Abstract
OBJECTIVE
To determine the correlates of the “metabolically healthy obese” (MHO) phenotype and the longitudinal risks of diabetes and cardiovascular disease (CVD)/stroke associated with this phenotype.
RESEARCH DESIGN AND METHODS
The North West Adelaide Health Study is a prospective cohort study of 4,056 randomly selected adults aged ≥18 years. Participants free of CVD/stroke and not underweight (n = 3,743) were stratified by BMI categories and metabolic risk, defined as having two or more International Diabetes Federation metabolic syndrome criteria, excluding waist circumference.
RESULTS
Correlates of the MHO (n = 454 [12.1%]) included smoking, socioeconomic disadvantage, and physical inactivity. Compared with metabolically healthy normal-weight subjects (n = 1,172 [31.3%]), the MHO were more likely to develop metabolic risk (15.5 vs. 33.1%, P < 0.001) and incident diabetes (odds ratio 2.09 [95% CI 0.87–5.03]) but not CVD/stroke (1.16 [0.58–2.29]) during 5.5–10.3 years of follow-up. These risks were not seen in MHO subjects maintaining metabolic health (n = 188 [67%]). Sustained metabolic health in obese participants was associated with age ≤40 years and lower waist circumference. Compared with the metabolically at-risk obese, MHO women demonstrated a significantly higher (mean [SE]) percentage of leg fat (49.9 [0.5] vs. 53.2 [0.7]) and lower waist circumference (104 [0.6] vs. 101 cm [0.8]), despite no significant differences in overall adiposity.
CONCLUSIONS
“Healthy” obesity was a transient state for one-third of subjects. Persistence of a MHO phenotype, which was associated with favorable outcomes, was related to younger age and a more peripheral fat distribution. The MHO phenotype may be sustained by promoting lower waist circumferences.
Given the scale of the obesity problem, the notion of “healthy obesity” (1) requires evaluation to avoid inappropriate public health messages. The metabolically healthy obese (MHO) phenotype, characterized by normal metabolic parameters, such as serum cholesterol and blood pressure, despite elevated BMI, has been reported in ∼10–15% of population samples, including the Framingham Offspring Study (2) and the United States (3) and Korean (4) National Health and Nutrition Examination Surveys (NHANES). A review concluded that the MHO demonstrated reduced cardiovascular disease (CVD) risks compared with metabolically at-risk obese (MRO) subjects (5). However, the longitudinal course of this phenotype is not well described or understood, because follow-up of the MHO phenotype has occurred in few studies. Where follow-up has occurred, there have been conflicting results regarding mortality outcomes (6–9).
Recent studies have reported that within the metabolically healthy, obese subjects demonstrated CVD risks (10,11) similar to those of normal-weight subjects. Some findings indicate that the CVD risk attributable to obesity requires the concomitant presence of metabolic risk factors (6,12,13); however, Meigs et al. (2) found that the MHO had a borderline increased risk of developing diabetes during an 11-year period. Outcomes in the MHO may be related to differences in body composition, fitness, and inflammatory profiles. In a recent large study of well-educated professional adults with low baseline levels of obesity, the MHO had better objectively assessed fitness than the MRO (6). Studies in small selected samples of postmenopausal obese women also suggest that the MHO may have more favorable inflammatory profiles (14), less visceral fat, and possibly less hepatic fat (15) than their counterparts with insulin resistance and other metabolic abnormalities (16).
The aim of this study was to determine the correlates of the MHO and the longitudinal outcomes of this phenotype derived from a large cohort of adults. We also examined diabetes and CVD outcomes in relation to the stability of the MHO over time, because previous studies have considered these phenotypes as static populations, ignoring weight and metabolic changes that occur over time. In addition, we examined health-related quality-of-life, body composition derived from dual energy X-ray absorptiometry (DXA), and socioeconomic status (SES) in these phenotypes because previous studies have not addressed these relationships in population samples.
