Abstract
OBJECTIVE
Reduced heart rate variability (HRV) and increased arterial stiffness (AS) are both present in youth with type 1 diabetes. However, it is unclear whether they are associated and whether their association is independent of cardiovascular disease (CVD) risk factors.
RESEARCH DESIGN AND METHODS
The SEARCH Cardiovascular Disease (SEARCH CVD) study explored the cross-sectional relationships between HRV and several measures of AS in youth with (n = 344) and without (n = 171) type 1 diabetes. The SphygmoCor device (AtCor Medical, Sydney, Australia) was used to measure HRV using SD of normal R-R interval (SDNN), as well as AS, using pulse wave velocity in the carotid to femoral segment (PWV-trunk) and augmentation index adjusted to a heart rate of 75 bpm (AIx75). Brachial distensibility (BrachD), another index of AS, was measured with a DynaPulse instrument (Pulse Metric, San Diego, CA). Multiple linear regression analyses explored the associations between HRV and each of the three AS measures, after adjusting for demographic characteristics and traditional CVD risk factors (blood pressure, lipids, obesity, microalbuminuria, and smoking) separately, for youth with and without type 1 diabetes.
RESULTS
Among youth with type 1 diabetes, lower SDNN was associated with peripheral AS (lower BrachD, P = 0.01; r2 = 0.30) and central AS (higher PVW-trunk, P < 0.0001; r2 = 0.37; and higher AIx75, P = 0.007; r2 = 0.08). These associations were attenuated with adjustment for CVD risk factors, but remained statistically significant for BrachD and PWV-trunk. While a similar association between HRV and BrachD was present in control youth, lower HRV was not associated with increased central AS or with AIx75.
CONCLUSIONS
Longitudinal studies are needed to understand the pathways responsible for these associations.
Cardiovascular disease (CVD) is the leading cause of mortality in persons with type 1 diabetes, and it occurs earlier in life than in the general population (1). The earlier CVD morbidity and mortality among people with type 1 diabetes are attributed primarily to an accelerated and more diffuse atherosclerotic process (2). Small clinical studies using B-mode imaging of carotid arteries have suggested that patients with type 1 diabetes have significant subclinical atherosclerosis as early as age 10–19 years, which is strongly associated with diabetes duration (3).
Arterial stiffness (AS), a marker of subclinical vascular disease that can be measured noninvasively, is an independent predictor of CVD mortality in adults with type 2 diabetes (4). However, little is known about the subclinical stages of vascular disease in individuals with type 1 diabetes. Moreover, increased AS has been documented among youth with type 1 diabetes as early as the second decade of life, as compared with healthy control subjects (5,6). Recent findings, including data from the SEARCH for Diabetes in Youth study (5), suggest that AS differs by measurement site, although there is still disagreement as to whether increased stiffness is more common in peripheral versus central vascular beds in youth with type 1 diabetes.
Cardiac autonomic neuropathy (CAN) is another complication of type 1 diabetes associated with increased morbidity and mortality (7,8). Recent studies, including data from the SEARCH Cardiovascular Disease (SEARCH CVD) study, suggest that youth with type 1 diabetes have signs of early autonomic dysfunction, specifically reduced overall heart rate variability (HRV) with parasympathetic loss and sympathetic override (9,10). The autonomic nervous system is responsible for regulating heart rate and vascular tone and, thus, may contribute to increased AS in individuals with type 1 diabetes. A recent report from the Epidemiology of Diabetes Complications (EDC) study among 144 adults with type 1 diabetes suggested an inverse association between markers of CAN and follow up measures of AS, independent of traditional CVD risk factors (11). However, these relationships have not been examined among contemporary adolescents and young adults with type 1 diabetes. Moreover, previous studies exploring this association (12–14) have not included a healthy comparison group. We aimed to explore the cross-sectional associations between cardiac autonomic function and markers of AS among youth with and without type 1 diabetes participating in the SEARCH CVD study. We hypothesized that altered cardiac autonomic function, specifically reduced overall HRV, will be associated with increased stiffness in both peripheral and central arterial beds, independent of traditional CVD risk factors. We further hypothesized that these associations will be observed in youth with type 1 diabetes but not in nondiabetic control subjects, indicating a common underlying mechanism predisposing individuals with type 1 diabetes to an increased risk of CVD outcomes.
RESEARCH DESIGN AND METHODS
Participants
SEARCH CVD is an ancillary study to the SEARCH for Diabetes in Youth Study, conducted in Colorado and Ohio. SEARCH is a multicenter study that conducts population-based ascertainment of nongestational cases of physician-diagnosed diabetes in youth age <20 years at diagnosis (15). A total of 402 SEARCH participants with type 1 diabetes, residents of Colorado and Ohio, who had a duration of diabetes of at least 5 years, were enrolled in the SEARCH CVD study between 2009 and 2011. During the same time period, a total of 204 frequency-matched (by age, sex, and race/ethnicity) youth without type 1 diabetes (control subjects) were also recruited in the study. Control participants were enrolled from the primary-care offices in the same geographical areas from which the patients with diabetes were referred and were confirmed free of diabetes by fasting glucose levels <126 mg/dL (16). The study was reviewed and approved by the local institutional review boards that had jurisdiction over the local study population, and all participants provided signed informed consent or assent.
