Abstract
OBJECTIVE
To examine the acute and 24-h glycemic responses to reductions in postexercise rapid-acting insulin dose in type 1 diabetic patients.
RESEARCH DESIGN AND METHODS
After preliminary testing, 11 male patients (24 ± 2 years, HbA1c 7.7 ± 0.3%; 61 ± 3.4 mmol/mol) attended the laboratory on three mornings. Patients consumed a standardized breakfast (1 g carbohydrate ⋅ kg−1 BM; 380 ± 10 kcal) and self-administered a 25% rapid-acting insulin dose 60 min prior to performing 45 min of treadmill running at 72.5 ± 0.9% VO2peak. At 60 min postexercise, patients ingested a meal (1 g carbohydrate ⋅ kg−1 BM; 660 ± 21 kcal) and administered a Full, 75%, or 50% rapid-acting insulin dose. Blood glucose concentrations were measured for 3 h postmeal. Interstitial glucose was recorded for 20 h after leaving the laboratory using a continuous glucose monitoring system.
RESULTS
All glycemic responses were similar across conditions up to 60 min postexercise. After the postexercise meal, blood glucose was preserved under 50%, but declined under Full and 75%. Thence at 3 h, blood glucose was highest under 50% (50% [10.4 ± 1.2] vs. Full [6.2 ± 0.7] and 75% [7.6 ± 1.2 mmol ⋅ L−1], P = 0.029); throughout this period, all patients were protected against hypoglycemia under 50% (blood glucose ≤3.9; Full, n = 5; 75%, n = 2; 50%, n = 0). Fifty percent continued to protect patients against hypoglycemia for a further 4 h under free-living conditions. However, late-evening and nocturnal glycemia were similar; as a consequence, late-onset hypoglycemia was experienced under all conditions.
CONCLUSIONS
A 25% pre-exercise and 50% postexercise rapid-acting insulin dose preserves glycemia and protects patients against early-onset hypoglycemia (≤8 h). However, this strategy does not protect against late-onset postexercise hypoglycemia.
Patients with type 1 diabetes are encouraged to engage in regular exercise as part of a healthy lifestyle (1,2). However, engaging in exercise is not without its difficulties (1). Defective glucose regulation presents a significant challenge in preventing hypoglycemia during, and particularly after, exercise (3,4). Exercise-induced hypoglycemia is both a frequent (5) and dangerous occurrence (6) and remains a major obstacle to patients who wish to engage in exercise (7).
Much of the literature has focused on providing strategies to help combat hypoglycemia during, and early after, exercise (8–17), with investigations focusing on altering exercise modality (14,18), carbohydrate consumption (12,16,17), and reductions to pre-exercise, rapid-acting insulin dose (10–12,17,19). Prior to moderate-intensity, continuous, aerobic exercise, it is recommended that patients should reduce their prandial rapid-acting insulin dose by ∼75% to prevent hypoglycemia during exercise (10–12). However, despite best preserving blood glucose, it has been shown that this strategy is not fully protective against postexercise hypoglycemia (11,12). This has, in part, been attributed to iatrogenic causes (11), whereby patients administer their usual doses of rapid-acting insulin in a heightened insulin-sensitive state, potentially leading to unexpected falls in blood glucose and, consequently, hypoglycemia (11).
A potential strategy to help minimize the risk of developing hypoglycemia after exercise could be to reduce the dose of rapid-acting insulin administered with the postexercise meal (20). Exercise increases the sensitivity of the body to insulin for many hours after exercise (3) and patients could be faced with a window of particularly high sensitivity around the postexercise meal, whereby greater rates of glucose uptake could occur to supplement the high metabolic priority of replenishing muscle glycogen (21). Thus, the meal consumed after exercise is important. With this in mind, it would be intuitive to reduce the amount of insulin administered with the meal consumed at this time; this may preserve glycemia and prevent postexercise hypoglycemia. Conversely, severe reductions in rapid-acting insulin dose may incur prolonged postexercise hyperglycemia, even more so if the pre-exercise dose is also reduced. However, there is a lack of data to confirm or refute these hypotheses. In addition, it would be prudent to examine the extent to which rapid-acting insulin dose adjustments may help combat late falls in glycemia after exercise, considering type 1 diabetic patients are susceptible to late-onset, postexercise hypoglycemia (3), suggested to be due to a biphasic response in glucose uptake occurring early and also late after exercise (22). Therefore, the aim of this study was to examine the acute and 24-h postexercise glycemic responses to reducing the postexercise rapid-acting insulin dose, when using the recommended pre-exercise insulin reductions, in type 1 diabetic patients.