RESEARCH DESIGN AND METHODS
Cohort participants and follow-up
The North West Adelaide Health Study (NWAHS) is a representative biomedical cohort study of adults of predominantly mixed European descent, aged at least 18 years. The study methods (17) and their validity to achieve an unbiased sample have been described (18). From December 1999 to July 2003, 5,850 adults provided baseline survey data, of whom 4,056 underwent a biomedical examination (69.4% of those completing the initial interview). The present analysis was conducted in 3,743 subjects free of existing CVD and not underweight at baseline.
The first follow-up (stage 2) of the cohort occurred between May 2004 and February 2006, with the median follow-up time being 4.0 years (range 1.7–5.8). Survey data were obtained for 88% (n = 3,574) and clinic data for 79% (n = 3,206). DXA was conducted at stage 2, involving 1,604 participants aged at least 50 years. The second follow-up (stage 3) occurred between June 2008 and August 2010. The median follow-up time was 8.2 years (range 5.5–10.3), and survey data were obtained for 67% (n = 2,710) and clinic data for 61% (n = 2,487) of the original clinic cohort. The same survey methodologies were used at all stages of the study.
Information was collected on demographics, doctor-diagnosed health conditions (diabetes, myocardial infarction, angina, stroke, transient ischemic attack), behavioral risk factors, family history of diabetes, the physical (PCS) and mental component summary (MCS) scores of the 36-item Short-Form (SF-36) health-related quality-of-life assessment, and use of therapies to lower lipid concentrations and blood pressure. Clinic assessment included measurement of height, weight, waist circumference, and blood pressure, and a fasting blood sample was drawn for assessment of glucose and lipids. Diabetes was identified by self-report of a doctor diagnosis or fasting plasma glucose ≥7.0 mmol/L.
Classification of metabolically at-risk was defined by having any two of the following, consistent with the International Diabetes Federation metabolic syndrome criteria (19): triglyceride level ≥1.7 mmol/L, HDL cholesterol level <1.0 mmol/L in men or <1.3 mmol/L in women or lipid-lowering medication use, blood pressure ≥130/85 mmHg or antihypertensive medication use, and fasting glucose level ≥5.6 mmol/L or self-reported diabetes. Participants with one risk factor or less were classified as metabolically healthy. BMI was categorized according to World Health Organization criteria (20). Subjects were then classified according to their BMI as normal (18.5–24.9 kg/m2) or obese (≥30.0 kg/m2) to identify metabolically healthy normal-weight (MHNW), MHO, and MRO phenotypes.
Recreational physical activity in the previous 2 weeks was ascertained by asking if the participant had engaged in 1) walking, and exercise that caused 2) moderate or 3) large increases in heart rate and breathing, as in vigorous activity. A score was calculated as the frequency of exercise sessions multiplied by the average time per session multiplied by (self-perceived) intensity, where walking has an intensity score of 3.5, moderate activity of 5, and vigorous activity of 7.5. Scores of <100 were classified as sedentary, 100–1,599 as low level, 1,600–3,199 or ≥3,200 and <2 h of vigorous exercise as moderate, and ≥3,200 and ≥2 h of vigorous exercise as high. Thus, moderate exercise could equate to a minimum of 7.5 h of walking per fortnight (17).
Sex-specific waist circumference criteria (20) were used to classify abdominal obesity as follows: men—normal: <95 cm; overweight: 95–101 cm; obese: ≥102 cm; women—normal: <80 cm; overweight: 80–87 cm; obese: ≥88 cm.
Individual-level SES variables included education level and household income. The Socio-Economic Indexes for Areas (SEIFA) Index of Relative Socio-economic Disadvantage (IRSD) is a measure of area socioeconomic position produced from the Australian Bureau of Statistics Census data derived from area attributes of percentages of people living with social disadvantage (21).
Densitometry and body composition
A subanalysis assessing the relationship between the phenotypes of interest and body composition measures was conducted using DXA measures that were obtained for 1,604 stage 2 participants aged at least 50 years, of whom 758 women and 588 men were free of CVD and not underweight. The relationship between phenotypes and grip strength was assessed in all stage 2 clinic participants (n = 3,206).