Anthropometric and metabolic measurements
Participants were invited for an outpatient research visit after an 8-h overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit until after the blood draw was complete. All participants were asked to refrain from any strenuous exercise, smoking, or consumption of any caffeinated drinks 12 h prior to the visit. Race/ethnicity was self-reported, and the participants were categorized into non-Hispanic white (NHW) and other racial/ethnic group (including Hispanic, African American, and Asian/Pacific Islander racial/ethnic groups). Participants completed standardized questionnaires including medical history, medication inventory, smoking status, physical activity, daily insulin dose, and family history of diabetes and CVD. Current cigarette smoking was defined as having smoked cigarettes on one or more of the 30 days preceding the survey. Individuals who had tried smoking or smoked regularly (at least one cigarette every day for 30 days) but were not current smokers were considered past smokers. Youth who had never smoked a whole cigarette were considered nonsmokers. Participants were asked the average number of days in a typical week that they participated in physical activity for at least 20 min that made them sweat or breathe hard and were then categorized as physically inactive (0–2 days/week) or physically active (3–7 days/week) (17). Height was measured in centimeters using a stadiometer and weight in kilograms using a standardized scale. BMI was calculated as weight in kilograms divided by the square of height in meters, and age- and sex-specific BMI z-scores were derived based on the Centers for Disease Control and Prevention national standards (18). Waist circumference was measured to the nearest 0.1 cm with the National Health and Nutrition Examination Survey protocol (19). Resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times, using an aneroid sphygmomanometer, while the subjects were seated for at least 5 min and the average of the three measurements was taken. Laboratory samples were obtained under conditions of metabolic stability, defined, for case subjects, as no episode of diabetic ketoacidosis during the previous month. A fasting blood draw was conducted for the assessment of the metabolic parameters (HbA1c, LDL cholesterol [LDL-c], HDL cholesterol [HDL-c], and triglyceride [TG] levels). Urinary albumin was measured from overnight timed urine samples by radioimmunoassay and expressed as albumin excretion rate (AER). AER levels between 20 and 199 µg/min were defined as microalbuminuria (20). High-performance liquid chromatography (TOSOH Bioscience, Inc., San Francisco, CA) was used to measure HbA1c. Measurements of TG and HDL-c were performed enzymatically on a Hitachi 917 autoanalyzer (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN). LDL-c was calculated by the Friedewald equation for individuals with TG concentration <400 mg/dL and the Beta Quantification procedure for those with TG concentration ≥400 mg/dL.
Assessment of cardiac autonomic function
HRV was measured in the morning between 7:00 and 11:00 a.m. in a room with a stable room temperature, with the participant lying in the resting supine position for 10 min, using the SphygmoCor device (AtCor Medical, Sydney, Australia). The device takes into account the normal heart beats, ignoring ectopic beats, to derive the statistical parameters of the normal R-R intervals (N-N intervals) of the electrocardiogram and estimates several time- and frequency-domain HRV indices (21). The time-domain indices of HRV used in the present analysis were SD of the R-R intervals (SDNN) and root mean square difference of successive normal R-R interval (RMSSD). SDNN is a measure of overall HRV, so lower SDNN levels indicate reduced overall HRV. RMSSD is a measure of parasympathetic autonomic function, and reduced RMSSD is a marker of parasympathetic loss.
Assessment of vascular function
Three AS measures were obtained after 5 min of rest in the supine position. Brachial distensibility (BrachD) was measured using a DynaPulse Pathway instrument (Pulse Metric, San Diego, CA), as previously described (22). This device derives brachial artery pressure curves using pulse dynamic analysis of arterial pressure signals obtained from a standard cuff sphygmomanometer to assess distensibility (% Δ/mmHg). BrachD was calculated using an empirical model to estimate baseline brachial artery diameter from sex, height, weight, and mean arterial pressure (22). Lower BrachD indicates increased peripheral AS.
Pulse wave velocity (PWV) in the carotid to femoral segment (PWV-trunk) was measured with the SphygmoCor device (AtCor Medical). PWV calculates the speed for the pressure wave generated by cardiac ejection to reach the periphery (m/s) (23). Three electrocardiogram leads were applied to the torso, and the average of three distances from the lowest portion of the sternal notch to the carotid and femoral artery sites was obtained. A pressure waveform was obtained for the proximal site (carotid), and a second was recorded from the femoral artery using an applanation tonometer. Waveforms were gated by the R wave on the simultaneously recorded electrocardiogram. PWV-trunk is the difference in the carotid-to-femoral path length divided by the difference in R wave-to-waveform foot times, using an average of at least 10 beats to cover a complete respiratory cycle (23). The average of three recordings of PWV-trunk was used in the analyses. Higher PWV-trunk indicates increased central AS.
Augmentation index (AIx) provides information concerning both wave reflections and AS (24). The applanation tonometer was placed over the right radial artery, and three radial pulse waves were obtained, as described previously (5). The pressure waves were calibrated using mean arterial pressure and DBP obtained in the same arm. The device then analyzed the pulse wave using a generalized transfer function (24). Wave forms collected over a 10-s period were averaged to produce peripheral and corresponding central (ascending aortic) pressure waveforms. Ascending aortic pressure and AIx were derived from the central pressure waveform. The AIx was calculated as the difference between the main outgoing wave and the reflected wave of the central arterial waveform, expressed as a percentage of the central pulse pressure. Because AIx is affected by heart rate, values were adjusted to a standard heart rate of 75 bpm (AIx75) (25). An average of three measures was used in the analyses. A higher AIx75 was used as surrogate for increased AS.