RESEARCH DESIGN AND METHODS
Patients
The study population consisted of 11 male type 1 diabetic patients (mean ± SEM; age 24 ± 2 years, BMI 22.6 ± 0.6, duration of diabetes 15 ± 2 years, HbA1c 7.7 ± 0.3%, 61 ± 3.4 mmol/mol, VO2peak 53 ± 1 mL ⋅ kg ⋅ min−1). Patients were eligible for inclusion if they were between 18 and 35 years of age with a duration of diabetes >2 years on enrollment. Patients were treated with a stable basal-bolus insulin regimen composed of either insulin glargine (n = 8) or detemir (n = 3) and fast-acting insulin analogs aspart (n = 10) or lispro (n = 1) for a minimum of 6 months. Patients were regularly and consistently active (participating in aerobic-based exercise for at least 30 min at a time, three times per week), free of diabetes-related complications, including hypoglycemia unawareness, and receiving no additional medication other than insulin. This study was approved by the local National Health Service Research Ethics Committee. All patients who participated provided written informed consent. Patients attended a preliminary screening visit, in which a comprehensive medical history and physical examination wasconducted. Additionally, patients completed a cardiopulmonary exercise stress test to assess cardiac function in response to exercise. All screening procedures complied with American College of Sports Medicine (ACSM) Guidelines for Exercise Testing and Prescription (23). Eligible patients underwent randomization by a computer program to determine the sequence of three crossover arms.
After the preliminary prescreening, patients attended four laboratory sessions. On visit 1, peak cardiorespiratory parameters were collected during the completion of an incremental-maximal treadmill run protocol (11,12). The treadmill run started at a velocity of 8 km ⋅ h−1 and increased 1 km ⋅ h−1 every 3 min to volitional exhaustion, as per West et al. (11,12). During this visit, anthropometric variables were also collected (body mass, stature, and BMI). Visits 2, 3, and 4 were experimental trials.
Prelaboratory phase
Patients were fitted with a continuous glucose monitoring system (CGMS; iPro2, Medtronic Diabetes, Northridge, CA) a minimum of 24 h before attending the laboratory and replicated their diet and activity patterns for 24 h prior to main trials.
CGMS
The iPro2 (Medtronic Diabetes, Northridge, CA) system consists of a needle-like sensor that is inserted into subcutaneous tissue. Sensor readings are obtained and stored in the memory of the monitor, which is attached to the indwelling sensor. Patients wore Enlite sensors (Medtronic Minimed, Northridge, CA), which were placed in the postural-lateral abdominal region to minimize the physiological time lags between blood and interstitial glucose (24). The sensor was inserted 24 h prior to each main trial, with the site of insertion replicated between trials. Sensors were removed 24 h after leaving the laboratory. During sensor wear, patients self-recorded capillary blood glucose concentrations at least four times per day for calibration purposes using a testing meter (GlucoMen LX; Menarini Diagnostics, Berkshire, U.K.). Sensor data were retrospectively processed using CareLink iPro software (Medtronic Diabetes) and iPro2 Retrospective Algorithm (Medtronic Diabetes) calibration routines.
Diet and activity replication
Patients arrived at the laboratory after an overnight fast, having replicated their diet in the previous 24 h (assessed using weighed dietary recording sheets). Additionally, patients were instructed to maintain their normal insulin regimen, with basal insulin dose standardized (dose, injection site, and time of injection) across trials. Patients were given a pedometer (Omron Healthcare Europe B.V., Hoofddorp, the Netherlands), which they were instructed to wear in the previous 24 h. Patients were required to avoid strenuous activity in the previous 48 h and maintain similar activity patterns between trials.
Testing procedure
All main trials were conducted on a morning (∼08:00 a.m.), with a minimum of 7 days between trials; patients replicated their trial start time across conditions. After arrival to the laboratory, patients were seated while a 20-gauge cannula (Vasofix; B. Braun Melsungen AG, Melsungen, Germany) was inserted into the antecubital vein of their nondominant arm. A 6-mL resting venous blood sample was taken, of which 20 μL was used for the immediate quantification of glucose and lactate (Biosen C-Line; EKF Diagnostic GmbH, London, U.K.) and 10 μL was analyzed for hemoglobin and hematocrit (Hemo Control; EKF Diagnostic GmbH), which was used to correct for changes in plasma volume (25). Venous blood (5 mL) was collected in a serum separation tube that was centrifuged for 15 min at 3,000 rev ⋅ min−1 and stored at −80°C for later analysis of rapid-acting insulin (Invitron Insulin Assay; Invitron, Monmouth U.K.) and β-hydroxybutyrate (Ranbut; Randox Laboratories, London, U.K.). Rapid-acting insulin was measured in patients using basal insulin glargine only. Immediately after this sample, patients administered a 25% (1.8 ± 0.1 IU) dose (i.e., a 75% reduction) of rapid-acting insulin into the abdomen, as recommended by Rabasa-Lhoret et al. (10) and West et al. (11). All patients were familiar with carbohydrate counting and were administering 1.0 ± 0.1 IU of rapid-acting insulin per 10 g of carbohydrate. The injection site was standardized across trials through administration at half the distance between the anterior-superior iliac crest and the naval. Insulin aspart and lispro were administered using Novopen3 (NovoNordisk, Bagsvaerd, Denmark) and HumaPen LUXURA (Eli Lilly, London, U.K.), respectively. After administration of the reduced rapid-acting insulin dose, patients consumed a cereal-based breakfast meal (frosted flakes, semiskimmed milk, and peaches) equating to 1 g carbohydrate ⋅ kg−1 BM (380 ± 10 kcal) within a 5-min period.
