Abstract
OBJECTIVE
Psoriasis is associated with increased risk of cardiovascular events and increased prevalence of cardiovascular risk factors. Diabetes mellitus (DM) is a major contributor to cardiovascular morbidity and mortality that may be associated with psoriasis, but conflicting results have been presented and nationwide data on the risk of new-onset DM in patients with psoriasis have not been reported.
RESEARCH DESIGN AND METHODS
The study comprised a Danish population ≥10 years of age on 1 January 1997 who were followed until new-onset DM, death, or 31 December 2009. Information on comorbidity, concomitant medication, and socioeconomic status was linked on an individual level. The primary study end point was DM requiring pharmacotherapy. Incidence rates for the development of DM events per 1,000 observational years were calculated and adjusted. Incidence rate ratios (IRRs) were estimated by Poisson regression.
RESULTS
A total of 4,614,807 subjects were eligible for analysis, with a maximum follow-up of 13 years. In the study period, 52,613 patients with psoriasis, including 6,784 patients with severe psoriasis, were identified. The overall incidence rates for new-onset DM were 3.67 (CI 3.65–3.69), 6.93 (6.63–7.25), and 9.65 (8.68–10.73) for the reference population, mild psoriasis, and severe psoriasis, respectively. Compared with the reference population, the IRR of new-onset DM was increased in all patients with psoriasis, i.e., IRR 1.49 (CI 1.43–1.56) and 2.13 (1.91–2.37) for those with mild and severe psoriasis.
CONCLUSIONS
In this nationwide cohort, psoriasis was associated with increased incidence rates of new-onset DM. The association remained statistically significant after adjustment for confounding factors.
Psoriasis is a multifactorial chronic inflammatory disorder affecting 1–3% of the world population (1). Studies have demonstrated that psoriasis is associated with cardiovascular disorders probably due, in part, to shared inflammatory pathways (2). Similarly, diabetes mellitus (DM) is a serious and growing public health problem worldwide with severe complications, including increased cardiovascular morbidity and mortality (3,4). Although previous studies have examined the association between psoriasis and risk of impaired glucose tolerance and DM, conflicting results have been reported, limited data are available on the impact of psoriasis severity on risk of DM, and nationwide data have not been presented (5–15). Therefore, our aim with the current study was to examine the association between psoriasis and new-onset DM, including the impact of psoriasis severity, in a nationwide setting.
RESEARCH DESIGN AND METHODS
Data sources and study population
The study was conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations (16). In Denmark, all citizens have a unique and life-long personal civil registration number that enables individual-level linkage of information across nationwide registers. All medications dispensed from pharmacies were obtained from the national prescription registry (the Danish Registry of Medicinal Product Statistics), where all dispensed prescriptions from Danish pharmacies have been recorded since 1995. The National Prescription Registry is directly linked to the system for reimbursement of drug expenses and has previously been validated (17). Deaths were identified from the Central Population Register, in which deaths are recorded within 2 weeks. Morbidity was obtained from the Danish National Patient Register, wherein all hospital admissions, out-patient consultations, diagnoses, and procedures have been recorded since 1978 according to the ICD (ICD-8 until 1994 and ICD-10 thereafter). Comorbidity at study entry was described by Charlson comorbidity index, as defined by 19 prespecified diagnoses at study entry and up to 1 year previously, and modified to ICD-10 (18). Socioeconomic status was defined by the individual average yearly gross income during a 5-year period prior to study inclusion, and patients were divided into quintiles according to their income. Data on death, comorbidity, concomitant medication, and socioeconomic status were linked on an individual case level.
The entire Danish population 10 years of age or older as of 1 January 1997 (baseline of study) was followed until 31 December 2009, emigration, new-onset DM, or death. Patients with psoriasis were identified by dispensed prescriptions of topical vitamin D derivatives, i.e., first-line treatment used exclusively for psoriasis and unavailable over the counter without prescription. Patients were classified as having severe psoriasis at the time of their third hospitalization or outpatient consultation for psoriasis (ICD-10 L40) or psoriatic arthritis (M070–M073). This method for identification and psoriasis severity classification has previously been validated (19,20). Patients with previous psoriasis and/or DM (defined by prior use of glucose-lowering drugs, see below) were excluded at the baseline to more accurately examine the time at risk and the chronology of disease onset.