All cardiac autonomic and vascular function tests were conducted fasting to prevent the possible influence of a surge in acute ambient glucose levels after a meal (26).
Statistical analyses
Statistical analyses were performed using SAS for Windows (version 9.2; SAS Institute, Cary, NC). SDNN, TG, and AER were log-transformed to better meet model assumptions (e.g., homogeneity of variance). Comparisons of demographic and clinical characteristics between youth with and without type 1 diabetes were examined using χ2 tests for categorical variables and t tests for normally distributed continuous variables. The associations between SDNN (main predictor variable) and AS outcomes were initially explored in a combined model, including both youth with and without type 1 diabetes, to test for effect modification by presence of type 1 diabetes by including an interaction term between SDNN and diabetes status. Since there was a suggestion of effect modification by presence of type 1 diabetes, subsequent analyses were stratified according to diabetes status. Multiple linear regression models were built separately for youth with and without type 1 diabetes to assess the relationships between SDNN (independent variable) and each of the three AS measures (dependent variables): peripheral (BrachD), central (PWV-trunk), and AIx75. Separate models for each AS outcome were constructed. A base model (Model 1) examined the relationship between SDNN and each AS measure after adjustment for demographic factors—age, sex, and race/ethnicity—and was the model used for our primary test of association between SDNN and each of the AS measures. Then, sequentially adjusted models explored the effect of adjustment for traditional CVD risk factors: DBP (Model 2), lipid (LDL-c, HDL-c, TG: Model 3), BMI (Model 4), microalbuminuria (Model 5), smoking status (Model 6), the combined model with all CVD risk factors (Model 7), and a final model with all covariates and HbA1c (Model 8). Finally, Model 9 explored potential mediation of the association between HRV and AS by heart rate. Similar analyses were conducted to assess the associations between RMSSD and each measure of AS.
RESULTS
