Abstract
OBJECTIVE
Insulin resistance has been described in type 1 diabetes mellitus, is related to risk of vascular complications, and may be more common in certain ethnic groups. Estimated glucose disposal rate (eGDR) is a validated clinical tool for estimating insulin sensitivity in type 1 diabetes. Because previous reports of eGDR in adults with type 1 diabetes have included few ethnic minorities, this study explored interethnic differences in eGDR and the relationship of eGDR with diabetic vascular complications.
RESEARCH DESIGN AND METHODS
We conducted a cross-sectional study using a sample that included 207 white, black, or Hispanic adults with prior clinical diagnosis of type 1 diabetes who were receiving care at an urban academic medical center. eGDR (milligrams per kilogram per minute) was calculated using HbA1c, waist circumference, and hypertensive status. Race/ethnicity was self-reported. Multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% CIs of association of eGDR with diabetes complications (cardiovascular disease, retinopathy, albuminuria, and chronic kidney disease above stage 3).
RESULTS
Forty-two percent of the participants were women, and mean age was 45 ± 15 years; 34% were white, 32% were Hispanic, and 34% were black. Ethnicity was significantly associated with eGDR; blacks had significantly lower eGDR (5.66 ± 2.34) than Hispanics (6.70 ± 2.29) and whites (7.20 ± 2.03) (P < 0.001). Patients with the lowest eGDR compared with the highest had a significantly greater risk of any diabetes complication (OR 3.1 [95% CI 1.2–8.1]) compared with the least insulin-resistant patients.
CONCLUSIONS
In an urban clinic population of patients with type 1 diabetes, blacks were significantly less insulin sensitive than whites or Hispanics, and lower eGDR was associated with diabetes complications. Further study is needed to determine whether using eGDR to target interventions can improve outcomes.
The presence of insulin resistance (IR), a key feature of type 2 diabetes mellitus, has been demonstrated in epidemiologic and metabolic studies of type 1 diabetes and is associated with greater vascular risk in these patients (1–5). IR and type 2 diabetes are more common among certain racial or ethnic groups. Among the general U.S. population, the risk of being diagnosed with diabetes is 66% higher in Hispanics and 77% higher in blacks compared with non-Hispanic whites (6,7). In populations with high rates of type 2 diabetes and obesity, individuals with type 1 diabetes may share genetic and environmental factors that result in reduced insulin sensitivity (8,9), a phenomenon sometimes referred to as “double diabetes” (10,11), although this clinical phenotype has not been studied rigorously.
The euglycemic-hyperinsulinemic clamp is the accepted standard for measurement of insulin sensitivity; however, it is not practical for use in the clinical setting. The estimated glucose disposal rate (eGDR) can be calculated using routine clinical measures: glycosylated hemoglobin (HbA1c), presence of hypertension, and waist circumference (12). The eGDR shows good correlation with IR measured by the euglycemic-hyperinsulinemic clamp and has been validated for the estimation of insulin sensitivity in individuals with type 1 diabetes (12,13). To date, studies examining the use of eGDR as a measure of IR in type 1 diabetes have been limited to mostly nonminority cohorts (13–15). Studies that have considered interethnic differences in insulin sensitivity have used more invasive methods, were restricted to pediatric populations, and may not have been powered adequately for comparisons among ethnic groups (6,16).
The rates of obesity and type 2 diabetes in our Bronx community are the highest in New York City and among the highest in the nation: 35% of adults are overweight, 31% are obese, and 12% have diagnosed diabetes (17). Furthermore, 54% of the residents of this community are self-described as Hispanic and 36% as black, groups that have high rates of type 2 diabetes (18). Given this background, we hypothesized that features of type 2 diabetes, including low eGDR, would be prevalent in our clinic population of patients with type 1 diabetes. Therefore, we conducted a cross-sectional study to assess the distribution of eGDR in a multiethnic population of patients with type 1 diabetes and the association between measured eGDR and diabetes complications.