Scans were performed on a Prodigy Dual Energy X-ray Absorptiometer, using acquisition and analysis software Encore version 9.15 (n = 1,259), or on a DPX+ Dual X-ray Absorptiometer using Lunar DPX version 4.7e software (n = 348 patients; both machines GE Lunar, Madison, WI). A system quality assurance test was performed at the beginning of each scan day to calibrate and verify correct operation of the scanner. In vitro precision for whole-body scans performed on the Prodigy was estimated by scanning a whole body phantom 10 times. The coefficients of variation were 0.48% for fat mass (FM) and 0.44% for fat free mass (FFM). Total-body bone density and body composition data were collected on all subjects, with acquisitions performed using the system-recommended scan mode for patients scanned on the Prodigy (thick or standard) and using the medium scan mode for all subjects scanned on the DPX. Radiation exposure to the region of interest with either machine was approximately 1 mrem for each total-body scan. FM and FFM were normalized for height by dividing by height squared to generate the FM index (FMI) and FFM index (FFMI) in kilograms per meter squared to avoid these measures being collinear with height.
Grip strength (kg), which correlates with lean mass, was available from all stage 2 clinic participants and was measured three times with each hand using a Jamar hand dynamometer (Lafayette Instrument Company, Lafayette, IN) while subjects were sitting with their arm supported by a horizontal surface. The best of the six measurements was used to define the maximum grip strength.
Statistical analysis
Data obtained were weighted to the Australian Bureau of Statistics’ Census (22) and Estimated Residential Population for South Australia (23), by region, age group, sex, and probability of selection in the household, to provide population representative estimates. Data were analyzed using SPSS 15.0 software (SPSS Inc, Chicago, IL). Differences in proportions were determined using the χ2 test and the Fisher exact test. Differences in means were determined using ANOVA. Variables significant at P < 0.25 at the univariate level were included in multivariable logistic regression analyses to identify correlates of the MHO phenotype and the longitudinal associations with incident diabetes and CVD/stroke events adjusted for confounders. The final model assessing the association of MHO with diabetes was adjusted for age, sex, household income, and family history of diabetes, with physical activity removed in the final model because it was not significant and the number with diabetes was small (n = 112). The model for incident CVD/stroke was adjusted for age, sex, smoking, household income, highest education level and physical activity, and LDL cholesterol. Multivariable ANOVA determined mean differences in body composition measures between groups adjusted for age and smoking.
The ethics committees of the North West Adelaide Health Service and the University of Adelaide gave approval for the conduct of the NWAHS, and all subjects gave written, informed consent.
RESULTS
At baseline, in 3,743 subjects free of existing CVD and not underweight, metabolic health was common (66.8%, n = 2,499) and present in 88.3% (n = 1,172) of normal-weight, 62.9% (n = 873) of overweight, and 44.2% (n = 454) of obese subjects. The prevalence of two or more cardiometabolic risk factors (defined as metabolically at-risk) was 33.2% (n = 1,244) and present in 11.7% (n = 155) of normal-weight, 37.1% (n = 516) of overweight, and 55.8% (n = 573) of obese subjects.
Demographic and biomedical characteristics of subjects classified as MHNW (31.3%), MHO (12.1%), and MRO (15.3%) are reported in Supplementary Table 1. Sex was not significantly related to obesity within the metabolically healthy subjects. Across the spectrum of health from MHNW to MHO to MRO, there were significant increases in age, people of lower SES, waist circumference, and health service use and reductions in smoking and physical activity. Despite their metabolic health, MHO subjects were significantly more likely to report poor to fair general health, as assessed by the SF-36 Short Form 1 (SF-1), and demonstrate significantly reduced (worse) SF-36 PCS and MCS scores compared with the MHNW group adjusted for age, sex, and smoking. However, the MHO demonstrated higher PCS and MCS scores than MRO subjects.