A total of 344 youth with type 1 diabetes and 171 healthy control subjects with complete measures of HRV and AS were included in these analyses. The clinical characteristics of study participants are presented in Table 1. Youth with and without type 1 diabetes had a similar age and sex distribution, though youth with diabetes were more likely to be NHW than control subjects (87 vs. 77%; P = 0.004). BMI, BMI z-score, and BP were similar among youth with and without type 1 diabetes. However, youth with type 1 diabetes had a worse metabolic profile with higher HbA1c (P < 0.0001) and LDL-c (P = 0.01) levels and a higher prevalence of microalbuminuria (10 vs. 2%; P = 0.007) as compared with healthy control subjects. The mean duration of diabetes was 10.1 ± 3.6 years. The heart rate was significantly higher among youth with type 1 diabetes as compared with their healthy counterparts (P < 0.0001). The prevalence of smoking and physical activity was similar in the two groups. Finally, SDNN and RMSSD were significantly lower among youth with type 1 diabetes (69.7 vs. 79.4 ms, P = 0.002; and 63.7 vs. 79.7 ms, P = 0.0003, respectively) as compared with their healthy counterparts.
Table 1.
Characteristics of youth with and without type 1 diabetes
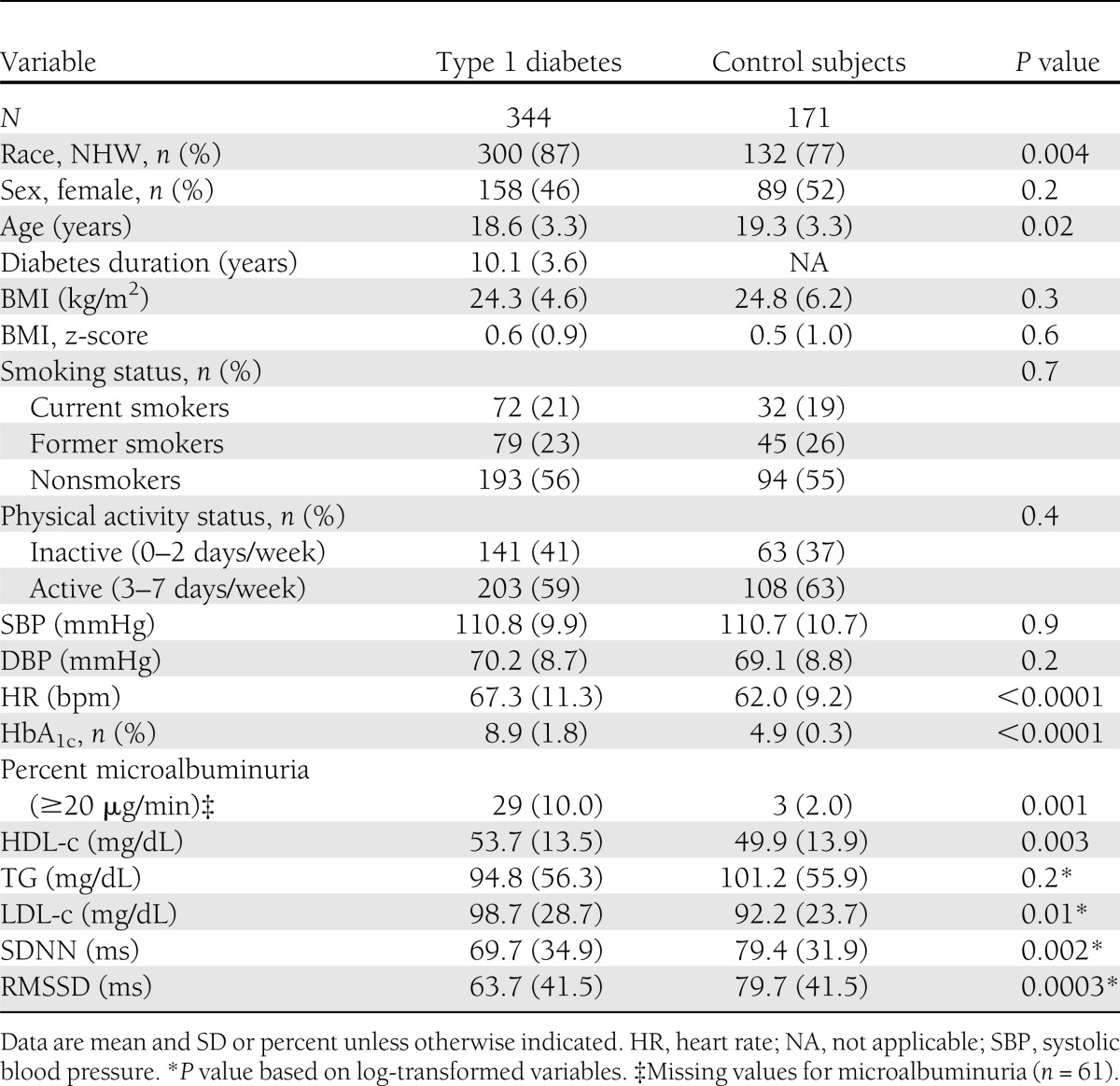
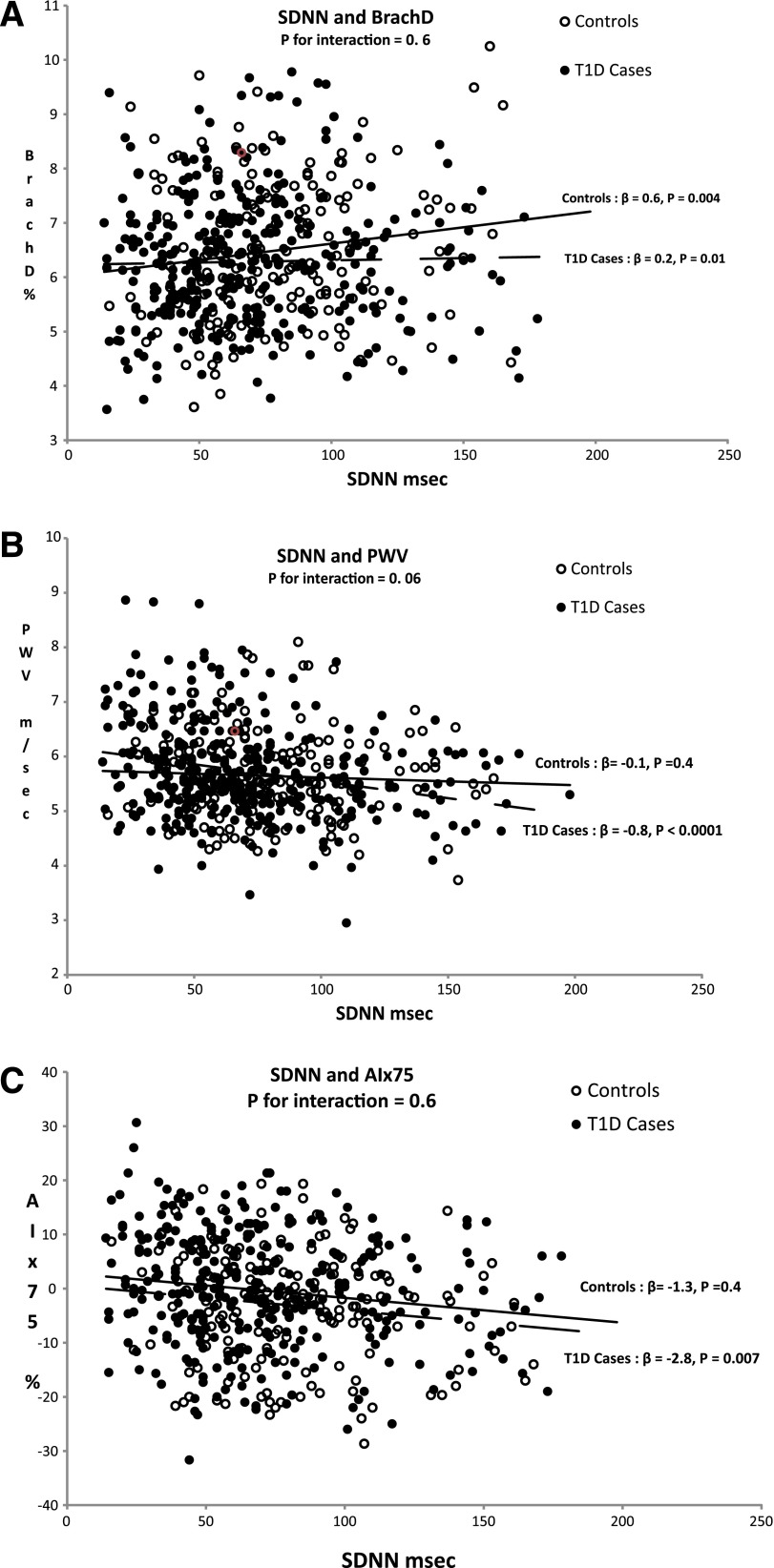
Figure 1 displays graphically the association between SDNN and each of the vascular stiffness measures, BrachD (Fig. 1A), PWV-trunk (Fig. 1B), and AIx75 (Fig. 1C), in youth with and without type 1 diabetes. Among youth with type 1 diabetes, reduced HRV (marked by lower SDNN) was significantly associated with all vascular stiffness outcomes: increased peripheral stiffness (lower BrachD, P = 0.01; Fig. 1A), increased central stiffness (higher PWV-trunk, P < 0.0001; Fig. 1B), and higher AIx75 (P = 0.007; Fig. 1C). In contrast, among control youth, the only significant association was between reduced HRV and peripheral stiffness (BrachD, P = 0.004; Fig. 1A). A formal test for effect modification by diabetes status of the association between SDNN and AS measures was marginally significant for central AS (PWV-trunk, P = 0.06; Fig. 1B).