RESEARCH DESIGN AND METHODS
Adult patients with a clinical diagnosis of type 1 diabetes were recruited from the endocrinology clinics and faculty practices at the Montefiore Medical Center (Bronx, NY). Patients were included if they received a clinical diagnosis of type 1 diabetes made by an attending endocrinologist. The following additional clinical characteristics also were assessed: initiation of insulin within the first year of diabetes diagnosis, diagnosis before age 30, past hospitalization for diabetic ketoacidosis (DKA), and a positive test for anti-GAD antibodies. Exclusions were the current use of oral antidiabetic agents, current pregnancy, and age <18 years. The research team measured waist circumference and BMI and obtained the following information through direct interviews and medical record review: family history of diabetes, cardiovascular risk factors, and presence of diabetes complications. The most recent results of laboratory tests (HbA1c, urine albumin-to-creatinine ratio, lipid profile) were obtained from the medical record. Laboratory tests were performed in the Montefiore Medical Center clinical laboratory for 195 of 207 subjects, and in all cases, HbA1c was measured in a laboratory certified by the National Glycohemoglobin Standardization Program. Race/ethnicity was obtained by self-report. Those not reporting ethnicity (n = 3) were excluded, as were Asians (n = 3) because of small numbers. The study was approved by the Montefiore Medical Center Institutional Review Board, and all participants provided written informed consent.
The eGDR was calculated as follows (12):
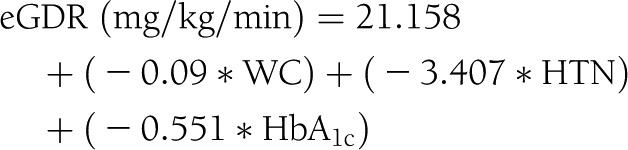 |
where HTN is the presence of hypertension (0 = no, 1 = yes) and WC is waist circumference. Hypertension was defined as a reported history or current diagnosis of physician-diagnosed hypertension or treatment with antihypertensive medication. Family history of type 2 diabetes was defined as having a first-degree family member with diabetes onset at >30 years and reported use of oral antidiabetic medication. Four types of diabetes complications and comorbidities were defined as binary variables: presence versus absence of cardiovascular disease (CVD; history of myocardial infarction, coronary artery bypass graft, percutaneous angioplasty, positive stress test, stroke, or peripheral vascular disease [documented by ankle-brachial index <0.9, angiography, or history of revascularization]); diabetic retinopathy (prior diagnosis of retinopathy, previous treatment with laser therapy for diabetes-related eye disease, or both); albuminuria (≥2 measurements of urine albumin-to-creatinine ratio >30 mg/g); and chronic kidney disease (CKD) stage 3 or higher based on the Modification of Diet in Renal Disease glomerular filtration rate calculator (19).
Statistical analyses were performed using StataC v.11 (StataCorp, College Station, TX) and SPSS 19 (IBM, Armonk, NY). All tests used a two-sided α of 0.05 to denote statistical significance. After assessing normality assumptions, eGDR (mean ± SD) was determined for the overall sample as well as for each ethnic group, and one-way ANOVA with post hoc pairwise comparisons were used to contrast means between ethnic groups (whites vs. Hispanics, Hispanics vs. blacks, and whites vs. blacks). P values were obtained by χ2 analysis for categorical variables. Continuous variables that did not meet normality assumptions were reported as median and interquartile range, and medians were compared by category of race/ethnicity with the Kruskal-Wallis test. The entire sample was divided into tertiles of eGDR (<5.39, 5.39–7.75, and >7.75), with the lowest eGDR tertile representing the most insulin resistant (IR) (highest IR) and the highest tertile representing the most insulin sensitive (lowest IR). Median (interquartile range) values for several demographic and metabolic variables were reported by eGDR tertile and a test for trend performed with Spearman rank correlation. In addition to assessing odds ratios (ORs) for eGDR tertiles, we assessed eGDR as a continuous variable. Because ORs of continuous variables with small unit measures are difficult to compare with those of categorical variables, we divided eGDR by its own SD (2.31) to make SD units, such that higher ORs reflect greater risk for complications.