After consuming breakfast, patients remained rested, with blood samples collected at 30 and 60 min after the ingestion of the meal. Sixty minutes after breakfast, patients performed 45 min of steady-state treadmill (Woodway, Weil am Rhein, Germany) running at 72.5 ± 0.9% VO2peak, an intensity that falls within the recommendations of ACSM (23) for exercising individuals with diabetes. Breath-by-breath respiratory parameters (MetaLyzer 3B; Cortex, Leipzig, Germany) and heart rate (S810; Polar, Kempele, Finland) were continuously recorded throughout exercise. After the cessation of exercise, blood samples were collected at 0, 5, 15, 30, and 60 min postexercise. At 60 min postexercise, in a randomized and counter-balanced fashion, patients administered a Full (7.5 ± 0.3 IU), 75% (5.6 ± 0.2 IU), or 50% (3.7 ± 0.1 IU) rapid-acting insulin dose, which was injected into the contralateral abdominal site to the pre-exercise, rapid-acting insulin dose. Patients then consumed a pasta-based lunch meal (pasta, tomato-based sauce, cheddar cheese), equating to 1 g carbohydrate · kg−1 BM (660 ± 21 kcal), and then remained at rest for 180 min postmeal. Blood samples were collected every 30 min throughout the postmeal period; patients could drink water ad libitum. Hypoglycemia was defined as a blood or interstitial glucose concentrations of ≤3.9 mmol · L−1 (15). If patients experienced hypoglycemia during the laboratory period, a 20-g carbohydrate bolus was administered (Lucozade; GlaxoSmithKline, Middlesex, U.K.).
Postlaboratory period
During CGMS wear, patients were required to replicate their diet across trials through the use of a weighed food diary. Dietary intake and insulin administration was self-recorded, with patients required to indicate if additional carbohydrate intake had been ingested to correct falling blood glucose. Patients were required to keep meal times and rapid-acting insulin dose consistent across trials. Patients continued to record a minimum of four capillary blood glucose concentrations daily for CGMS calibration.
Data analysis
Statistical analysis was performed using PASW statistics software (IBM PASW version 18; IBM, Armonk, NY). A repeated-measures ANOVA on three levels (condition × time) was conducted, with Bonferroni-corrected pairwise comparisons and paired samples Student t test used to examine time and treatment effects, respectively. Statistical significance was accepted at P ≤ 0.05. Area under the curve was calculated using the methods described by Wolever and Jenkins (26).
RESULTS
Prelaboratory phase
Patients displayed similar glycemic control during the 24 h before arriving to the laboratory, with similar mean (Full, 8.6 ± 0.4; 75%, 7.7 ± 0.5; 50%, 8.1 ± 0.6 mmol ⋅ L−1; P = 0.451) and total interstitial glucose area under the curve (Full, 12,389 ± 705; 75%, 10,986 ± 765; 50%, 11,385 ± 791 mmol ⋅ L−1 ⋅ min−1; P = 0.400) across trials. During this time, dietary patterns were similar, with no differences in total energy intake (Full, 2,249 ± 110; 5%, 2,290 ± 178; 50%, 2,130 ± 128 kcal; P = 0.750), with percentage contribution from carbohydrate (Full, 52 ± 3; 75%, 49 ± 4; 50%, 49 ± 4%; P = 0.844), fat (Full, 29 ± 3; 75%, 30 ± 3; 50%, 33 ± 3%; P = 0.958), and protein (Full, 18 ± 2; 75%, 21 ± 3; 50%, 18 ± 2%; P = 0.843) similar between conditions. No differences were observed in activity patterns with similar total steps recorded across conditions (Full, 7,507 ± 124; 75%, 8,028 ± 119; 50%, 8,056 ± 138 steps; P = 0.627).
Laboratory phase
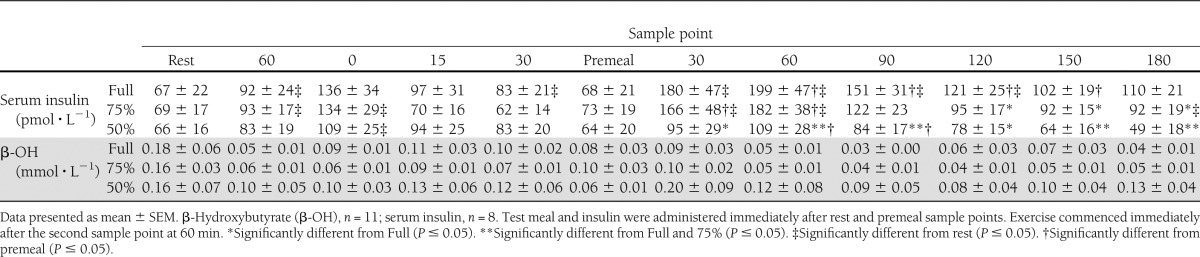
The absolute blood glucose responses are presented in Fig. 1. There was a significant time effect (P < 0.001, partial-η2 = 0.643) and a significant condition × time interaction for absolute blood glucose concentrations (P < 0.001, partial-η2 = 0.290). Fasting blood glucose concentrations were similar between conditions (Full, 7.2 ± 0.9; 75%, 7.1 ± 0.7; 50%, 7.1 ± 0.6 mmol ⋅ L−1; P = 0.594). After the ingestion of the breakfast meal and reduced rapid-acting insulin dose, blood glucose peaked at 60 min and was similar across conditions (Fig. 1). Serum insulin and β-hydroxybutyrate responses are presented in Table 1. There were no differences in fasting serum insulin (P = 0.989) or β-hydroxybutyrate (P = 0.962) concentrations across conditions (Table 1). Pre-exercise serum insulin and β-hydroxybutyrate responses were similar across conditions (P > 0.05) (Table 1).