Figure 1.
The associations between lower HRV and AS measures (panel A: BrachD; panel B: PWV-trunk; panel C: AIx75). The P value for interaction is in the combined sample including case and control subjects. The β coefficients and P values are from the linear regression models shown separately for case and control subjects adjusted for age, sex, and race/ethnicity. Filled circles, case subjects; open circles, control subjects. P value for interaction is derived from the combined model including case and control subjects. T1D, type 1 diabetes. (A high-quality color representation of this figure is available in the online issue.)
Table 2 shows results of multiple linear regression analyses exploring the association between SDNN and the three AS outcomes, stratified by diabetes status, in sequentially adjusted models.
Table 2.
Associations between SDNN and measures of AS (BrachD, PWV-trunk, and AIx75) in sequentially adjusted linear regression models
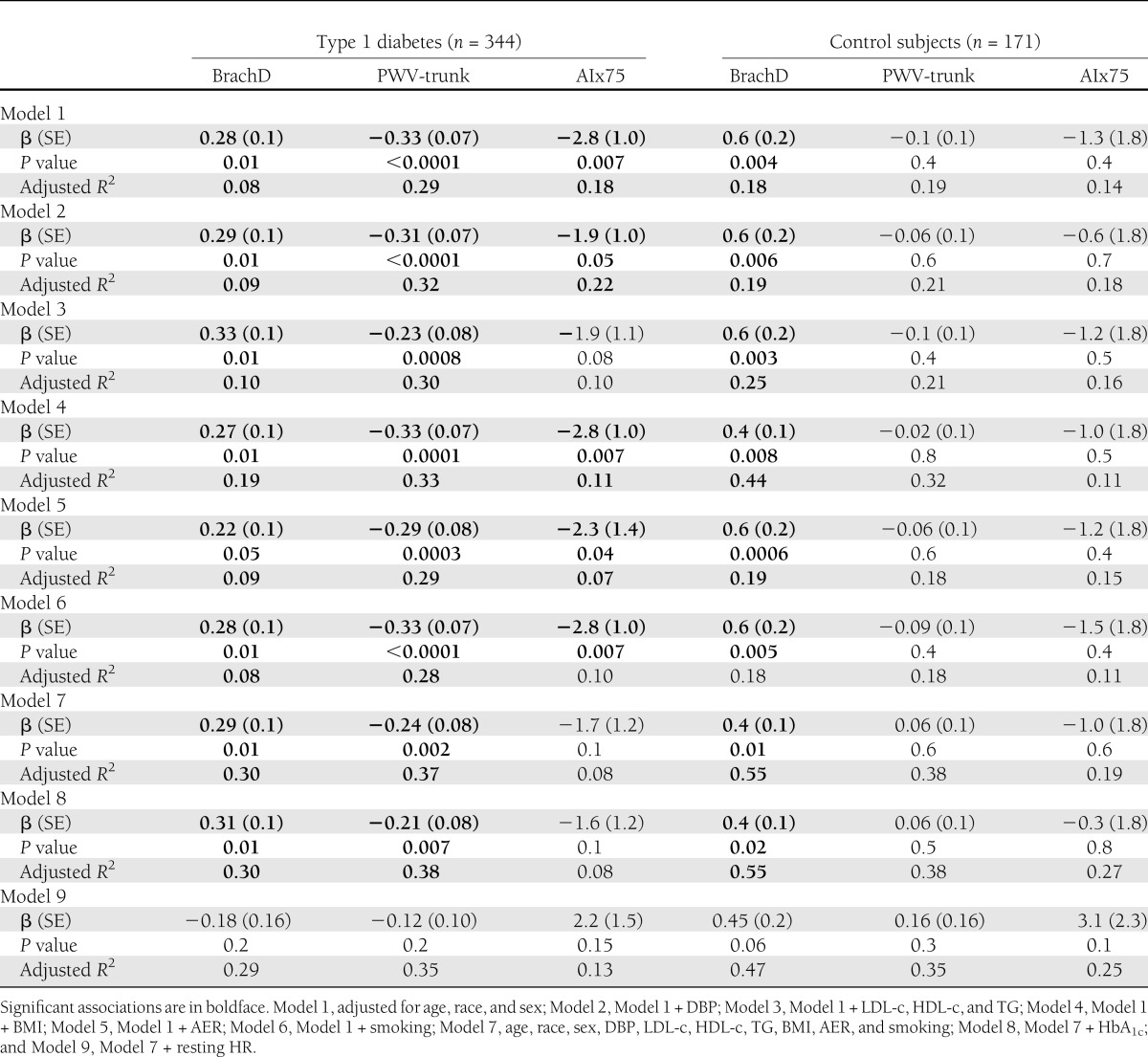
Among youth with type 1 diabetes, lower SDNN was significantly associated with lower BrachD (P = 0.01), higher PWV-trunk (P < 0.0001), and higher AIx75 (P = 0.007) independent of demographic variables (Model 1). These associations were unchanged on additional adjustment for DBP (Model 2), lipid levels (Model 3), obesity-related parameter (BMI, Model 4), microalbuminuria (AER, Model 5), and smoking (Model 6). In the fully adjusted model (Model 7), with all the covariates included, the relationships between SDNN and PWV-trunk/BrachD among youth with type 1 diabetes were virtually unchanged, but the association with AIx75 was reduced (P = 0.1). Above and beyond the variability included in the base model, blood pressure and obesity-related variables explained most of the variability in BrachD (change in adjusted R2 of 13 and 11%, respectively, from base model) and PWV (change in adjusted R2 of 7 and 4%, respectively, from base model). Addition of traditional CVD risk factors did not improve prediction of AIx75. Addition of HbA1c (Model 8) resulted in minimal improvement from the model with all CVD risk factors. Finally, on additional adjustment for resting heart rate in Model 9, the associations between SDNN and markers of AS became nonsignificant, with resting heart rate remaining significantly associated with AS in all models (data not shown).
Among healthy control subjects, lower SDNN was significantly associated with BrachD (P = 0.004) and remained unchanged and statistically significant in the sequentially adjusted and final models (P = 0.01). Obesity-related variables explained most of the variability in BrachD in youth without diabetes, with an increase of 26% in adjusted R2 from the base model.
Supplementary Table 1 shows results of multiple linear regression analyses exploring the association between RMSSD and the three AS outcomes, stratified by diabetes status, in sequentially adjusted models. Among youth with type 1 diabetes, a significant inverse association was found between RMSSD and all measures of AS (PWV-trunk, BrachD, and AIx75). No such associations were found in the healthy control group.
CONCLUSIONS