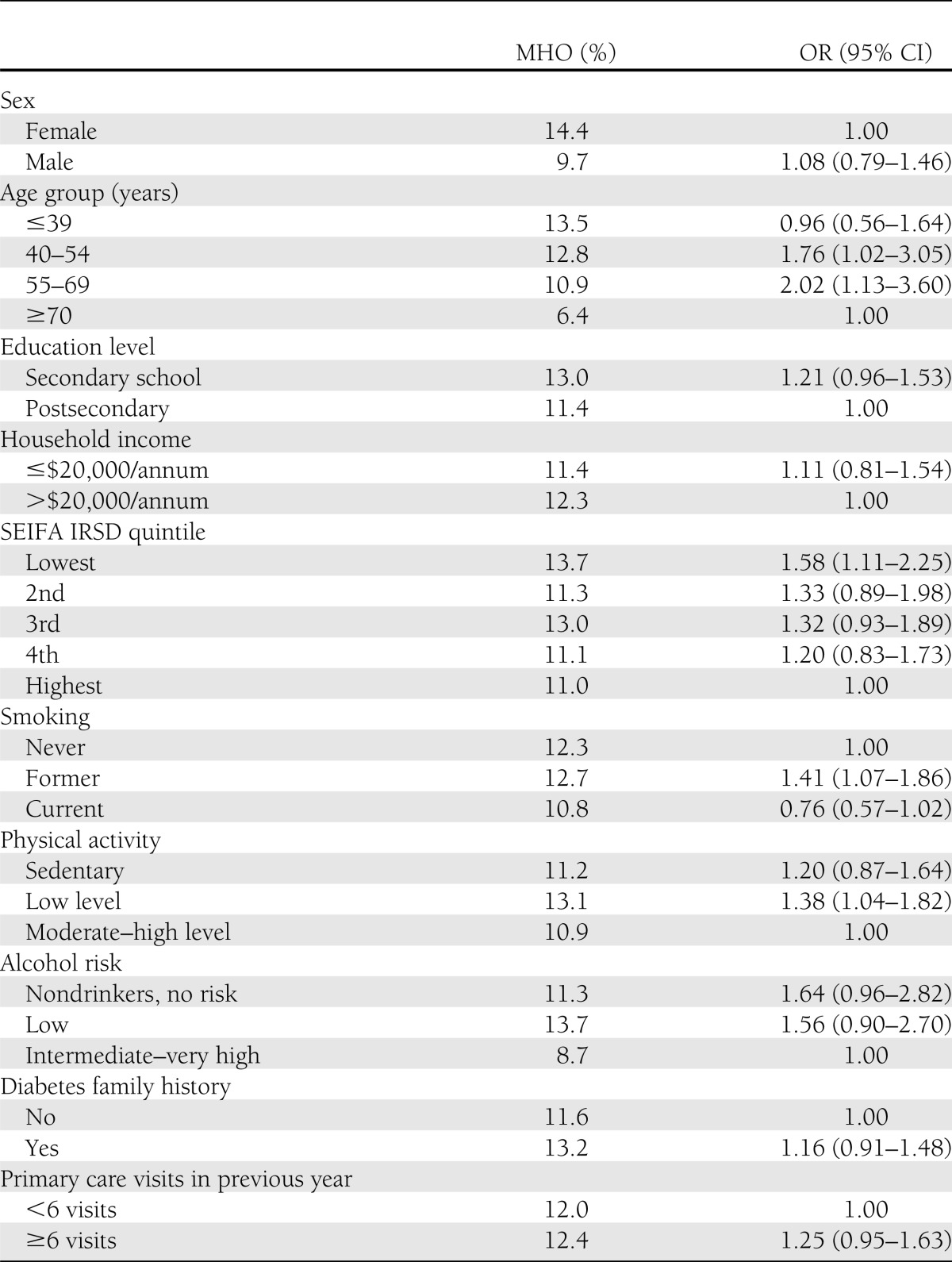
In cross-sectional multivariable analysis at baseline (Table 1), compared with the MHNW, the MHO phenotype was significantly associated with middle age, neighborhood disadvantage, former smoking, and low levels of physical activity. An association with no alcohol use approached significance. However, when compared with MRO subjects as the reference population, consistent with the analysis of NHANES 1999–2004 data (3), the MHO were more likely to be female (odds ratio [OR] 1.96 [95% CI 1.56–2.44]), younger (age <40 years: 4.58 [3.26–6.44]; age 40–54 years: 1.86 [1.35–2.56]), with lower levels of waist circumference (normal: 3.20 [2.40–4.25]; overweight: 2.33 [1.90–2.85]), be engaging in moderate- to high-level physical activity (1.39 [1.10–1.76]), and residing in high SES neighborhoods (OR for highest quintile of SEIFA-ID 1.52 [1.15–2.01]; fourth quintile: 1.26 [0.98–1.63], P = 0.07).
