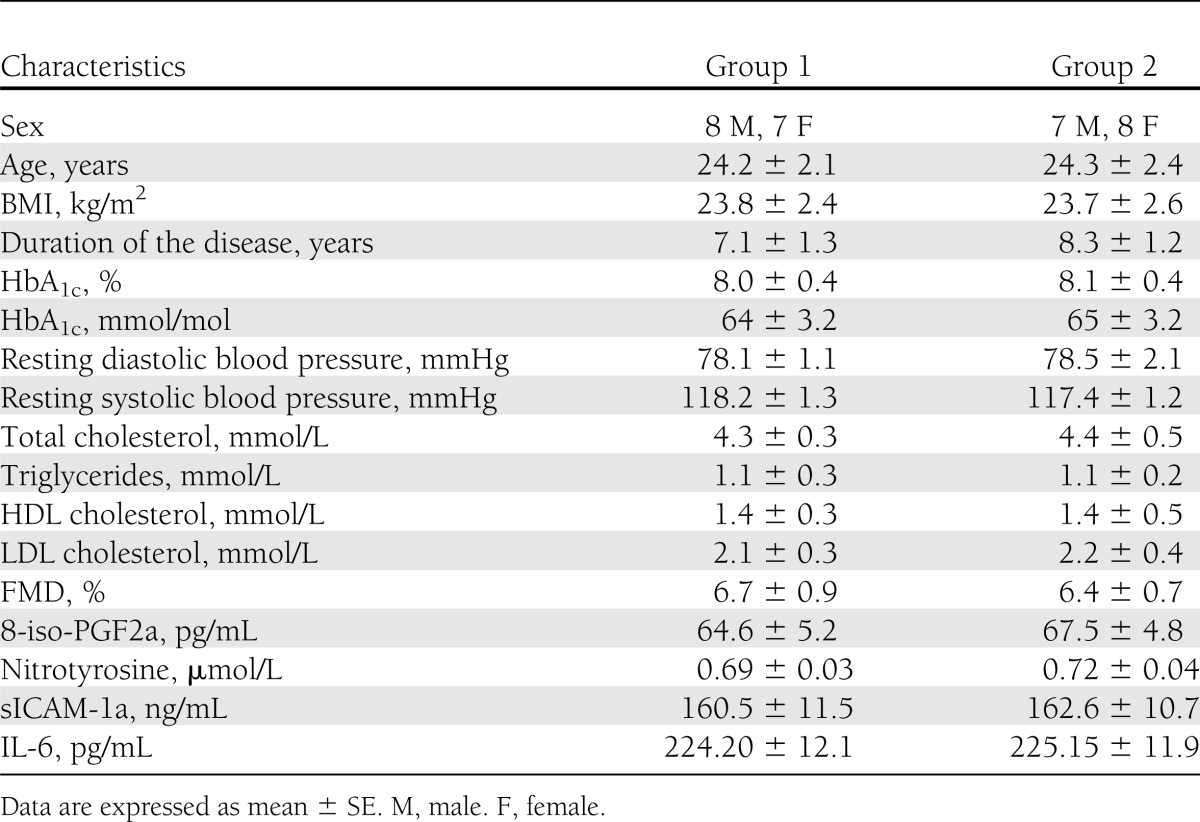
Two groups of 15 matched subjects with type 1 diabetes were studied (Table 1). They had bedside tests of autonomic function (17) (based on the methods of Gold et al. [18]) yielding normal results and did not have hypoglycemia unawareness, and they had no major macrocomplications or microcomplications of diabetes. They were treated with multiple daily insulin injections. All subjects were nonsmokers and had normal blood cell counts, plasma lipids, plasma electrolytes, and liver and renal function, and they were normotensive. No subject was using medications known to affect neuroendocrine responses to hypoglycemia or that were anti-inflammatory. Studies were approved by the ethical committees of the authors' institutions, and all participants gave written informed consent.
Table 1.