Pharmacotherapy
Drugs are registered in the national prescription registry according to the international Anatomical Therapeutic Chemical (ATC) classification system. Patients with psoriasis were identified by their claimed prescriptions of topical vitamin D derivates (ATC D05AX). Baseline treatment with antidepressive medication (N06A), nonsteroidal anti-inflammatory drugs (NSAIDs; M01A), platelet inhibitors (B01AC), cholesterol-lowering drugs (C10A), systemic glucocorticoids (H02AB), β-blockers (C07), thiazides (C03AA), ACE inhibitors/angiotensin 2 receptor blockers (ARBs) (C09), vitamin K antagonists (B01AA), loop diuretics (C03C), and spironolactone (C03D) was defined by dispensed prescriptions up to 6 months prior to the study inclusion date.
Outcome
The primary end point was the development of DM needing pharmacotherapy, which was defined by initiation of glucose-lowering drugs (A10), identified by claimed prescriptions from pharmacies, as done previously (19,21,22). Glucose-lowering drugs are not available over the counter in Denmark. In addition, the incidence rates of all-cause mortality and cardiovascular mortality (I0-I99) were examined.
Statistical analysis
Baseline characteristics were summarized as means with standard deviations or frequencies and percentages, as appropriate. Incidence rates were summarized as new-onset DM cases per 1,000 patient-years. We estimated the incidence rate ratio (IRR) for each study end point with Poisson regression models adjusted for confounding factors, including age, sex, calendar year, concomitant medication, comorbidity, and socioeconomic status. Cox regression is routinely used for epidemiological cohort studies of censored data but is less efficient when large datasets are analyzed. Therefore, we used Poisson regression analyses as done previously (23).
Psoriasis was included as a time-dependent variable. Age and calendar year were also included as time-dependent variables. Comorbidity and concomitant medication were included as fixed variables obtained at baseline.
A sensitivity analysis, to determine whether a potential risk of surveillance bias affected our primary results, was carried out by excluding all subjects with a history of hospitalizations and prescription claims. Patients with psoriatic arthritis may be more likely to receive treatment with glucocorticoids; hence, to attenuate the potential impact of glucocorticoids on the risk of DM, we excluded patients with psoriatic arthritis in a sensitivity analysis.