Table 1.
Multivariable logistic regression analyses of baseline biomedical and demographic characteristics associated with obesity (MHO, n = 454) within metabolically healthy subjects
Longitudinal associations
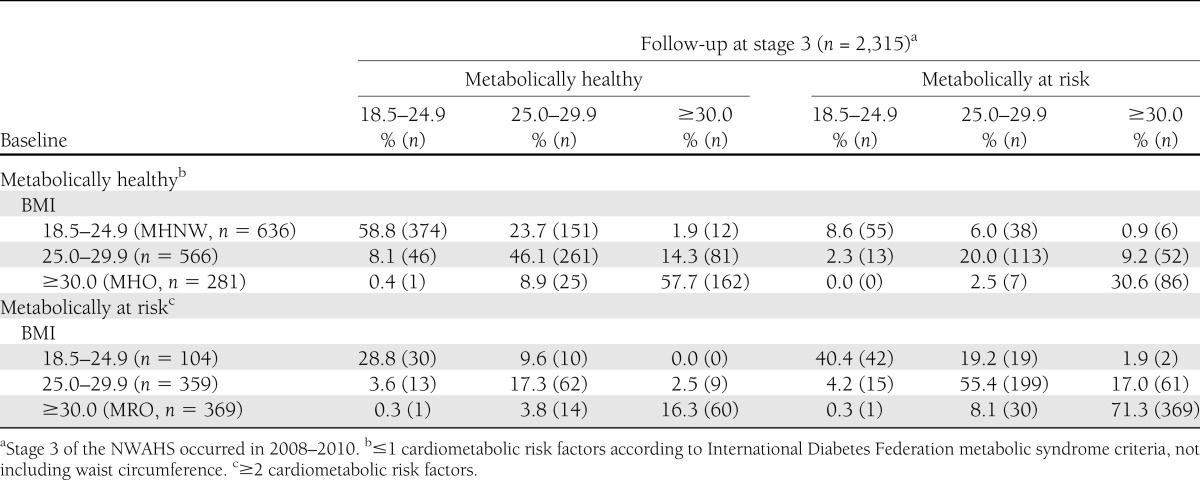
Table 2 reports the stability of these phenotypes during the follow-up period. Participants underwent changes in BMI category and cardiometabolic risk, with 59% of the MHNW, 58% of MHO, and 71% of MRO subjects maintaining their baseline status. Within those who were metabolically healthy at baseline, the development of two or more cardiometabolic risk factors was significantly more likely to occur in obese (baseline MHO, 33.1%, n = 93) compared with normal-weight (MHNW, 15.5%, n = 99, P < 0.001) subjects.
Table 2.
Combined BMI (kg/m2) and metabolic risk factor status at follow-up in relation to baseline status
At follow-up, maintenance of baseline metabolic health (≤1 risk factor) occurred in 67% (n = 188) of MHO participants and was associated with the following baseline variables: younger age (<40 years, OR 8.38 [95% CI 2.13–33.0]), lower levels of waist circumference (OR per cm increase in waist circumference 0.97 [0.95–0.99]), and with low to middle quintiles of area-level SES (2nd SEIFA IRSD quintile OR 2.34 [0.93–5.88], P = 0.07; 3rd SEIFA IRSD quintile 2.72 [1.14–6.50] vs. lowest quintile). No significant association with moderate to high levels of physical activity (1.32 [0.69–2.52]), low/no risk alcohol consumption (2.96 [0.77–11.4]), or smoking behavior was observed. Physical activity showed no association with maintenance of metabolic health when follow-up physical activity was also considered (data not shown).