ORs were used to estimate the relative risk of each major complication separately (CVD, retinopathy, albuminuria, CKD stage ≥3, as well as any combination of these four), comparing the lowest and middle eGDR tertiles with the highest, which served as the reference group. ORs were estimated from logistic models with covariates including age, sex, race/ethnicity, duration of diabetes, HDL cholesterol, total serum cholesterol, triglycerides, smoking history, and history of DKA. Logistic model fit was assessed with Hosmer-Lemeshow statistics and potential interactions were assessed with product terms. Interactions were tested by creating interaction product terms, multiplying eGDR as a continuous variable with dichotomized categorical variables for race (white/nonwhite), smoking (yes/no), and sex (male/female), as well as with the continuous variable for age. These were assessed for statistical significance in models adjusting only for the two main effects terms.
RESULTS
All participants (n = 207) previously had received a clinical diagnosis of type 1 diabetes by an attending endocrinologist and were receiving insulin treatment at the time of enrollment. Of these, 89% had at least one additional clinical characteristic of type 1 diabetes (85% initiated insulin within 1 year of diagnosis; 60% had a prior hospitalization for DKA, and 70% were diagnosed before age 30), and 70% met all three criteria. By ethnicity, 79% of whites, 94% of Hispanics, and 86% of blacks had at least one additional characteristic; by eGDR tertiles, 81% of tertile 1, 86% of tertile 2, and 91% of tertile 3 had at least one additional clinical characteristic.
Table 1 shows the patient characteristics by race/ethnicity: 34 self-identified as white, 32 as Hispanic, and 34% as black. The groups differed by age, with whites being somewhat older than blacks or Hispanics. Continuous variables were not normally distributed within the race/ethnicity groups, except for eGDR. Median BMI (26.5 kg/m2), median duration of diabetes (21 years), family history of type 2 diabetes (32%), sex (42% female), and median total daily insulin dose (0.59 units/kg/day) were similar across race/ethnicities. Of the individuals tested for GAD antibody (n = 65), 65% were positive, which was similar across ethnic groups. Median HbA1c levels varied among the groups and was 7.6% in whites, 8.1% in Hispanics, and 8.7% in blacks (P < 0.001). Serum triglycerides were similar across ethnic groups, whereas total and HDL cholesterol were highest in blacks and lowest in Hispanics. CVD, diabetic retinopathy, and eGFR <60 (CKD stage ≥3) were seen in the same proportions across race/ethnicities, whereas albuminuria and hypertension were most prevalent in blacks and least prevalent in whites. Use of statins, ACE inhibitors/angiotensin receptor blockers, aspirin, and history of smoking were similar among the groups. eGDR was higher in whites compared with blacks or Hispanics (P < 0.001).
Table 1.
Demographic and metabolic characteristics by race/ethnicity

Table 2 shows similar patient characteristics according to eGDR tertile. Tertile 1 represents the lowest level of eGDR (most IR) and tertile 3 the highest (most insulin sensitive). Tertile 1 had a higher proportion of blacks than whites or Hispanics, whereas tertile 3 had the lowest proportion of blacks. BMI, age, duration of diabetes, HbA1c, daily insulin dose, and serum triglycerides were inversely associated with eGDR. Family history of type 2 diabetes was more frequent in the lower eGDR tertiles, and there was no apparent association between total cholesterol or smoking status and eGDR. Patients with lower eGDR had higher rates of statin, aspirin, and ACE inhibitor/angiotensin receptor blocker use than those with higher eGDR. All microvascular complications, as well as CVD, were seen at significantly higher proportions in tertile 1 compared with tertile 3.
Table 2.