To address the impact of bias caused by the increased health care consumption associated with our study criteria for psoriasis diagnosis, we also conducted analyses with altered criteria of psoriasis inclusion criteria. In accordance with the altered criteria, patients with psoriasis were identified by their first vitamin D prescription claim and classified as having severe disease at the time of their first in- or outpatient hospitalization with this diagnosis, i.e., a definition of psoriasis considerably less conservative and less related to recurrent physician and hospital visits than the one used in the primary analyses.
Finally, an additional analysis with the exclusion of subjects <40 years of age (to focus more exclusively on type 2 DM) was performed. A two-sided P value <0.05 was considered statistically significant. Model assumptions, including absence of interaction between covariates, were tested. A statistically significant interaction between severe psoriasis and age was found; however, age stratification did not provide further clinically relevant information and therefore overall results (i.e., not age stratified) are presented. All statistical analyses were performed with SAS statistical software version 9.2 and STATA software version 11.
RESULTS
Baseline characteristics and death rates
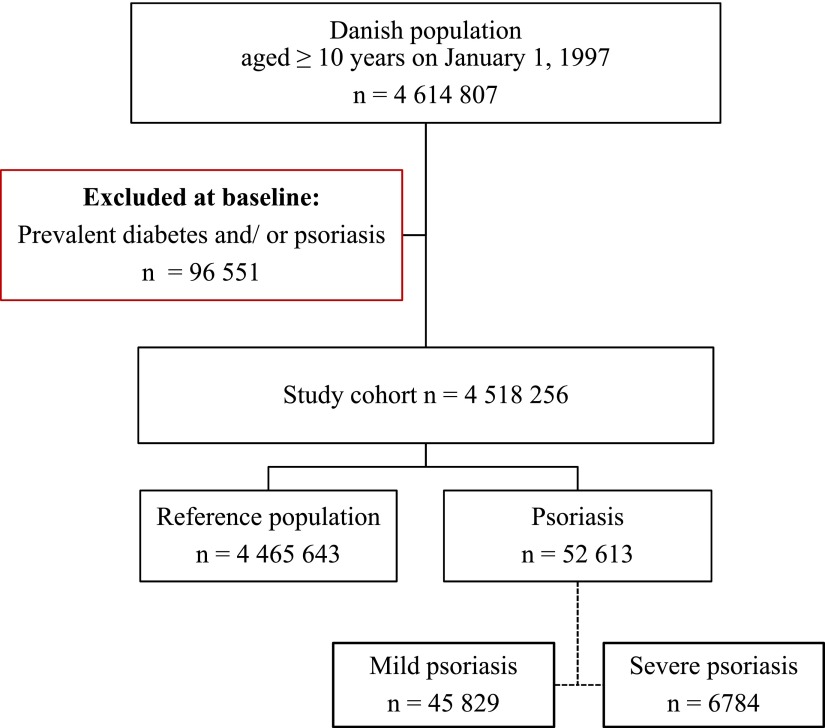
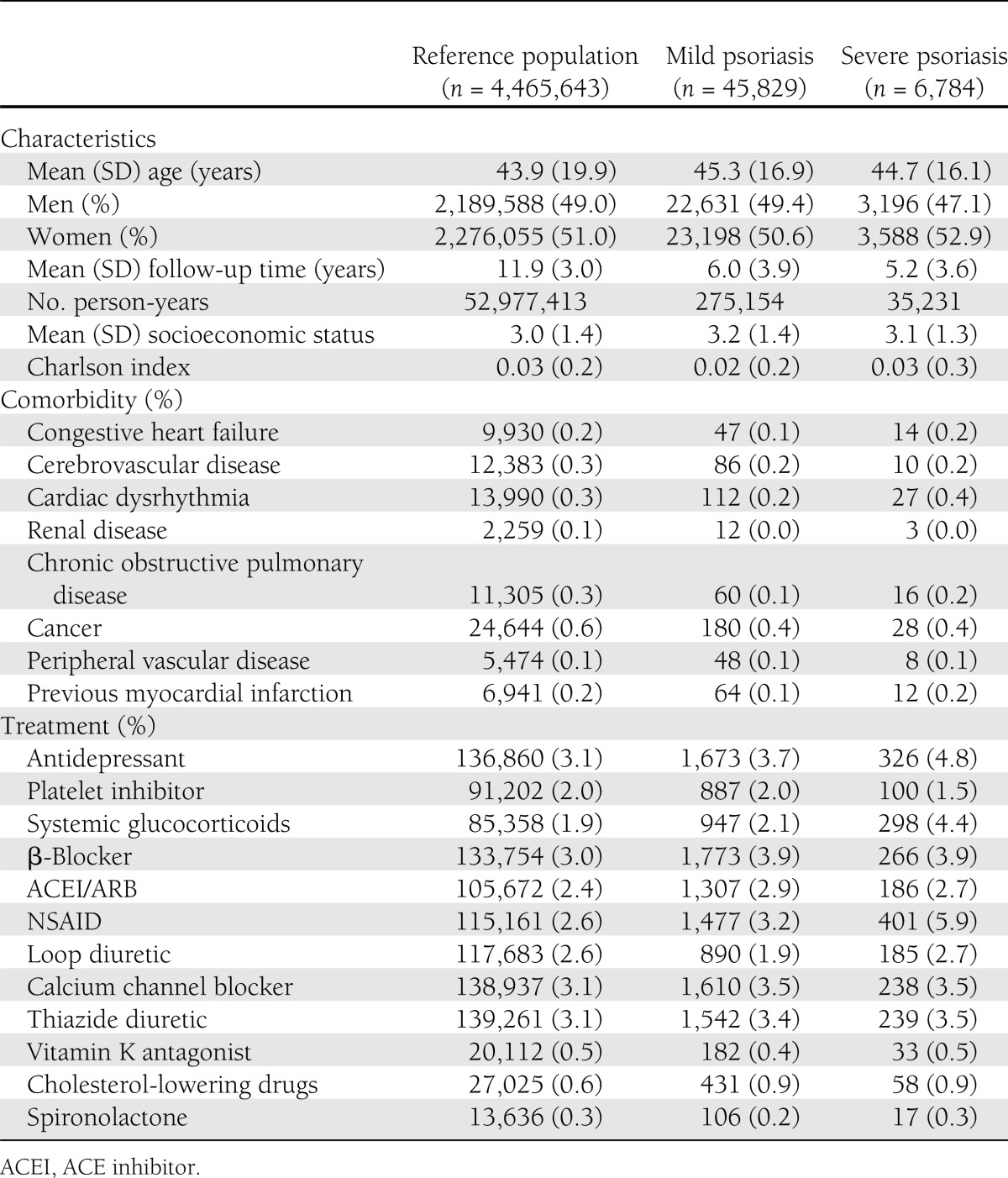
The study comprised 4,614,807 subjects with a maximum follow-up of 13 years. A total of 96,551 subjects with a previous history of DM and/or psoriasis were excluded from the study at baseline. During the study period, 45,829 subjects with mild and 6,784 subject with severe psoriasis were identified, including 2,197 with psoriatic arthritis. They were compared with the reference population of 4,562,194 individuals. An illustration of the study population selection is presented in Fig. 1. Compared with the reference population, psoriasis patients had a similar use of cardioprotective medications and comorbidity at baseline, whereas use of NSAIDs, cholesterol-lowering drugs, and antidepressive drugs was slightly increased in patients with severe psoriasis compared with mild psoriasis and the reference population, respectively. Follow-up in individual groups and baseline characteristics of the study population are summarized in Table 1.