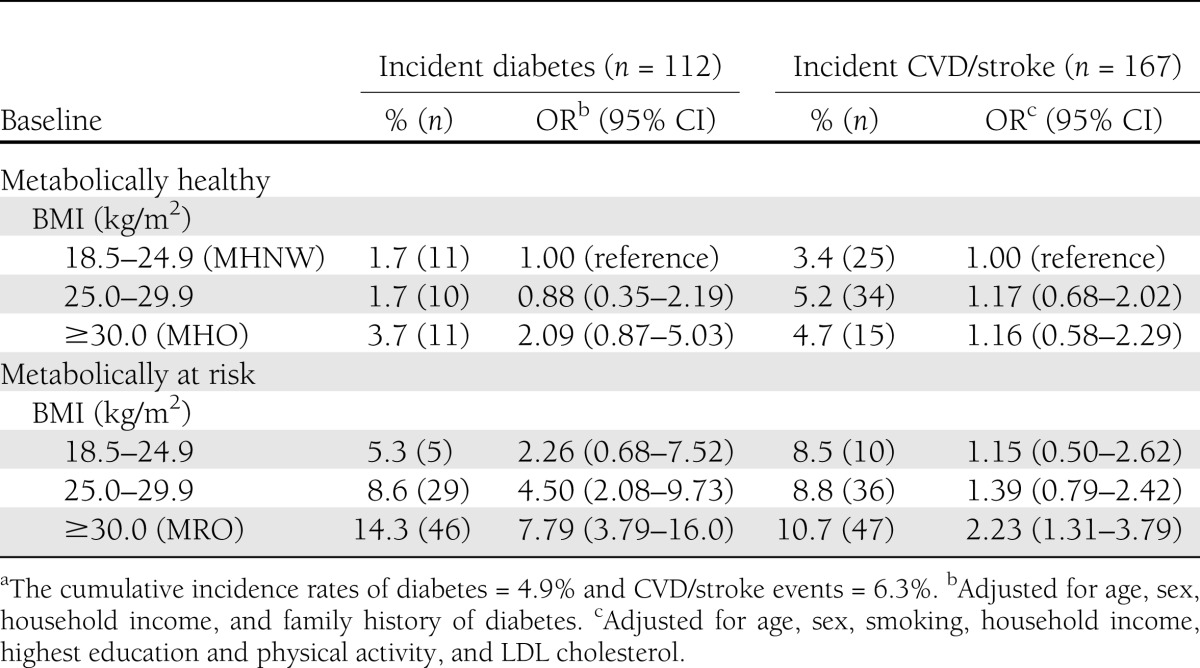
Longitudinal risks of incident diabetes and CVD/stroke in relation to baseline MHO and MRO status are reported in Table 3. The cumulative incidence rates of diabetes and CVD/stroke events were 4.9 and 6.3%, respectively, in the overall NWAHS population during the follow-up period. Compared with the MHNW group, MHO subjects demonstrated an increased risk of developing diabetes bordering on significance (OR 2.09, P = 0.09; Table 3), but not CVD/stroke events, after adjustment for confounders. In contrast, the MRO were significantly more likely to develop diabetes and CVD/stroke events.
Table 3.
Adjusted ORs and 95% CIs for incident diabetes and CVD/stroke in relation to baseline BMI and metabolic risk statusa
Given the instability of these phenotypes over time, we assessed incident events in relation to change in baseline status for those classified as MHNW, MHO, and MRO (Supplementary Table 2). In the MHO who remained stable over time, incident diabetes (1.1%, n = 2) and CVD/stroke cases (3.7%, n = 7) were uncommon and similar in frequency to those who remained MHNW over time. In contrast, participants progressing from the MHO to the MRO phenotype were significantly more likely to develop diabetes but not CVD/stroke events. The burden of incident events was borne largely by subjects displaying a stable MRO phenotype; however, the burden was reduced in those MRO who improved.