Figure 1.
Time-course changes in blood glucose from rest. Data presented as mean ± SEM. Triangles, Full; squares, 75%; diamonds, 50%. Transparent sample point within a condition indicates significant difference from premeal concentrations (P ≤ 0.05). *Significantly different from Full (P ≤ 0.05); **significantly different from Full and 75% (P ≤ 0.05). Thatched area indicates exercise, and vertical dashed line break indicates postexercise intervention. Note that test meal and insulin were administered immediately after rest and 60-min postexercise sample points.
Table 1.
Serum insulin and β-hydroxybutyrate responses during the trials
There were no differences in exercising rates of % VO2peak (Full, 73.8 ± 0.01; 75%, 72.8 ± 0.01; 50%, 70.8 ± 0.01%; P = 0.575) or peak heart rate (Full, 80 ± 2; 75%, 79 ± 3; 50%, 79 ± 1%; P = 0.631). The decline in blood glucose with exercise was similar across conditions (Full, Δ = −6.8 ± 0.03; 75%, Δ = −6.9 ± 0.03; 50%, Δ = −6.2 ± 0.03 mmol ⋅ L−1; P = 0.891); moreover, patients elicited similar peak lactates immediately postexercise (Full, 3.7 ± 0.6; 75%, 3.8 ± 0.5; 50%, 3.7 ± 0.5 mmol ⋅ L−1; P = 0.965). There were no incidences of hypoglycemia during exercise, with all patients completing the exercise protocol on each occasion.
Postexercise intervention
After the postexercise meal and rapid-acting insulin dose, total serum insulin area under the curve was significantly lower under 50% (21,748 ± 4,799 pmol ⋅ L−1 ⋅ min−1) when compared with Full (38,166 ± 6,716 pmol ⋅ L−1 ⋅ min−1, P = 0.004) and 75% (33,351 ± 5,634 pmol ⋅ L−1 ⋅ min−1, P = 0.031). Individual peak serum insulin concentrations were lowest under 50% (83 ± 29 pmol ⋅ L−1 ⋅ min−1) compared with 75% (196 ± 42 pmol ⋅ L−1 ⋅ min−1, P = 0.017) and Full (229 ± 44 pmol ⋅ L−1 ⋅ min−1, P = 0.020). Blood glucose concentrations decreased under Full and 75%; however, under 50%, blood glucose was preserved, eliciting similar concentrations to premeal (Fig. 1). During this time, five patients experienced hypoglycemia under Full and two under 75%, whereas under 50%, all patients were protected. Furthermore, some participants experienced multiple bouts of hypoglycemia, with total episodes across each trial greatest under Full (Full, n = 9; 75%, n = 6; 50%, n = 0). On average, blood glucose concentrations were 2.8 ± 0.2 mmol ⋅ L−1 before receiving carbohydrate supplementation. Upon leaving the laboratory on 180 min postmeal, blood glucose concentrations were greater under 50% when compared with Full and 75% (Fig. 1). There were no conditional differences in blood lactate responses (P = 0.996) or serum β-hydroxybutyrate (P = 0.076) (Table 1).
Postlaboratory phase
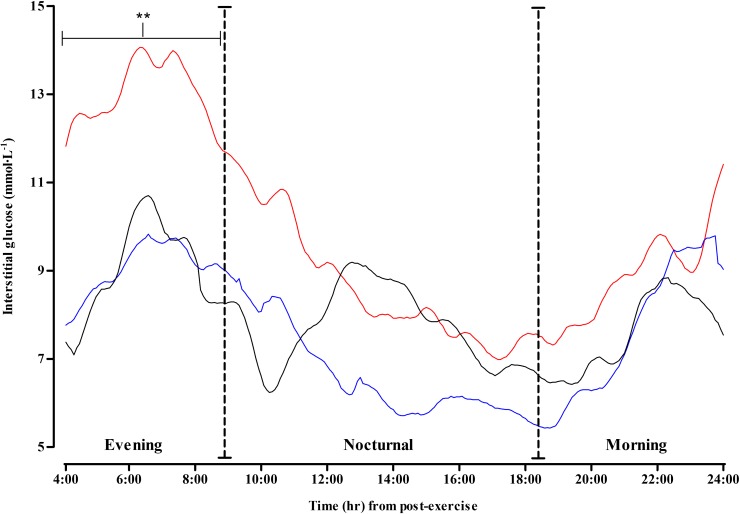
The interstitial glucose responses are presented in Fig. 2. After leaving the laboratory, 50% prevented hypoglycemia for a further 4 h, meaning the first hypoglycemic episode under 50% occurred at 8 h postexercise. During the evening (Fig. 2), total interstitial glucose area under the curve was significantly greater under 50% (2,709 ± 245 mmol ⋅ L−1 ⋅ min−1) when compared with Full (1,706 ± 247 mmol ⋅ L−1 ⋅ min−1, P < 0.001) and 75% (1,860 ± 244 mmol ⋅ L−1 ⋅ min−1, P < 0.001).