Demographic and metabolic characteristics by eGDR tertiles
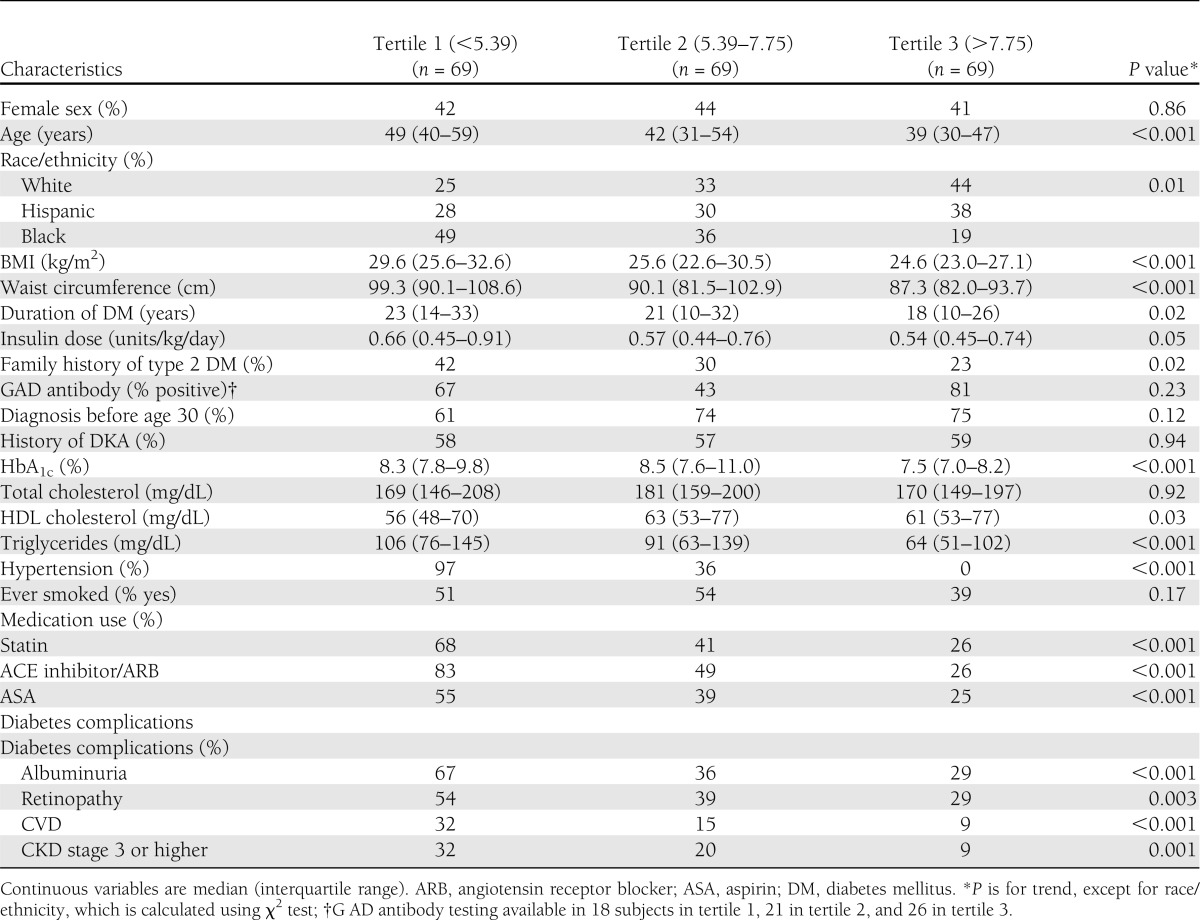
Table 3 shows the OR and 95% CI for each diabetes-related complication, and a composite of any of them combined, compared among tertiles, using tertile 3 (highest eGDR) as a reference. Model 1 adjusted for age, sex and ethnicity, whereas model 2 also adjusted for duration of diabetes, total and HDL cholesterol, triglycerides, and smoking. When compared with tertile 3, tertile 1 had significantly higher risk of CVD, retinopathy, albuminuria, CKD stage 3 or higher, and the composite of any diabetes-related complication. ORs for tertile 1 remained statistically significant for albuminuria, CKD stage 3 or higher, and the composite of any diabetes-related complication after further adjustment in model 2. Point estimates for CVD and ORs for retinopathy showed modest attenuation and lost statistical significance in model 2. Tertile 2 showed a significantly increased risk for retinopathy in model 1 only (OR 1.5 [95% CI 0.7–3.1]). There was no significantly higher odds of complications in tertile 2 compared with tertile 3 in either model. In a sensitivity analysis, ORs were unchanged when only patients with at least one additional clinical feature of type 1 diabetes (positive for GAD antibody, history of DKA, diagnosis before age 30, or initiation of insulin within 1 year of diagnosis) were included (data not shown). When eGDR is included in the model as a continuous variable (model 3), a similar pattern of association of eGDR with complications is observed. Testing interactions of eGDR (continuous) with race, smoking, sex, and age showed no evidence of significant interaction. Furthermore, when the models were run separately within the black, Hispanic, and white subgroups, results were similar and consistent (data not shown).