Figure 1.
Flowchart of selection of study population. (A high-quality color representation of this figure is available in the online issue.)
Table 1.
Baseline characteristics of the study population
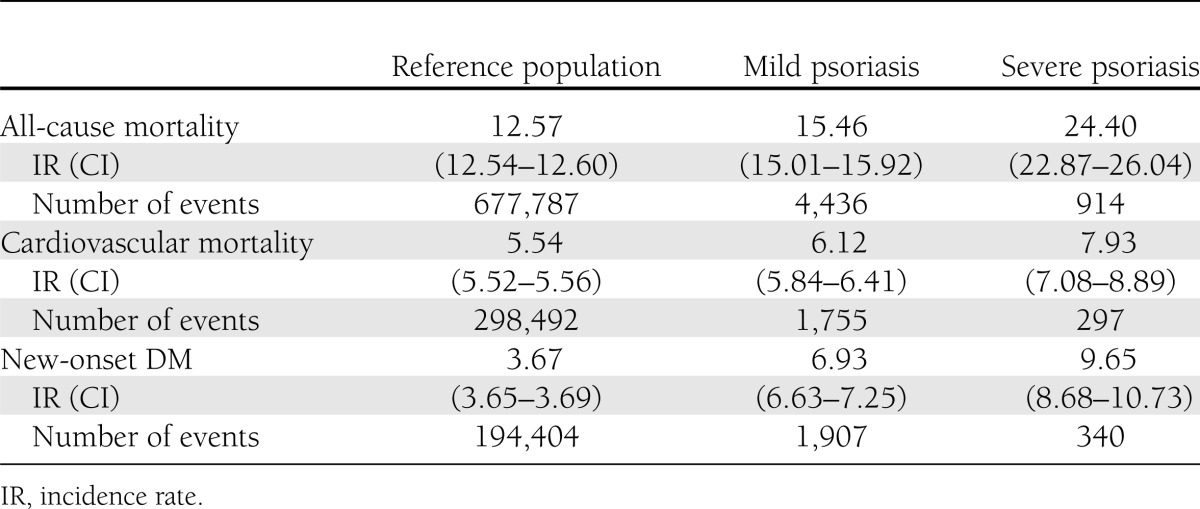
Psoriasis was associated with increased incidence rates of all-cause mortality, cardiovascular mortality, and new-onset DM (Table 2). The overall incidence rates per 1,000 person-years for all-cause mortality were 12.57 (CI 12.54–12.60), 15.46 (15.01–15.92), and 24.40 (22.87–26.04) for the reference population and patients with mild and severe psoriasis, respectively. Correspondingly, the rates for cardiovascular mortality were 5.54 (5.52–5.56), 6.12 (5.84–6.41), and 7.93 (7.08–8.89).
Table 2.