Figure 2.
Time-course changes in interstitial glucose throughout the postlaboratory period. Data presented as mean (SEM error bars have been removed for reader clarity). Black line, Full; blue line, 75%; red line, 50%. **Interstitial glucose area under the curve is significantly different from Full and 75% (P ≤ 0.05). Vertical dashed line breaks indicate evening, nocturnal, or morning periods.
By 9 h postexercise, glycemia under 50% had decreased over time, with concentrations lower than those elicited upon leaving the laboratory (Fig. 2). After 9 h, interstitial glucose concentrations became comparable across conditions and were lowest during the night (Fig. 2). Nighttime and morning glycemic responses were also similar across trials (Fig. 2).
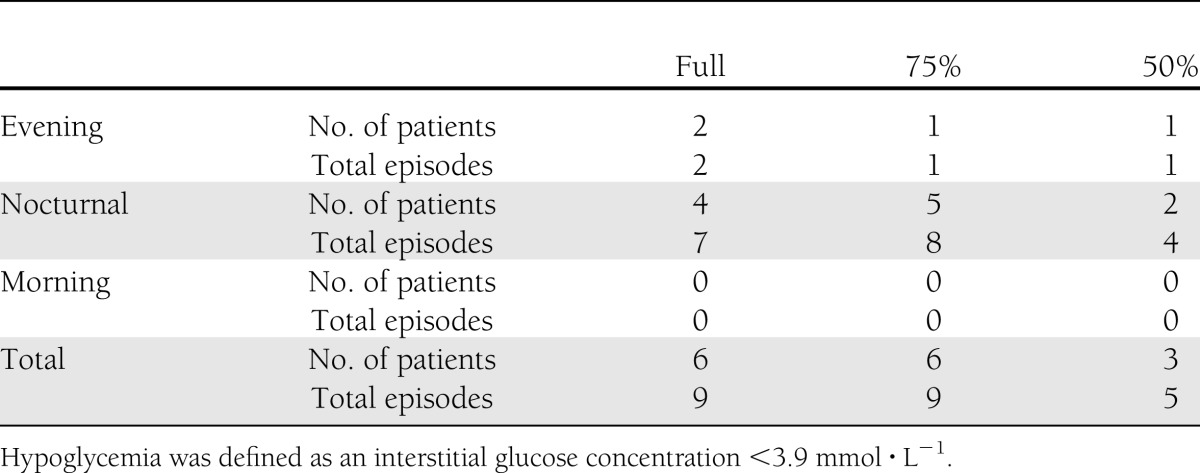
All patients experiencing hypoglycemia during the laboratory period also experienced further hypoglycemic episodes during sleep. The total number of hypoglycemic episodes during the postlaboratory phase was lowest under 50% (Table 2). Of the total hypoglycemic episodes across all trials, 82% occurred nocturnally (Table 2). Additionally, the number of patients who self-corrected blood glucose to prevent further declines in glycemia was less under 50% (n = 2) when compared with 75% (n = 6) and Full (n = 6).
Table 2.
The total number of patients experiencing hypoglycemia and the total number of hypoglycemic episodes during the postlaboratory period
During the postlaboratory period, total energy consumed (Full, 1,588 ± 234; 75%, 1,769 ± 275; 50%, 1,731 ± 141 kcal; P = 0.836), with contribution from carbohydrate (Full, 55 ± 4; 75%, 47 ± 5; 50%, 48 ± 4%; P = 0.516), fat (Full, 30 ± 4; 75%, 31 ± 4; 50%, 34 ± 4%; P = 0.916), and protein (Full, 16 ± 2; 75%, 22 ± 6; 50%, 18 ± 2%; P = 0.827), was similar between conditions. Moreover, there were no differences in the total amount of insulin administered or in the insulin to carbohydrate ratio (P > 0.05). No differences were observed in activity patterns during the 24-h postexercise period, with similar steps recorded (Full, 5,341 ± 95; 75%, 4,786 ± 87; 50%, 5,543 ± 108 steps; P = 0.877) across conditions.
CONCLUSIONS
The aim of this study was to examine the acute and 24-h glycemic responses to reductions in postexercise rapid-acting insulin dose, when the recommended pre-exercise insulin reductions were used, in patients with type 1 diabetes. The results demonstrate that despite a large reduction in the pre-exercise, rapid-acting insulin dose, it is important that the postexercise dose is also heavily reduced. We have demonstrated that a reduction in postexercise rapid-acting insulin of 50% best preserves glycemia and provides the greatest protection against hypoglycemia early after exercise (∼8 h). However, beyond this time, this strategy becomes less effective. A potential mechanism explaining the late fall in glycemia could be the administration of further unchanged doses of rapid-acting insulin and/or unchanged circulating basal insulin concentrations during a second period of elevated glucose uptake (22).