Table 3.
Odds ratios (95% CIs) for diabetes complications by eGDR

ORs for albuminuria and the composite of any diabetes-related complication by ethnicity are shown in Table 4. Model 1 adjusted for age and sex; model 2 added duration of diabetes, total and HDL cholesterol, triglycerides, smoking, and history of DKA; and model 3 also adjusted for eGDR. Compared with whites, Hispanics and blacks were more likely to have albuminuria in each of the models. When compared with whites, Hispanics were more likely to exhibit any diabetes complication. While blacks compared with whites had a similar trend to higher odds of any complication, these associations were not statistically significant. Results of these models were similar when patients lacking an additional clinical feature of type 1 diabetes were excluded from the analysis (data not shown).
Table 4.
Odds ratios (95% CIs) for selected complications by race/ethnicity

CONCLUSIONS
In an ethnically diverse population of patients with type 1 diabetes, we found that low eGDR was associated with family history of type 2 diabetes, obesity, and nonwhite race/ethnicity. Furthermore, we confirmed that low eGDR is associated with an increased risk of diabetes vascular complications. Although the concept of IR among individuals with type 1 diabetes is not new (3–5), few studies have explored this phenomenon in nonwhite populations or assessed interethnic differences (6,16). To our knowledge, this is the first study of adults with type 1 diabetes that was adequately powered to analyze eGDR, demographic and metabolic confounders, and diabetes complications among three major ethnic groups.
A combination of obesity prevalence and demographic composition of our local community may place our type 1 diabetes population at particularly high risk for IR as well as related macro- and microvascular complications (3,20). Given the high rates of type 2 diabetes often found among Hispanics and blacks, it is not surprising that a significant percentage of our type 1 diabetes clinic cohort shows features of “double diabetes,” that is, evidence of type 2 diabetes features (including greater waist circumference, higher triglycerides, and lower HDL) in the lowest eGDR tertile (10,11).
Unadjusted mean eGDR was lower in blacks than in Hispanics or whites in our cohort. This is consistent with a multiethnic study of 1,086 nondiabetic individuals; Haffner et al. (6) showed that African Americans and Hispanics were more IR (using the frequently sampled intravenous glucose tolerance test) than non-Hispanic whites. Danielson et al. (16) examined insulin sensitivity using eGDR in a pediatric multiethnic cohort with type 1 diabetes (n = 79) and found less IR among non-Hispanic whites compared with other ethnic groups; in contrast to our study, the average age of patients in the study by Danielson et al. was 13.5 years, ranging from 3.2–32.5 years, and all patients had onset of type 1 diabetes before age 18.