We found that cardiac autonomic dysfunction, as measured by lower HRV, is associated with increased vascular stiffness in both central and peripheral vascular beds, among youth with type 1 diabetes. These associations are independent of traditional CVD risk factors, including blood pressure, lipid levels, obesity-related parameters, microalbuminuria, and smoking. No associations between HRV and measures of AS were found in the nondiabetic control group, except for an association between SDNN (though not RMSSD) and peripheral stiffness (as measured by BrachD).
Our data extend the findings from previous studies that have demonstrated an association between cardiac autonomic function and various measures of AS in adults with type 1 diabetes (12–14). Ahlgren et al. (12) found that heart rate variation during deep breathing, the expiration/inspiration ratio, correlated with increased aortic stiffness in 40 women (r = −0.49; P = 0.002) but not in 38 men (r = −0.14; P = 0.4) with type 1 diabetes, independent of duration of diabetes. The Stockholm Diabetes Intervention Study found, in a group of 59 adults with type 1 diabetes, that HRV correlated with arterial wall stiffness of the right common carotid artery (13). Of note, all these studies were cross-sectional and did not examine the relationship independent of the traditional CVD risk factors. However, in the Pittsburgh EDC Study, lower baseline expiration/inspiration ratio correlated strongly with both greater AS (measured by both AIx and augmentation pressure) and with reduced estimated myocardial perfusion (measured by subendocardial viability ratio) in 144 adults with type 1 diabetes some 18 years later (11). Moreover, these associations were independent of other CVD risk factors. Although EDC did not measure AIx and augmentation pressure at baseline, the authors suggested, based on their findings, that cardiovascular autonomic neuropathy may play an important pathophysiological role in the development of arterial stiffening in adults with type 1 diabetes. Similarly, an association between lower HRV and progression of coronary artery calcification in both adults with type 1 diabetes and nondiabetic control subjects was recently reported by the Coronary Artery Calcification in Type 1 Diabetes study (27).
Our finding of a strong correlation between reduced HRV and increased central AS in youth with type 1 diabetes, but not among healthy control subjects, provides indirect support to the hypothesis of a pathophysiological role for CAN in the development of AS. However, given the cross-sectional design of our study, we cannot directly test this hypothesis. Data from a limited number of animal (28) and human studies (29,30) suggests that the amplified sympathetic tone present in the early stage of CAN, also identified previously in our youth with type 1 diabetes (9), could potentially increase the vascular tone and thus contribute to increased vascular stiffness, thus providing biologic plausibility for the above hypothesis. Indeed, on additional adjustment for resting heart rate (elevated in youth with type 1 diabetes versus control subjects), the associations between SDNN and markers of AS became nonsignificant, with resting heart rate remaining significantly associated with AS in all models). This suggests that increased resting heart rate may be on the causal pathway linking the reduced HRV with altered sympathovagal balance (parasympathetic loss with sympathetic override) in youth with type 1 diabetes to increased AS. This hypothesis needs to be further tested in longitudinal studies.