All patients completed a bout of moderate-intensity aerobic exercise (∼73% VO2peak), running at a velocity of 9.3 ± 0.3 km · h−1 and expending 708 ± 16 kcal, with no incidences of hypoglycemia during exercise. This complements the recommendations of prior literature (10–12) and emphasizes the importance of reducing the rapid-acting insulin dose before exercise. Furthermore, patients were, on average, within euglycemic ranges for 60 min after exercise, suggesting this strategy exposes patients to only transient periods of hyperglycemia prior to exercise (Fig. 1).
After the consumption of the postexercise meal, blood glucose was best preserved with a 50% reduced rapid-acting insulin dose, which protected all patients from hypoglycemia throughout the postexercise meal period, whereas blood glucose concentrations declined under both Full and 75% doses (Fig. 1). The exact mechanisms underpinning these findings are not entirely known. Moderate-intensity running exercise requires a contribution from muscle glycogen (27,28), of which the replenishment is of a high metabolic priority (21), with muscle glycogen depletion suggested to have a role in the development of postexercise hypoglycemia (29). After exercise, glycogen synthesis is increased dramatically, thought to be driven primarily by depleted glycogen (30,31), an upregulation of insulin signaling pathways (32), and a prolonged increase in the permeability of the muscle cell membrane to glucose (33). Taken together, an increased rate of glucose transport into the muscle and an increased capacity to convert glucose to glycogen results in a window where there is likely an increased potency of the administered rapid-acting insulin (34).
To suggest that the glycemic responses of our patients under Full and 75% are solely due to depleted muscle glycogen stores is difficult, as large carbohydrate boluses (1.0 g carbohydrate · kg−1 BM) were ingested before and after exercise, which would have helped supplement glycogen reserves (21). However, additional explanations for why hypoglycemia was experienced during the Full and 75% conditions may be that the administration of larger doses of rapid-acting insulin created a milieu of relative hyperinsulinemia, in turn suppressing hepatic glucose production and enhancing glucose uptake (34), potentially into nonexercised tissue (27). Irrespective of the mechanisms at play, we have demonstrated the importance of reducing the dose of rapid-acting insulin administered with the meal after exercise. In contrast to current recommendations that advocate a reduction of 30% (35), we found a 50% reduction was necessary to prevent falls in blood glucose early after exercise. Although serum β-hydroxybutyrate throughout the postmeal period was, on average, greatest under 50% (Table 1), these concentrations were of little clinical significance, with concentrations well below those deemed hyperketonemic (>1.0 mmol−1) (36). From a clinical viewpoint, a 50% reduction in rapid-acting insulin after exercise, when the pre-exercise dose was also heavily reduced, does not increase ketone body concentrations.
Interstitial glucose concentrations revealed a preservation of glycemia under 50% for a further 4 h after leaving the laboratory, meaning patients had been protected against hypoglycemia for 8 h postexercise. However, patients were exposed to hyperglycemia during this time. In comparison, glycemia during the evening (between 4 and 9 h postexercise) (Fig. 2) was significantly lower under both Full and 75% conditions, with patients experiencing further hypoglycemic episodes under these conditions. However, at ∼7 h postexercise, there was a decline in interstitial glucose concentrations under 50%, such that at ∼9 h postexercise, glycemia was similar between conditions. The decline in interstitial glucose was consistent across trials (Fig. 2) and was coincidental with evening meal time. Patients replicated dietary habits (i.e., meal composition, time of consumption, and rapid-acting insulin dose), and their basal insulin regimen was standardized across trials. Type 1 diabetic patients face particular difficulty in avoiding hypoglycemia 7–11 h postexercise, as the requirement for glucose to maintain euglycemia is increased (26,37). Considering that increased insulin sensitivity may persist for >48 h after exercise, an additional strategy could be to reduce the basal insulin dose. However, this strategy requires planning exercise in advance and offers little flexibility regarding the timing of exercise; if unforeseen circumstances prevent planned exercise from taking place, the patient is likely to be exposed to prolonged periods of hyperglycemia (38). Therefore, it may be more appropriate to further adjust the rapid-acting insulin dose during this time.
Of the total number of hypoglycemic episodes experienced across all trials during the postlaboratory period, 82% occurred nocturnally. Therefore, sleep may be a contributing factor to the increased incidence of hypoglycemia during the night (3). As well as recent exercise (5, 20), antecedent hypoglycemia (6) may blunt symptomatic and autonomic responses to hypoglycemia later in the day, particularly during sleep (39). This is important, as those patients who experienced hypoglycemia in the laboratory eliciting blood glucose concentrations <3 mmol ⋅ L−1 also experienced hypoglycemia again during sleep. These data suggest that if patients experience hypoglycemia early after exercise, there is potential that they are at an increased risk of blood glucose falling again during the night. Although only 18% of patients experienced nocturnal hypoglycemia under 50%, the late fall in glycemia means the risk, albeit less, may still be present for patients despite the large reductions in pre- and postexercise rapid-acting insulin dose. Experiencing hypoglycemia during sleep is a real fear for type 1 diabetic patients (39), which is likely to be exacerbated when exercise is performed during the day. Therefore, there is a need to further refine current exercise recommendations for type 1 diabetic patients such that patients can engage in regular exercise with a reduced risk, and fear, of postexercise nocturnal hypoglycemia.