Baseline characteristics of the two groups of type 1 diabetic patients
All study patients were asked to avoid any exercise and to consume their usual weight-maintaining diet for 3 days before each experiment. All people were asked to perform intensive home blood glucose monitoring and to avoid hypoglycemia for at least 5 days before a study. On the day before a study, intermediate or long-acting insulin was discontinued and replaced by injections of regular insulin before breakfast and lunch. Each subject was admitted to the research center the evening before an experiment. At that time, two intravenous cannulas were inserted under 1% lidocaine local anesthesia. One cannula was placed to be used for blood drawing. The other cannula was placed in the contralateral arm for infusions. All subjects received an evening meal and a continuous low-dose infusion of insulin to normalize plasma glucose. The insulin infusion was adjusted overnight to maintain blood glucose between 4.4 and 7.2 mmol/L.
Hypoglycemia experiments
All the subjects of group 1 were studied after an overnight 10-h fast. Two different experiments were planned for each subject in randomized order, a period of 2 h of hypoglycemia with or without GLP-1 infusion. Each subject underwent each experiment within at least 2-week interval, but within 4 weeks.
At time zero, a primed constant (9.0 pmol·kg−1·min−1) infusion of insulin (Actrapid; NovoNordisk, Copenhagen, Denmark) was started and continued for 120 min. The rate of decline of glucose was controlled (∼0.08 mmol/min) and the glucose nadir (2.9 mmol/L) was achieved using a modification of the glucose clamp technique. During the clamp period, plasma glucose was measured every 5 min and a 20% dextrose infusion was adjusted so that plasma glucose levels were held constant at 2.9 ± 0.1 mmol/L (19). Potassium chloride (20 mmol/L) was infused during the clamp to reduce insulin-induced hypokalemia. The experiment was repeated with GLP-1 infusion at the rate of 0.4 pmol·kg−1·min−1, according to Nauck et al. (20).
Hyperglycemia experiments
All the subjects of group 2 were studied after an overnight 10-h fast. Two different experiments were planned for each subject in randomized order, a period of 2 h of hyperglycemic clamp at the level of hyperglycemia of 15 mmol/L (21) with or without GLP-1 (0.4 pmol·kg−1·min−1) (20) infusion. Each subject underwent each experiment within at least 2-week interval, but within 4 weeks.
At baseline and after 1 and 2 h, blood samples were withdrawn for biochemical assays to measure glycemia, plasma nitrotyrosine, and plasma 8-iso prostaglandin F2alpha (8-iso-PGF2a), which are markers of oxidative stress, and to measure soluble intercellular adhesion molecule-1 (sICAM-1) and interleukin-6 (IL-6), which are markers of inflammation, whereas endothelial function was measured by flow-mediated dilation (FMD).
Biochemical and clinical measurements
Cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, and plasma nitrotyrosine were measured as previously described (22). Plasma glucose was measured by the glucose-oxidase method, HbA1c was measured by high-performance liquid chromatography, insulin was measured by microparticle enzyme immunoassay (Abbott Laboratories, Wiesbaden, Germany). Plasma 8-iso-PGF2a (Cayman Chemical, Ann Arbor, MI), sICAM-1 (British Bio-technology, Abington, Oxon, UK), and IL-6 (R&D Systems, Minneapolis, MN) were determined with commercially available kits.
Endothelial function
Endothelial function was evaluated measuring the FMD of the brachial artery (15). At the end of each test, the subjects laid quietly for 15 min. Then, sublingual nitroglycerin (0.3 mg) was administered, and 3 min later the last measurements were performed. Response to nitroglycerin was used as a measure of endothelium-independent vasodilation.
Statistical analysis
The sample size was selected according to previous studies (4–15). Data are expressed as means ± SE. The Kolmogorov-Smirnov algorithm was used to determine whether each variable had a normal distribution. Comparisons of baseline data among the groups were performed using unpaired Student t test or Mann-Whitney U test, when indicated. The changes in variables during the tests were assessed by two-way ANOVA with repeated measures or Kolmogorov-Smirnov test, when indicated. If differences reached statistical significance, then post hoc analyses with two-tailed paired t test or Wilcoxon signed rank test for paired comparisons were used to assess differences at individual time periods in the study. Correlations between FMD changes and plasma levels of nitrotyrosine, 8-iso-PGF2a, sICAM-1, and IL-6 during each experiment were examined using linear regression analysis. Statistical significance was defined as P < 0.05.