Incidence rates with 95% CIs per 1,000 person-years and numbers of events for all-cause mortality, cardiovascular mortality, and new-onset DM
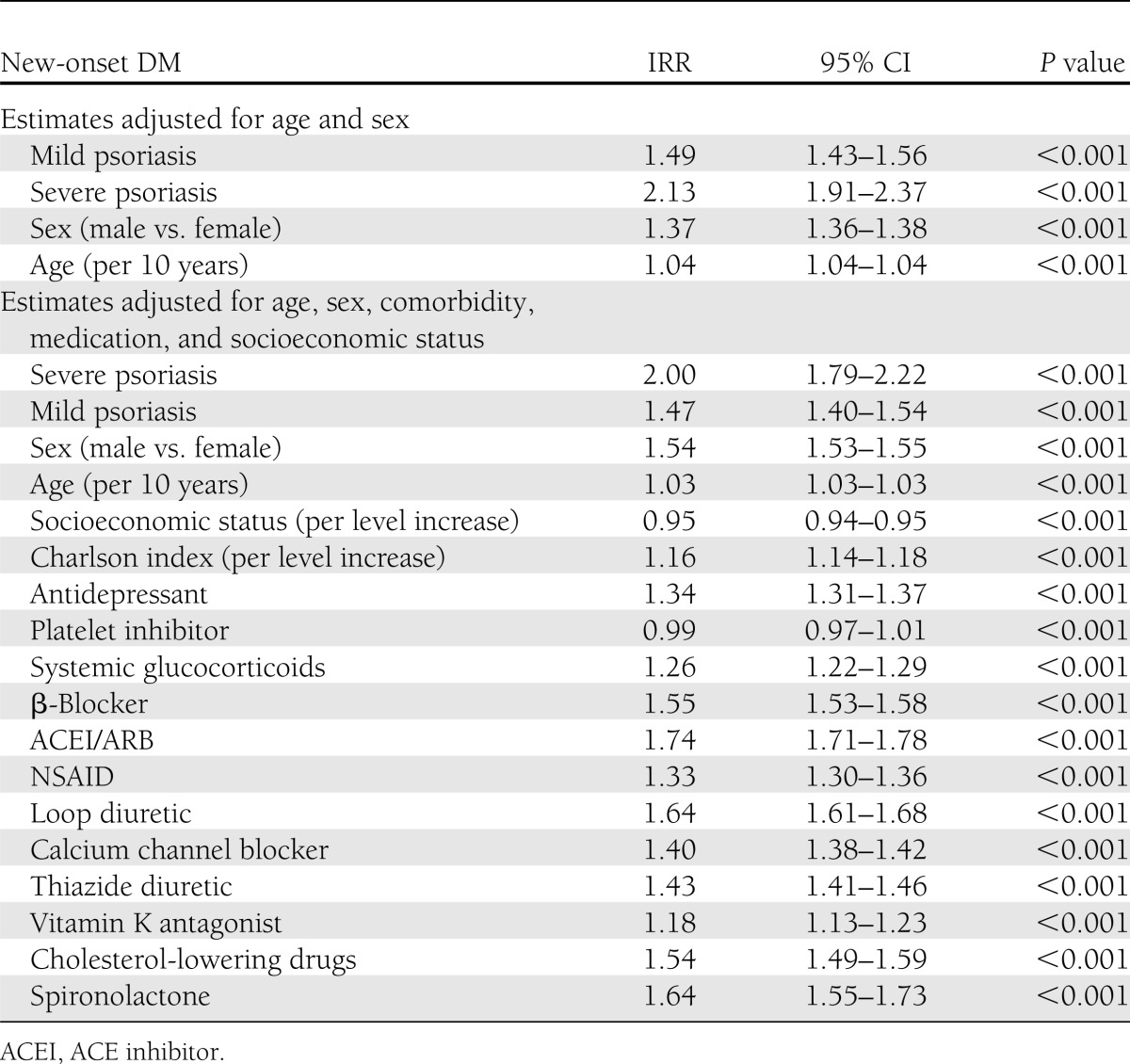
New-onset DM
The incidence rates per 1,000 person-years for new-onset DM were 3.67 (CI 3.65–3.69), 6.93 (6.63–7.25), and 9.65 (8.68–10.73) for the reference population and patients with mild and severe psoriasis, respectively. The Poisson regression analyses, adjusted for age and sex, confirmed increased incidence rates for new-onset DM in all patients with psoriasis compared with the reference population with IRR 1.49 (1.43–1.56) and 2.13 (1.91–2.37) for mild and severe psoriasis, respectively. The IRRs associated with psoriasis remained statistically significant in fully adjusted statistical models controlling for age, sex, calendar year, comorbidity, concomitant medications, and socioeconomic status (Table 3).
Table 3.
New-onset DM: IRRs and 95% CIs for mild and severe psoriasis along with individual covariates
Sensitivity analyses
When we excluded subjects with prior hospitalizations and/or prescription claims, the resulting IRRs for new-onset DM were fully comparable to the primary results, i.e., IRR 1.49 (CI 1.43–1.56) and 2.12 (1.90–2.36) for mild and severe psoriasis, respectively. After exclusion of patients with psoriatic arthritis, the results were not altered significantly. In addition, exclusion of subjects <40 years of age yielded similar results, i.e., IRR 1.49 (1.43–1.57) and 2.02 (1.80–2.27) for mild and severe psoriasis, respectively.
When the criteria for identifying psoriasis were altered to the first vitamin D prescription claim for mild psoriasis and first hospitalization for severe psoriasis, a total of 66,476 patients with mild psoriasis and 17,271 with severe psoriasis were identified. In this analysis, the results were unaffected by the criteria alteration and fully comparable to results of the primary analyses; IRR 1.41 (CI 1.35–1.47) and 1.86 (1.73–2.00) for mild and severe psoriasis, respectively.