Sport and recreational activities vary greatly in terms of their physiological demands and metabolic responses. The effects of changing the intensity, duration, or mode of exercise may therefore necessitate manipulating the rapid-acting insulin dose accordingly to account for these changes. Moreover, the feeding pattern used in this study (60 min before and after exercise) may not be typical of the normal dietary habits of exercising patients. Worthy of note, the patients examined in this study were in good glycemic control (HbA1c 7.7 ± 0.3%, 61 ± 3.4 mmol/mol) and had a good level of aerobic fitness (VO2peak 53 ± 1 mL · kg · min−1). Thus, there is potential that the glycemic response to pre- and postexercise rapid-acting insulin reductions may differ with patient age, fitness, and glycemic control. Although it was not possible to address these issues within this study, this should not detract from the clinical application of our findings. To our knowledge, we are the first to investigate postexercise rapid-acting insulin dose reductions after running exercise in patients with type 1 diabetes, when using the recommended pre-exercise insulin dose reductions. We have demonstrated the importance of large reductions in the pre- and postexercise rapid-acting insulin dose in preserving glycemia early after exercise and, through investigation under free-living conditions, further highlight that the difficulties related to delayed and, in particular, nocturnal postexercise hypoglycemia are still existent despite the application of large rapid-acting insulin dose reductions before and after exercise. Therefore, future research should further investigate strategies to normalize glycemia in the late postexercise period, preventing late-onset hypoglycemia.
In summary, the results of the current study suggest that a 50% reduction in postexercise rapid-acting insulin dose, when combined with a large pre-exercise rapid-acting insulin reduction, preserves glycemia and prevents hypoglycemia for ∼8 h after exercise. However, patients should be made aware that they may be exposed to periods of hyperglycemia, but not hyperketonemia, after the postexercise meal, and there is still a risk of developing late-onset hypoglycemia. Patients are therefore recommended to refine these strategies according to their own individual exercise routine and glycemic responses.
Acknowledgments
This study was funded by Diabetes UK.
No potential conflicts of interest relevant to this article were reported.
M.D.C. contributed to the conception and design of the study, researched data, and wrote the manuscript. M.W. aided in participant recruitment and reviewed and edited the manuscript. M.I.T. and D.G.J. aided in data collection and reviewed and edited the manuscript. E.J.S. contributed to the design of the study and reviewed and edited the manuscript. R.M.B. contributed to the analysis of CGM data and aided in preparation of the manuscript. S.C.B. reviewed and edited the manuscript. D.J.W. contributed to the conception and design of the study, aided in researching data, and contributed to the writing of the manuscript. D.J.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the study participants for their time, effort, and commitment and the research team at the National Institute for Health Research Clinical Research Facility (Newcastle University) for their assistance.
Footnotes
Clinical trial reg. no. NCT01531855, Clinicaltrials.gov.
References
- 1.Zinman B, Ruderman N, Campaigne BN, Devlin JT, Schneider SH, American Diabetes Association Physical activity/exercise and diabetes mellitus. Diabetes Care 2003;26(Suppl. 1):S73–S77 [DOI] [PubMed] [Google Scholar]
- 2.Chu L, Hamilton J, Riddell MC. Clinical management of the physically active patient with type 1 diabetes. Phys Sportsmed 2011;39:64–77 [DOI] [PubMed] [Google Scholar]
- 3.MacDonald MJ. Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care 1987;10:584–588 [DOI] [PubMed] [Google Scholar]
- 4.Tsalikian E, Mauras N, Beck RW, et al. Diabetes Research In Children Network Direcnet Study Group Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr 2005;147:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briscoe VJ, Tate DB, Davis SN. Type 1 diabetes: exercise and hypoglycemia. Appl Physiol Nutr Metab 2007;32:576–582 [DOI] [PubMed] [Google Scholar]
- 6.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008;31:2108–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubé MC, Weisnagel SJ, Prud’homme D, Lavoie C. Exercise and newer insulins: how much glucose supplement to avoid hypoglycemia? Med Sci Sports Exerc 2005;37:1276–1282 [DOI] [PubMed] [Google Scholar]
- 9.Gallen I. Exercise in type 1 diabetes. Diabet Med 2003;20(Suppl. 1):2–5 [DOI] [PubMed] [Google Scholar]
- 10.Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care 2001;24:625–630 [DOI] [PubMed] [Google Scholar]
- 11.West DJ, Morton RD, Bain SC, Stephens JW, Bracken RM. Blood glucose responses to reductions in pre-exercise rapid-acting insulin for 24 h after running in individuals with type 1 diabetes. J Sports Sci 2010;28:781–788 [DOI] [PubMed] [Google Scholar]