IR may alter risk profiles such that individuals with type 1 diabetes and greater IR are at higher risk for macro- and microvascular complications. In this study, when adjusting for age, sex, and race/ethnicity, lower eGDR was associated with greater prevalence of retinopathy, CVD, nephropathy, and a composite of any complication. Chillarón et al. (13) examined 91 white patients with type 1 diabetes in Spain and found that patients with any diabetes-related complication had a lower eGDR and that eGDR was significantly lower in patients with diabetic neuropathy, retinopathy, or nephropathy. In a cohort of patients with type 1 diabetes from the Pittsburgh Epidemiology of Diabetes Complications study, eGDR was a better predictor of coronary artery disease (CAD) end points than glycemic control (HbA1c) and was prognostic of all-cause mortality, predicting death to the same extent as ischemia on electrocardiogram, and only slightly less than having a prior CAD event or frank nephropathy (21). Baseline eGDR also predicted the eventual development of CAD and microvascular events in the Diabetes Control and Complications Trial, while meeting the criteria for metabolic syndrome and total daily insulin dose did not significantly predict the same diabetes outcomes (16,22). Similarly, our study showed only small and borderline significant variation in total daily insulin dose across eGDR tertiles. However, eGDR remained a significant predictor of both albuminuria and the composite of any diabetes complication, even after adjusting for lipids, smoking, and duration of diabetes. The point estimates for associations with CVD and CKD stage 3 or higher were of similar magnitude, although not significant.
In our cohort, both eGDR and race/ethnicity are related to diabetes complications, making it difficult to determine the independent contribution of eGDR. In sequential models controlling for demographics and established vascular risk factors (Table 4), the addition of eGDR resulted in a modest attenuation of the OR for microalbuminuria in blacks versus whites. This suggests that eGDR explains some, but not all, of the increased risk of microalbuminuria among blacks. A similar, although less sizable, pattern was observed for the Hispanic versus white comparisons.
Although eGDR has been proposed as a means of risk stratification of patients with type 1 diabetes, a specific eGDR threshold has not been defined, and direct comparisons of eGDR across studies may be problematic because of differences in clinical variables, such as how type 1 diabetes was determined. Nonetheless, others have reported similar relationships between eGDR category and clinical outcomes. For instance, in the study by Chillarón et al. (13), diabetes complications occurred exclusively in patients in their lowest eGDR tertile (<8.16). Olson et al. (21) reported that eGDR in the lowest quintile (<6.22) was an independent predictor of overall mortality in the cohort of patients with type 1 diabetes in the Pittsburgh Epidemiology of Diabetes Complications study.
Limitations of our study include retrospective data collection and cross-sectional data analysis. The data collected relied in part on self-report and were supported by review of existing medical records, but systematic adjudication of clinical outcomes was not performed. Self-report of outcomes could lead to misclassification, but such misclassification is likely to be random and thus would bias results toward the null. Eligibility for inclusion was based on a clinical diagnosis of type 1 diabetes, and confirmatory laboratory indicators (e.g., anti-GAD antibody) were available for only a subset of patients. However, such missing data did not differ by race/ethnicity, suggesting it was not a source of significant bias. Limiting our patient population to those treated only with insulin may have excluded the patients with type 1 diabetes with the highest IR, who might have been treated with metformin. Such combination therapy for type 1 diabetes is uncommon in our medical center and is unlikely to have excluded many patients. Limited reliable clinical data on neuropathy were available, so we were unable to include this microvascular complication in our analysis. Finally, our analysis was designed to evaluate eGDR in relation to diabetes complications and race/ethnicity and not to compare eGDR with its component variables.
In conclusion, this study corroborates previous reports that a low eGDR is associated with macro- and microvascular complications in type 1 diabetes. Assessment of eGDR could be useful in identifying individuals who might benefit the most from early and aggressive preventative strategies. Future studies should determine whether the ethnic differences in eGDR and diabetes complications that we observed can be replicated in other populations. In addition, larger prospective cohort studies can further assess the utility of eGDR in risk stratification for diabetes complications and how eGDR compares to currently used measures such as HbA1c.
Acknowledgments
This publication was supported in part by the Clinical and Translational Science Award (CTSA) Grant 8UL1 TR000086 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
No potential conflicts of interest relevant to this article were reported.