CONCLUSIONS
In the current study, we compared the incidence rates of new-onset DM in patients with psoriasis with those of the general population in an unselected, nationwide register–based cohort followed for a maximum of 13 years. After adjusting for age, sex, concomitant medication, comorbidity, and socioeconomic status, the IRRs of new-onset DM were significantly increased in all patients with psoriasis compared with the general population. Importantly, the IRR of new-onset DM increased with psoriasis severity.
Psoriasis is a chronic inflammatory disease characterized by regulatory T-cell dysfunction and activation of T-helper 1 (Th1) and Th17 T cells producing proinflammatory cytokines (24,25). Similarly, inflammatory processes play a pivotal role in the development and progression of DM, and increased levels of proinflammatory cytokines, such as tumor necrosis factor-α, are associated with insulin resistance and type 2 DM (26,27). Furthermore, chronic inflammation in psoriatic individuals gives rise to increased levels of insulin-like growth factor-II, which promote epidermal proliferation and are linked to DM and atherosclerosis (28–30). Indeed, systemic inflammation may contribute to both DM and cardiovascular disease in patients with psoriasis (31). Along this line, increasing evidence has linked other chronic inflammatory diseases, e.g., rheumatoid arthritis, to enhanced risk of DM, insulin resistance, and cardiovascular disease (32,33). Furthermore, recent studies have suggested that the use of tumor necrosis factor-α antagonists in patients with rheumatoid arthritis or psoriasis improves insulin sensitivity and reduces the risk of DM and cardiovascular disease (34,35). From this perspective, our findings of an elevated risk of new-onset DM in patients with psoriasis further emphasize the considerable overlap of disease mechanisms shared by chronic inflammatory diseases and DM.
Previous case-control and cross-sectional studies have demonstrated an increased probability of developing DM among psoriatic individuals (5–8,11–13,15,36). In contrast, a few studies have shown no association between psoriasis and DM (9,10,14). Among the reasons for these conflicting results could be methodological issues, e.g., inadequate population size, information bias, and inadequate adjustments for covariates. Although a few cohort studies of selected populations have been carried out, results from nationwide cohort studies have not been reported previously (5,11,37). Additionally, very little data are available on the potential impact of psoriasis disease severity on the risk of DM, and the evidence of a relationship to psoriasis severity is sparse (5,8,38). Our results, however, are clearly in agreement with previous studies demonstrating a link between chronic inflammatory diseases, including psoriasis and rheumatoid arthritis, and augmented risk of DM (5–7,11–13,32,33,37). The increased risk of DM in patients with psoriasis is likely to contribute to the increased risk of cardiovascular morbidity and mortality observed in these patients (including the population examined in the current report), albeit this risk has been found to be increased even after adjustments for DM and other risk factors in agreement with the notion of independent effects of inflammation per se (19,20,39–41). Taken together, our results add to current evidence that patients with psoriasis are at increased risk of DM and cardiovascular disease and they would appear to underline the importance of regular evaluation of cardiovascular risk factors, including blood glucose levels, in these patients (42).
Major strengths of the current study include the use of nationwide prospectively recorded data, completeness of follow-up, and use of validated measures of exposure and outcome. In addition, the exclusion of subjects with prevalent psoriasis and DM at the baseline ensured the most accurate allocation of time at risk. Importantly, health care in Denmark is essentially free of charge and therefore, in principle, equally accessible to all. The inclusion of the entire Danish population in the current study also attenuated the risk of selection bias related to age, sex, socioeconomic status, and health care insurance status. In addition, the demonstration of a dose response to psoriasis disease severity is suggestive of a direct relationship between psoriasis and risk of DM, and inflammation is likely to be a shared causal mechanism.