- 12.West DJ, Stephens JW, Bain SC, et al. A combined insulin reduction and carbohydrate feeding strategy 30 min before running best preserves blood glucose concentration after exercise through improved fuel oxidation in type 1 diabetes mellitus. J Sports Sci 2011;29:279–289 [DOI] [PubMed] [Google Scholar]
- 13.Campaigne BN, Wallberg-Henriksson H, Gunnarsson R. Glucose and insulin responses in relation to insulin dose and caloric intake 12 h after acute physical exercise in men with IDDM. Diabetes Care 1987;10:716–721 [DOI] [PubMed] [Google Scholar]
- 14.Guelfi KJ, Jones TW, Fournier PA. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 2005;28:1289–1294 [DOI] [PubMed] [Google Scholar]
- 15.Yardley JE, Kenny GP, Perkins BA, et al. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care 2012;35:669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West DJ, Morton RD, Stephens JW, et al. Isomaltulose improves postexercise glycemia by reducing CHO oxidation in T1DM. Med Sci Sports Exerc 2011;43:204–210 [DOI] [PubMed] [Google Scholar]
- 17.De Feo P, Di Loreto C, Ranchelli A, et al. Exercise and diabetes. Acta Biomed 2006;77(Suppl. 1):14–17 [PubMed] [Google Scholar]
- 18.Bussau VA, Ferreira LD, Jones TW, Fournier PA. The 10-s maximal sprint: a novel approach to counter an exercise-mediated fall in glycemia in individuals with type 1 diabetes. Diabetes Care 2006;29:601–606 [DOI] [PubMed] [Google Scholar]
- 19.Grimm J. Exercise in type 1 Diabetes. In Exercise and Sport in Diabetes. Nagi D, Ed. Chichester, John Wiley & Sons, 2005, p. 25–43 [Google Scholar]
- 20.Sandoval DA, Guy DLA, Richardson MA, Ertl AC, Davis SN. Acute, same-day effects of antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am J Physiol Endocrinol Metab 2006;290:E1331–E1338 [DOI] [PubMed] [Google Scholar]
- 21.Jentjens R, Jeukendrup AE. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med 2003;33:117–144 [DOI] [PubMed] [Google Scholar]
- 22.McMahon SK, Ferreira LD, Ratnam N, et al. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab 2007;92:963–968 [DOI] [PubMed] [Google Scholar]
- 23.ACSM Clinical Exercise Testing. In ACSM's Guidelines for Exercise Testing and Prescription. Walter R, Gordon NF, Pescatello LS, Eds. Baltimore, American College of Sports Medicine, 2010 [Google Scholar]
- 24.Keenan DB, Mastrototaro JJ, Zisser H, et al. Accuracy of the Enlite 6-day glucose sensor with guardian and Veo calibration algorithms. Diabetes Technol Ther 2012;14:225–231 [DOI] [PubMed] [Google Scholar]
- 25.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974;37:247–248 [DOI] [PubMed] [Google Scholar]
- 26.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 1986;43:167–172 [DOI] [PubMed] [Google Scholar]
- 27.Chokkalingam K, Tsintzas K, Norton L, Jewell K, Macdonald IA, Mansell PI. Exercise under hyperinsulinaemic conditions increases whole-body glucose disposal without affecting muscle glycogen utilisation in type 1 diabetes. Diabetologia 2007;50:414–421 [DOI] [PubMed] [Google Scholar]
- 28.Jenni S, Oetliker C, Allemann S, et al. Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus—a prospective single-blinded randomised crossover trial. Diabetologia 2008;51:1457–1465 [DOI] [PubMed] [Google Scholar]
- 29.Lumb AN, Gallen IW. Diabetes management for intense exercise. Curr Opin Endocrinol Diabetes Obes 2009;16:150–155 [DOI] [PubMed] [Google Scholar]
- 30.Nielsen JN, Derave W, Kristiansen S, Ralston E, Ploug T, Richter EA. Glycogen synthase localization and activity in rat skeletal muscle is strongly dependent on glycogen content. J Physiol 2001;531:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikines KJ, Sonne B, Farrell P, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988;254:E248–E259 [DOI] [PubMed] [Google Scholar]
- 32.Maarbjerg SJ, Sylow L, Richter EA. Current understanding of increased insulin sensitivity after exercise - emerging candidates. Acta Physiol (Oxf) 2011;202:323–335 [DOI] [PubMed] [Google Scholar]
- 33.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy J. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol 1989;256:E494–E499 [DOI] [PubMed] [Google Scholar]
- 34.Zinman B, Murray FT, Vranic M, et al. Glucoregulation during moderate exercise in insulin treated diabetics. J Clin Endocrinol Metab 1977;45:641–652 [DOI] [PubMed] [Google Scholar]
- 35.Lumb AN, Gallen IW. Insulin dose adjustment and exercise in type 1 diabetes: what do we tell the patient? The British Journal of Diabetes & Vascular Disease 2009;9:273–277 [Google Scholar]
- 36.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999;15:412–426 [DOI] [PubMed] [Google Scholar]
- 37.Tamborlane WV. Triple jeopardy: nocturnal hypoglycemia after exercise in the young with diabetes. J Clin Endocrinol Metab 2007;92:815–816 [DOI] [PubMed] [Google Scholar]
- 38.Lumb A. The role of newer technologies (CSII and CGM) and novel strategies in the management of type 1 diabetes for sport and exercise. In Type 1 Diabetes, Clinical Management of the Athlete Gallen I, Ed. London, Springer-Verlag London Ltd., 2012, p. 101–113 [Google Scholar]
- 39.Cryer PE, Childs BP. Negotiating the barrier of hypoglycemia in diabetes. Diabetes Spectr 2002;15:20–27 [Google Scholar]