We found an association between lower HRV and increased central stiffness in youth with type 1 diabetes but not in control subjects, and this association was independent of traditional CVD risk factors (lipids, blood pressure, microalbuminuria, obesity, and smoking).These results may indicate, rather than a causal relationship, a common pathway, possibly hyperglycemia, leading in parallel to both cardiac autonomic dysfunction and increased AS in individuals with type 1 diabetes. There is ample biologic plausibility for the role of hyperglycemia as a common mediator of both abnormalities (31,32). Hyperglycemia has been shown to promote accumulation of advanced glycation end products, causing cross-linking and polymerization of collagen molecules within the vessel wall (33), thus resulting in loss of elasticity with subsequent reduction in arterial and myocardial compliance. Moreover, hyperglycemia was also shown to induce abnormal signaling of the autonomic neurons via accumulation of advanced glycation end products and microangiopathy, causing ischemic atrophy of the autonomic nerve fibers innervating cardiac and vascular tissue (34).
Other mechanisms, in addition to hyperglycemia, are likely to be involved. Similar to EDC, we also found an association between lower HRV and increased AIx75. However, in our study, the association was attenuated on adjustment for traditional CVD risk factors. This is not unexpected since, although cardiac autonomic dysfunction and arterial stiffening are both accelerated in the hyperglycemic environment, they likely share additional CVD risk factors. For example, elevated blood pressure levels and smoking were shown to be associated with both AS and cardiac autonomic dysfunction in subjects with type 1 diabetes (11,35–37).
Finally, we found a significant association between HRV and peripheral AS, independent of demographic and CVD risk factors, in both youth with and without type 1 diabetes. Further research is needed to understand the potential pathways responsible for this association. Similar findings were observed by Nakao et al. (38) among 382 young healthy Japanese men, with a significant negative association between brachial-ankle PWV and HRV parameters for parasympathetic nervous activity.
Our study has several limitations. First, the cross-sectional nature of the study limits our ability to evaluate causality and order of events. We therefore intend to prospectively follow this cohort to better understand the progression of cardiac autonomic dysfunction and vascular stiffening over time. Second, our sample of healthy control subjects was approximately half of that of youth with type 1 diabetes. However, the nonsignificant associations between HRV and AS measures (PWV-trunk and AIx75) were not equivocal among our control sample and likely not the result of a type II error (lack of power). Finally, the HRV measure used in our study was derived from a 10-min recording of the baseline electrocardiogram. While this is a relatively short length of recording, it is considered standard practice for clinical and research purposes, and it is advocated by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (21), as opposed to the HRV measures derived from the 24-h Holter recordings. Our study also has several unique strengths: the setting of this study in a young adult contemporary population, the large and diverse sample of patients with type 1 diabetes, inclusion of a healthy control sample, and the simple noninvasive, bedside assessment of multiple measures of AS, including both central and peripheral arteries.
In summary, we found a strong association between cardiac autonomic dysfunction and both central and peripheral AS in youth with type 1 diabetes, independent of traditional CVD risk factors. While lower HRV was also associated with increased peripheral stiffness in nondiabetic control youth, the association with central stiffness may be unique to individuals with type 1 diabetes. Regardless of the mechanisms responsible for this association, which need to be further explored in longitudinal studies, this association may contribute to the increased and premature cardiovascular disease burden in people with type 1 diabetes.
Acknowledgments
This study was funded by National Institutes of Health Grant R01-DK-078542 (principal investigator, D.D.).
No potential conflicts of interest relevant to this article were reported.
M.J. analyzed data and wrote the manuscript. E.M.U. and D.D. helped with the research and writing of the manuscript. R.P.W., R.B.D., R.F.H., S.R.D., S.M.M., and L.M.D. contributed to the discussion and reviewed and edited the manuscript. J.W.T. helped with the research of data. T.E.F. assisted in the analyses of data, contributed to the discussion, and reviewed and edited the manuscript. D.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The SEARCH for Diabetes in Youth Study is indebted to the many youth, families, and health care providers, whose participation made this study possible.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0923/-/DC1.