Major limitations of the current study include the use of hospitalizations to classify severe psoriasis, as this may have increased the presence of comorbidities and decreased the threshold for detection of the study outcome in this patient group because of surveillance bias. We addressed this key concern by making multiple adjustments for confounding variables, including the Charlson comorbidity index and concomitant medication. Importantly, we applied sensitivity analyses with altered inclusion criteria and exclusion of patients with a history of hospitalizations and/or prescription claims and observed no considerable changes in the results. This method of classifying severe psoriasis was previously validated by examining the medical records of 50 randomly selected patients with psoriasis consecutively referred for first-time hospital-based treatment. At presentation, these patients were mainly treated with topical agents and had a mean psoriasis area and severity index score of 10, which was consistent with severe psoriasis (19). Also, the registries used in the current study did not allow for the identification of some cardiometabolic risk factors (e.g., obesity, smoking, and lipid profile). Although the study design accounted for some of these effects by multivariable adjustments, e.g., socioeconomic status and concomitant medication, the effect of residual confounding cannot be ruled out. The use of vitamin D prescription claims to identify patients with psoriasis preludes conclusions regarding patients treated with other first-line topical treatments. However, this method of identification was validated previously by examining the hospital records of 155 randomly selected patients with psoriasis, where 73.5% were to found to have a history of vitamin D derivative use (20). Indeed, the latter is the preferred first-line treatment for psoriasis in Denmark and any bias related to such potential misclassification is expected to be minor and would arguably draw the results toward the null. Furthermore, the current study used claimed prescriptions for glucose-lowering drugs to define new-onset DM; consequently, the patients with DM managed with diet alone were not identified. The results are therefore only valid for patients with DM requiring pharmacotherapy.
It was not possible to differentiate between type 1 and type 2 DM in the current study, as the registries do not hold data on, for example, antiglutamic acid decarboxylase antibodies or C-peptide levels. However, the vast majority of patients treated with glucose-lowering medications have type 2 DM, and therefore, the results mainly reflect an increased risk of type 2 DM. Finally, the majority of the Danish population is of Caucasian descent, which may limit the extrapolation of the results to other ethnicities.
This nationwide cohort study suggests that patients with psoriasis are at an increased risk of new-onset DM compared with the general population. The incidence rates were highest in individuals with severe psoriasis. The results emphasize the importance of considering psoriasis a systemic inflammatory disorder rather than an isolated skin disease. Clinicians should be aware and may want to consider early screening and treatment of these risk factors. Moreover, prospective studies aimed at the effects of such interventions on hard outcomes are urgently needed.
Acknowledgments
This study was supported by unrestricted grants from the LEO foundation, the Axel Muusfeldts Foundation, DERMBIO, and the Danish Psoriasis Association. The sponsors had no influence on data collection, no access to the data, and no influence on the decision to submit.
L.S. has received honoraria as a consultant and/or speaker for Abbott, Janssen-Cilag, MSD, Pfizer, and LEO Pharma. O.A. has received honoraria as a speaker for Abbott, Pfizer, and Janssen-Cilag. No other potential conflicts of interest relevant to this article were reported.
U.K. and O.A. conceived and designed the experiments, analyzed the data, wrote the manuscript, and revised the manuscript for critical contents. P.R.H. and G.H.G. conceived and designed the experiments, analyzed the data, and revised the manuscript for critical contents. J.L., S.L.K., and S.A.W. analyzed the data and revised the manuscript for critical contents. L.S. conceived and designed the experiments and revised the manuscript for critical contents. C.T.-P. conceived and designed the experiments, analyzed the data, and revised the manuscript for critical contents. All authors approved the final manuscript. O.A. is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in oral form at the annual European Society of Cardiology Congress, Munich, Germany, 25–29 August 2012.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361:496–509 [DOI] [PubMed] [Google Scholar]
- 2.Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol 2009;161:1–7 [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007;115:1544–1550 [DOI] [PubMed] [Google Scholar]
- 4.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol 2012;148:995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brauchli YB, Jick SS, Meier CR. Psoriasis and the risk of incident diabetes mellitus: a population-based study. Br J Dermatol 2008;159:1331–1337 [DOI] [PubMed] [Google Scholar]
- 7.Cohen AD, Dreiher J, Shapiro Y, et al. Psoriasis and diabetes: a population-based cross-sectional study. J Eur Acad Dermatol Venereol 2008;22:585–589 [DOI] [PubMed] [Google Scholar]