RESULTS
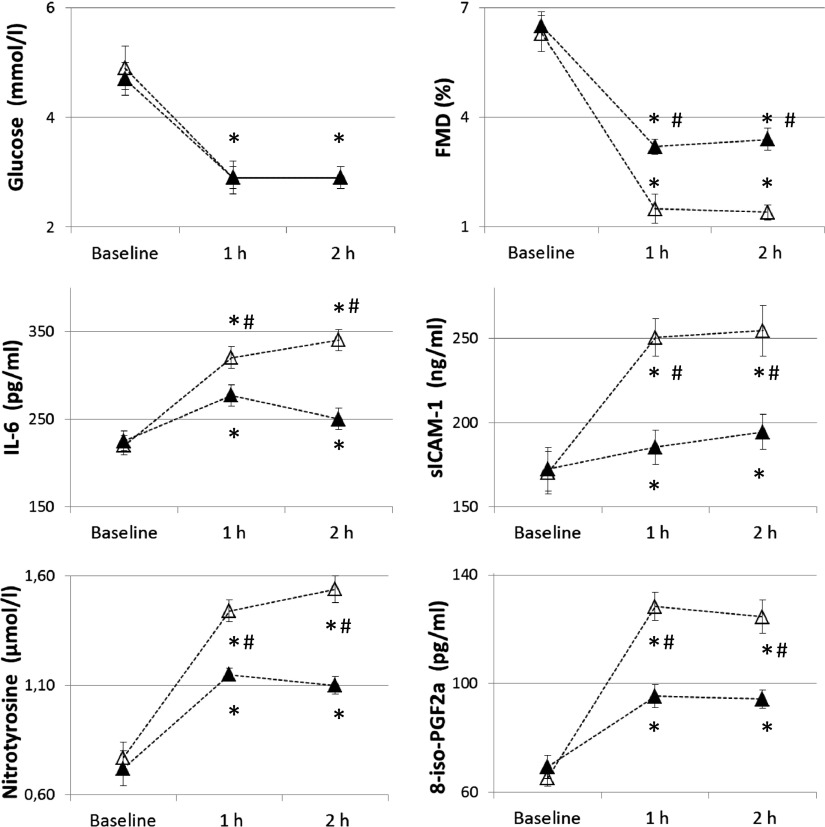
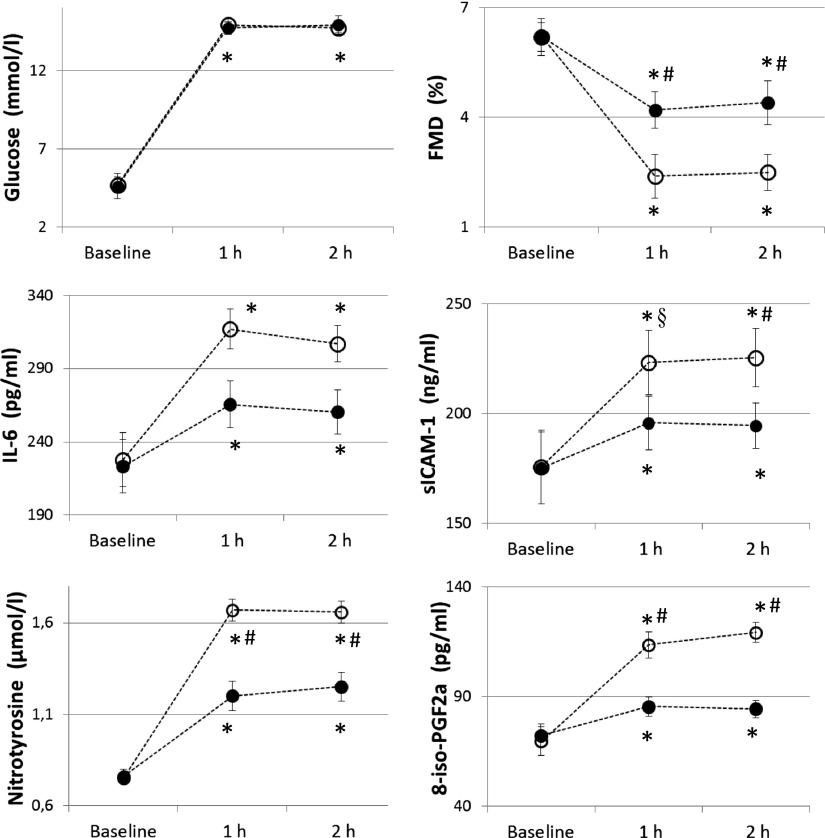
Similar to previous studies (4,5), after 2 h of hypoglycemia FMD significantly decreased, whereas sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 significantly increased compared with basal values (Fig. 1). When hypoglycemia was accompanied by the simultaneous infusion of GLP-1, all these phenomena were significantly attenuated; FMD decreased less, and sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 were not as increased (Fig. 1). Similar results were obtained in the hyperglycemic clamp experiments. According to previous studies (23), after 2 h of hyperglycemia FMD significantly decreased and sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 significantly increased compared with basal values (Fig. 2). When hyperglycemia was accompanied by the simultaneous infusion of GLP-1, all these phenomena were significantly attenuated (Fig. 2).