E.J.E. and J.P.C. designed the study and the survey, participated in data collection/patient interviews, analyzed the data, and drafted and edited the manuscript. J.L.O. participated in data collection/patient interviews, completed data entry and preliminary data analyses, reviewed the literature, and drafted and edited the manuscript. H.W.C. and S.N.R. conducted data analyses and assisted with manuscript editing. O.L. assisted with study design and data collection/patient interviews. E.J.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Preliminary data from this study were presented in poster form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We acknowledge the contributions of Jordan Nestor and Rebecca Geliebter (students at Albert Einstein College of Medicine) as well as the attending physicians and nursing staff of the Montefiore Medical Center diabetes clinics and faculty practice sites.
Footnotes
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1982;23:313–319 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 1982;31:795–801 [DOI] [PubMed] [Google Scholar]
- 3.Chillarón JJ, Flores-Le-Roux JA, Goday A, et al. Metabolic syndrome and type-1 diabetes mellitus: prevalence and associated factors. Rev Esp Cardiol 2010;63:423–429 [in Spanish] [PubMed] [Google Scholar]
- 4.Thorn LM, Forsblom C, Fagerudd J, et al. FinnDiane Study Group Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 2005;28:2019–2024 [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues TC, Canani LH, Gross JL. Metabolic syndrome, insulin resistance and cardiovascular disease in type-1 diabetes mellitus. Arq Bras Cardiol 2010;94:134–139 [in Portuguese] [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 1996;45:742–748 [DOI] [PubMed] [Google Scholar]
- 7.National Diabetes Statistics, 2011 [Internet]. Bethesda, MD: National Diabetes Information Clearinghouse, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Available from http://diabetes.niddk.nih.gov/dm/pubs/statistics/ Accessed 22 February 2010
- 8.Evelly J. Bronx health: the high cost of undiagnosed diabetes. Bronx News Network 29 November 2010.Available from http://www.bronxnewsnetwork.org/2010/11/bronx-health-high-cost-of-undiagnosed.html Accessed 21 December 2010
- 9.Diabetes data & trends. Diagnosed diabetes. Atlanta, GA: Centers for Diseases Control and Prevention (CDC), 27 January 2012. Available at http://www.cdc.gov/diabetes/statistics/prevalence_national.htm Accessed 14 April 2011
- 10.Libman IM, Becker DJ. Coexistence of type 1 and type 2 diabetes mellitus: “double” diabetes? Pediatr Diabetes 2003;4:110–113 [DOI] [PubMed] [Google Scholar]
- 11.Gilliam LK, Brooks-Worrell BM, Palmer JP, Greenbaum CJ, Pihoker C. Autoimmunity and clinical course in children with type 1, type 2, and type 1.5 diabetes. J Autoimmun 2005;25:244–250 [DOI] [PubMed] [Google Scholar]
- 12.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000;49:626–632 [DOI] [PubMed] [Google Scholar]
- 13.Chillarón JJ, Goday A, Flores-Le-Roux JA, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab 2009;94:3530–3534 [DOI] [PubMed] [Google Scholar]
- 14.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 15.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707–712 [DOI] [PubMed] [Google Scholar]
- 16.Danielson KK, Drum ML, Estrada CL, Lipton RB. Racial and ethnic differences in an estimated measure of insulin resistance among individuals with type 1 diabetes. Diabetes Care 2010;33:614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, Berger D, Matte T. Diabetes in New York City: public health burden and disparities [report online], 2006. New York: New York City Department of Health and Mental Hygiene. Available from http://www.nyc.gov/html/doh/downloads/pdf/epi/diabetes_chart_book.pdf. Accessed 4 February 2013 [Google Scholar]
- 18.United States Census 2010 [homepage]. Washington, DC: US Census Bureau. Available from http://www.census.gov/2010census/. Accessed 4 February 2013
- 19.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(Suppl 1):S1–S266 [PubMed] [Google Scholar]
- 20.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care 2006;29:1891–1896 [DOI] [PubMed] [Google Scholar]
- 21.Olson JC, Erbey JR, Williams KV, et al. Subclinical atherosclerosis and estimated glucose disposal rate as predictors of mortality in type 1 diabetes. Ann Epidemiol 2002;12:331–337 [DOI] [PubMed] [Google Scholar]
- 22.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]