- 8.Husted JA, Thavaneswaran A, Chandran V, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken) 2011;63:1729–1735 [DOI] [PubMed] [Google Scholar]
- 9.Inerot A, Enerbäck C, Enlund F, et al. Collecting a set of psoriasis family material through a patient organisation; clinical characterisation and presence of additional disorders. BMC Dermatol 2005;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondratiouk S, Udaltsova N, Klatsky AL. Associations of psoriatic arthritis and cardiovascular conditions in a large population. Perm J 2008;12:4–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Han J, Hu FB, Curhan GC, Qureshi AA. Psoriasis and risk of type 2 diabetes among women and men in the United States: a population-based cohort study. J Invest Dermatol 2012;132:291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006;55:829–835 [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol 2009;145:379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynoso-von Drateln C, Martínez-Abundis E, Balcázar-Muñoz BR, Bustos-Saldaña R, González-Ortiz M. Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. J Am Acad Dermatol 2003;48:882–885 [DOI] [PubMed] [Google Scholar]
- 15.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. Arch Dermatol. 15 October 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 17.Gaist D, Sørensen HT, Hallas J. The Danish prescription registries. Dan Med Bull 1997;44:445–448 [PubMed] [Google Scholar]
- 18.Nuttall M, van der Meulen J, Emberton M. Charlson scores based on ICD-10 administrative data were valid in assessing comorbidity in patients undergoing urological cancer surgery. J Clin Epidemiol 2006;59:265–273 [DOI] [PubMed] [Google Scholar]
- 19.Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011;270:147–157 [DOI] [PubMed] [Google Scholar]
- 20.Ahlehoff O, Gislason GH, Jorgensen CH, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J 2012;33:2054–2064 [DOI] [PubMed] [Google Scholar]
- 21.Andersson C, Norgaard ML, Hansen PR, et al. Heart failure severity, as determined by loop diuretic dosages, predicts the risk of developing diabetes after myocardial infarction: a nationwide cohort study. Eur J Heart Fail 2010;12:1333–1338 [DOI] [PubMed] [Google Scholar]
- 22.Norgaard ML, Andersen SS, Schramm TK, et al. Changes in short- and long-term cardiovascular risk of incident diabetes and incident myocardial infarction—a nationwide study. Diabetologia 2010;53:1612–1619 [DOI] [PubMed] [Google Scholar]
- 23.Lindhardsen J, Ahlehoff O, Gislason GH, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011;70:929–934 [DOI] [PubMed] [Google Scholar]
- 24.Davidovici BB, Sattar N, Prinz JC, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol 2010;130:1785–1796 [DOI] [PubMed] [Google Scholar]
- 25.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008;128:1207–1211 [DOI] [PubMed] [Google Scholar]
- 26.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 27.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004;53:693–700 [DOI] [PubMed] [Google Scholar]
- 28.Yoo H, Kim SJ, Kim Y, Lee H, Kim TY. Insulin-like growth factor-II regulates the 12-lipoxygenase gene expression and promotes cell proliferation in human keratinocytes via the extracellular regulatory kinase and phosphatidylinositol 3-kinase pathways. Int J Biochem Cell Biol 2007;39:1248–1259 [DOI] [PubMed] [Google Scholar]
- 29.Zaina S, Nilsson J. Insulin-like growth factor II and its receptors in atherosclerosis and in conditions predisposing to atherosclerosis. Curr Opin Lipidol 2003;14:483–489 [DOI] [PubMed] [Google Scholar]
- 30.Wakkee M, Thio HB, Prens EP, Sijbrands EJ, Neumann HA. Unfavorable cardiovascular risk profiles in untreated and treated psoriasis patients. Atherosclerosis 2007;190:1–9 [DOI] [PubMed] [Google Scholar]
- 31.Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol 2011;20:303–307 [DOI] [PubMed] [Google Scholar]
- 32.Gabriel SE, Crowson CS. Risk factors for cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol 2012;24:171–176 [DOI] [PubMed] [Google Scholar]
- 33.Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine 2011;78:179–183 [DOI] [PubMed] [Google Scholar]
- 34.Dixon WG, Symmons DP. What effects might anti-TNFalpha treatment be expected to have on cardiovascular morbidity and mortality in rheumatoid arthritis? A review of the role of TNFalpha in cardiovascular pathophysiology. Ann Rheum Dis 2007;66:1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg JD, Kremer JM, Curtis JR, et al. CORRONA Investigators Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:576–582 [DOI] [PubMed] [Google Scholar]
- 36.Cheng J, Kuai D, Zhang L, Yang X, Qiu B. Psoriasis increased the risk of diabetes: a meta-analysis. Arch Dermatol Res 2012;304:119–125 [DOI] [PubMed] [Google Scholar]
- 37.Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis 2010;69:2114–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2006;33:2167–2172 [PubMed] [Google Scholar]
- 39.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735–1741 [DOI] [PubMed] [Google Scholar]
- 40.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 2010;31:1000–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahlehoff O, Gislason GH, Lindhardsen J, et al. Prognosis following first-time myocardial infarction in patients with psoriasis: a Danish nationwide cohort study. J Intern Med 2011;270:237–244 [DOI] [PubMed] [Google Scholar]
- 42.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–331 [DOI] [PubMed] [Google Scholar]