Figure 1.
Glycemia, FMD, sICAM-1, nitrotyrosine, IL-6, and 8-iso-PGF2a in type 1 diabetes during hypoglycemia experiments. Open triangles (△) indicate hypoglycemia and filled triangles (▲) indicate hypoglycemia plus GLP-1. *P < 0.01 vs. basal. #P < 0.01 vs. hypoglycemia plus GLP-1.
Figure 2.
Glycemia, FMD, sICAM-1, nitrotyrosine, IL-6, and 8-iso-PGF2a in type 1 diabetes during hyperglycemia experiments. Open circles (○) indicate hyperglycemia and filled circles (●) indicate hyperglycemia plus GLP-1. *P < 0.01 vs. basal. #P < 0.01 vs. hyperglycemia plus GLP-1. §P < 0.01 vs. hyperglycemia plus GLP-1.
Endothelial-independent vasodilatation was not affected in any of the experiments. No correlation was found between FMD changes and plasma levels of nitrotyrosine, 8-iso-PGF2a, sICAM-1, and IL-6 during each experiment.
CONCLUSIONS
This study confirms that both hyperglycemia and hypoglycemia induce endothelial dysfunction, oxidative stress, and inflammation in people with type 1 diabetes. However, this study, for the first time, shows that GLP-1 administration during hyperglycemia or hypoglycemia can counterbalance the deleterious effects.
Evidence suggests that both hyperglycemia and hypoglycemia can induce endothelial dysfunction and inflammation, producing oxidative stress (3,4,7). Furthermore, studies are accumulating showing that GLP-1 and its analogs used in clinical practice have antioxidant activity (10,15,16). Therefore, it is reasonable that GLP-1 should, by reducing oxidative stress generation, improve endothelial dysfunction and inflammation generated by both hyperglycemia and hypoglycemia.
It is of interest that our study confirms that both hyperglycemia and hypoglycemia can be considered equivalent as proatherosclerotic risk factors, and that they seem to work through the same pathways and mainly by generating oxidative stress (1,7).
The possibility that GLP-1 might directly affect the level of glycemia cannot be completely excluded. However, in our opinion, this possibility has been largely minimized by continuously clamping the level of glycemia in both hyperglycemia and hypoglycemia.
Correlations have not been found in the various parameters during the experiments, particularly between oxidative stress and inflammation and endothelial dysfunction. This can be easily explained. Insulin, which has been used during the clamping, has antioxidant activity, although weak (24), and it already has been shown that when insulin is introduced in the experiments any kind of association between oxidative stress and another parameter is lost (24).
In our opinion, this report has important practical implications. The risk of cardiovascular disease in type 1 diabetes, although somewhat neglected, is very high (25).
The role of hyperglycemia favoring cardiovascular disease in type 1 diabetes seems to be relevant; however, many other classical and less classical risk factors also seem to be involved (7).
However, the role of the oxidative stress, in particular, seems relevant in the pathogenesis of these complications in type 1 diabetes. It is well-known that hyperglycemia generates oxidative stress (7); however, data support the hypothesis that the haptoglobin genotype influences cardiovascular risk in type 1 diabetes, favoring the generation of the oxidative stress (26). Consistent with this hypothesis, the evidence that high α-tocopherol levels among patients with renal disease and among those using vitamin supplements was associated with lower cardiovascular risk in type 1 diabetes (27). Finally, recent findings suggest that hypoglycemia, a frequent event in type 1 diabetes that is emerging as a cardiovascular risk factor, also can produce oxidative stress (28).