References
- 1.Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol 1987;59:750–755 [DOI] [PubMed] [Google Scholar]
- 2.Valsania P, Zarich SW, Kowalchuk GJ, Kosinski E, Warram JH, Krolewski AS. Severity of coronary artery disease in young patients with insulin-dependent diabetes mellitus. Am Heart J 1991;122:695–700 [DOI] [PubMed] [Google Scholar]
- 3.Kanters SD, Algra A, Banga JD. Carotid intima-media thickness in hyperlipidemic type I and type II diabetic patients. Diabetes Care 1997;20:276–280 [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–2090 [DOI] [PubMed] [Google Scholar]
- 5.Urbina EM, Wadwa RP, Davis C, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr 2010;156:731–737 [DOI] [PubMed] [Google Scholar]
- 6.Krantz JS, Mack WJ, Hodis HN, Liu CR, Liu CH, Kaufman FR. Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J Pediatr 2004;145:452–457 [DOI] [PubMed] [Google Scholar]
- 7.Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med 1993;10:820–824 [DOI] [PubMed] [Google Scholar]
- 8.Ziegler D, Zentai CP, Perz S, et al. KORA Study Group Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care 2008;31:556–561 [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal M, Urbina EM, Wadwa RP, et al. Reduced Heart Rate Variability in Youth with Type 1 Diabetes: The SEARCH CVD Study. Diabetes Care 2013:36:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javorka M, Javorkova J, Tonhajzerova I, Javorka K. Parasympathetic versus sympathetic control of the cardiovascular system in young patients with type 1 diabetes mellitus. Clin Physiol Funct Imaging 2005;25:270–274 [DOI] [PubMed] [Google Scholar]
- 11.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 2010;33:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlgren AR, Sundkvist G, Wollmer P, Sonesson B, Länne T. Increased aortic stiffness in women with type 1 diabetes mellitus is associated with diabetes duration and autonomic nerve function. Diabet Med 1999;16:291–297 [DOI] [PubMed] [Google Scholar]
- 13.Jensen-Urstad K, Reichard P, Jensen-Urstad M. Decreased heart rate variability in patients with type 1 diabetes mellitus is related to arterial wall stiffness. J Intern Med 1999;245:57–61 [DOI] [PubMed] [Google Scholar]
- 14.Liatis S, Alexiadou K, Tsiakou A, Makrilakis K, Katsilambros N, Tentolouris N. Cardiac autonomic function correlates with arterial stiffness in the early stage of type 1 diabetes. Exp Diabetes Res 2011;2011:957901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SEARCH Study Group SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 17.Kann L, Kinchen SA, Williams BI, et al. State and Local YRBSS Coodinators. Youth Risk Behavior Surveillance System Youth risk behavior surveillance—United States, 1999. MMWR CDC Surveill Summ 2000;49:1–32 [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 2002;11:1–190 [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention The Third National Health and Nutrition Examination Survey (NHANES III 1988-94) reference manuals and reports (CD ROM). Bethesda, MD, National Center for Health Statistics, 2005 [Google Scholar]
- 20.Mogensen CE, Chachati A, Christensen CK, et al. Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest 1985-1986;9:85–95 [DOI] [PubMed] [Google Scholar]
- 21.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–1065 [PubMed] [Google Scholar]
- 22.Urbina EM, Brinton TJ, Elkasabany A, Berenson GS. Brachial artery distensibility and relation to cardiovascular risk factors in healthy young adults (The Bogalusa Heart Study). Am J Cardiol 2002;89:946–951 [DOI] [PubMed] [Google Scholar]
- 23.Laurent S, Cockcroft J, Van Bortel L, et al. European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605 [DOI] [PubMed] [Google Scholar]
- 24.O'Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl 1996;14:S147–S157 [PubMed] [Google Scholar]
- 25.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbina EM, Williams RV, Alpert BS, et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 2009;54:919–950 [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues TC, Ehrlich J, Hunter CM, Kinney GL, Rewers M, Snell-Bergeon JK. Reduced heart rate variability predicts progression of coronary artery calcification in adults with type 1 diabetes and controls without diabetes. Diabetes Technol Ther 2010;12:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosson E, Valensi P, Laude D, Mesangeau D, Dabire H. Arterial stiffness and the autonomic nervous system during the development of Zucker diabetic fatty rats. Diabetes Metab 2009;35:364–370 [DOI] [PubMed] [Google Scholar]
- 29.Failla M, Grappiolo A, Emanuelli G, et al. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J Hypertens 1999;17:1117–1123 [DOI] [PubMed] [Google Scholar]
- 30.Nemes A, Takács R, Gavallér H, et al. Correlations between aortic stiffness and parasympathetic autonomic function in healthy volunteers. Can J Physiol Pharmacol 2010;88:1166–1171 [DOI] [PubMed] [Google Scholar]
- 31.Romney JS, Lewanczuk RZ. Vascular compliance is reduced in the early stages of type 1 diabetes. Diabetes Care 2001;24:2102–2106 [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer MA, Weinberg CR, Cook DL, et al. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care 1984;7:447–453 [DOI] [PubMed] [Google Scholar]
- 33.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 2003;21:3–12 [DOI] [PubMed] [Google Scholar]
- 34.Verrotti A, Loiacono G, Mohn A, Chiarelli F. New insights in diabetic autonomic neuropathy in children and adolescents. Eur J Endocrinol 2009;161:811–818 [DOI] [PubMed] [Google Scholar]
- 35.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre-hypertension in youth. J Clin Hypertens (Greenwich) 2011;13:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayad F, Belhadj M, Pariés J, Attali JR, Valensi P. Association between cardiac autonomic neuropathy and hypertension and its potential influence on diabetic complications. Diabet Med 2010;27:804–811 [DOI] [PubMed] [Google Scholar]
- 37.Scallan C, Doonan RJ, Daskalopoulou SS. The combined effect of hypertension and smoking on arterial stiffness. Clin Exp Hypertens 2010;32:319–328 [DOI] [PubMed] [Google Scholar]
- 38.Nakao M, Nomura K, Karita K, Nishikitani M, Yano E. Relationship between brachial-ankle pulse wave velocity and heart rate variability in young Japanese men. Hypertens Res 2004;27:925–931 [DOI] [PubMed] [Google Scholar]