It is currently suggested that GLP-1 analogs could be helpful in the management of type 1 diabetes, mainly because they contribute to improving metabolic control and to reducing insulin requirements (12). Our data suggest that another potential reason to use GLP-1 analogs in the management of type 1 diabetes might be their potential to reduce oxidative stress, which is generated during both hyperglycemia and hypoglycemia.
In conclusion, this study shows that GLP-1 can counterbalance the deleterious effect of both hyperglycemia and hypoglycemia on oxidative stress generation, inflammation, and endothelial function, and it supports the usefulness of GLP-1 and its analogs in the management of type 1 diabetes.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
A.C., A.N., and E.O. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. S.C. contributed to discussion and reviewed and edited the manuscript. L.L.S. researched data and contributed to discussion. G.P. researched data and contributed to discussion. K.E. researched data, contributed to discussion, and reviewed and edited the manuscript. D.G. and S.G. contributed to discussion and reviewed and edited manuscript. A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008;24:353–363 [DOI] [PubMed] [Google Scholar]
- 2.Singh P, Jain A, Kaur G. Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem 2004;260:153–159 [DOI] [PubMed] [Google Scholar]
- 3.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010;33:1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010;33:1529–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Alexanian A, Ying R, et al. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 2012;32:712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceriello A. Hyperglycaemia and the vessel wall: the pathophysiological aspects on the atherosclerotic burden in patients with diabetes. Eur J Cardiovasc Prev Rehabil 2010;17(Suppl 1):S15–S19 [DOI] [PubMed] [Google Scholar]
- 8.Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects [corrected in Metabolism 2009;58:1046]. Metabolism 2009;58:443–448 [DOI] [PubMed] [Google Scholar]
- 9.Nandish S, Wyatt J, Bailon O, Smith M, Oliveros R, Chilton R. Implementing cardiovascular risk reduction in patients with cardiovascular disease and diabetes mellitus. Am J Cardiol 2011;108(Suppl):42B–51B [DOI] [PubMed] [Google Scholar]
- 10.Peters A. Incretin-based therapies: review of current clinical trial data. Am J Med 2010;123(Suppl):S28–S37 [DOI] [PubMed] [Google Scholar]
- 11.Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes 2010;59:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Issa CM, Azar ST. Possible role of GLP-1 and its agonists in the treatment of type 1 diabetes mellitus. Curr Diab Rep 2012;12:560–567 [DOI] [PubMed] [Google Scholar]
- 13.Mudaliar S, Henry RR. Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med 2010;123(Suppl):S19–S27 [DOI] [PubMed] [Google Scholar]
- 14.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A, Esposito K, Testa R, Bonfigli AR, Marra M, Giugliano D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 2011;34:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 2010;30:1407–1414 [DOI] [PubMed] [Google Scholar]
- 17.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985;8:491–498 [DOI] [PubMed] [Google Scholar]
- 18.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 19.Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS. Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987;316:1376–1383 [DOI] [PubMed] [Google Scholar]
- 20.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 22.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia 2001;44:834–838 [DOI] [PubMed] [Google Scholar]
- 23.Ceriello A, Esposito K, Ihnat M, Thorpe J, Giugliano D. Effect of acute hyperglycaemia, long-term glycaemic control and insulin on endothelial dysfunction and inflammation in Type 1 diabetic patients with different characteristics. Diabet Med 2010;27:911–917 [DOI] [PubMed] [Google Scholar]
- 24.Monnier L, Colette C, Mas E, et al. Regulation of oxidative stress by glycaemic control: evidence for an independent inhibitory effect of insulin therapy. Diabetologia 2010;53:562–571 [DOI] [PubMed] [Google Scholar]
- 25.Orchard TJ, Costacou T. When are type 1 diabetic patients at risk for cardiovascular disease? Curr Diab Rep 2010;10:48–54 [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes 2008;57:1702–1706 [DOI] [PubMed] [Google Scholar]
- 27.Costacou T, Zgibor JC, Evans RW, Tyurina YY, Kagan VE, Orchard TJ. Antioxidants and coronary artery disease among individuals with type 1 diabetes: Findings from the Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications 2006;20:387–394 [DOI] [PubMed] [Google Scholar]
- 28.Ceriello A, Novials A, Ortega E, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 2012;61:2993–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]