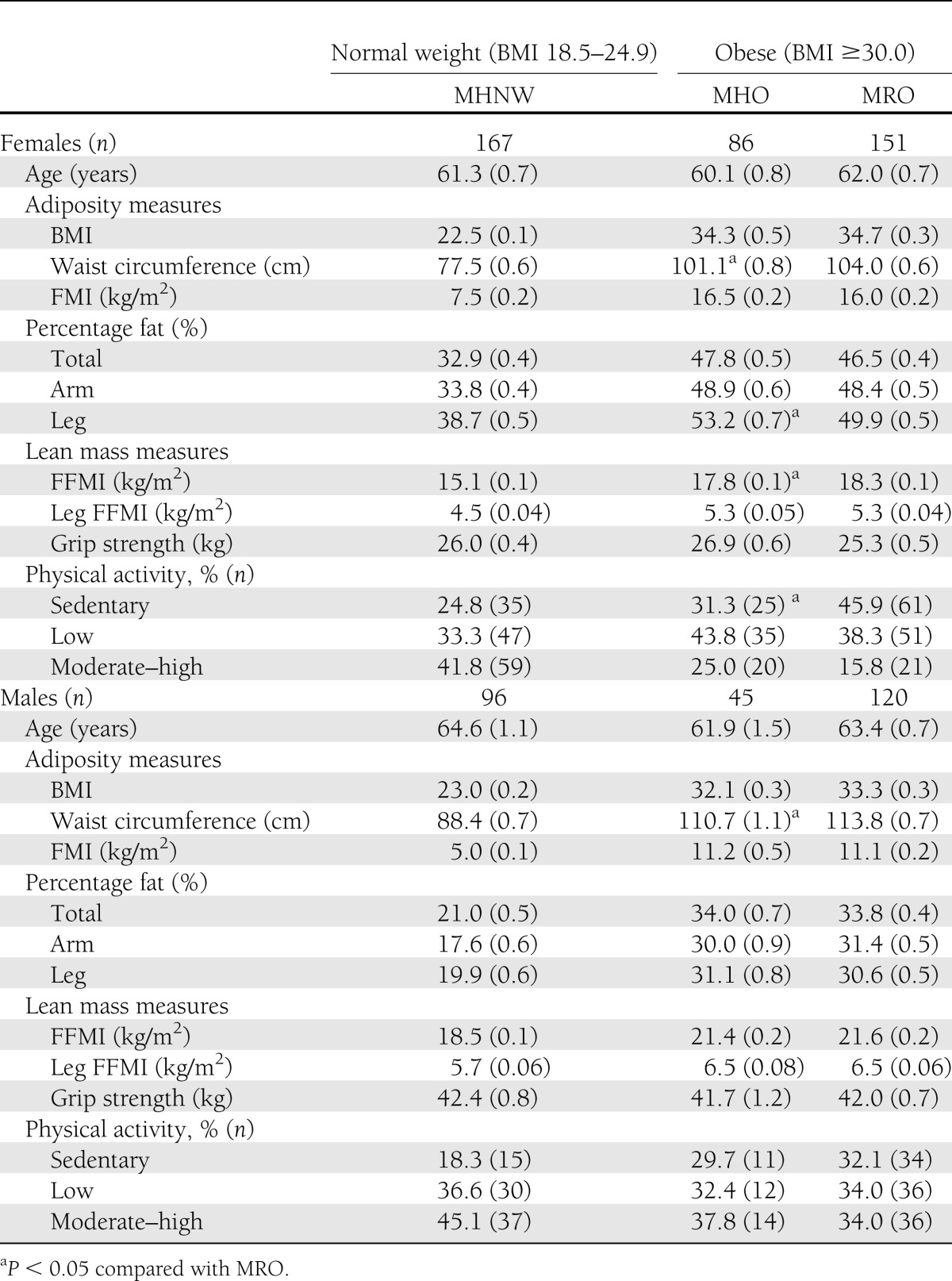
Table 4 reports the age- and smoking-adjusted mean (SE) adiposity and lean mass measures in MHNW, MHO, and MRO participants. MHO women demonstrated significantly higher levels of percentage of leg fat and significantly lower levels of waist circumference, despite no significant differences in overall adiposity, as measured by BMI, FMI, and percentage of total fat, compared with MRO women. MHO women also demonstrated a significantly lower FFMI, but no other differences in lean mass measures were observed despite significantly increased rates of engaging in physical activity. In men, the MHO demonstrated lower waist circumferences than the MRO, but no significant differences in physical activity levels or body composition measures were observed.
Table 4.
Mean (SE) adiposity and lean mass measures in relation to BMI (kg/m2) and metabolic risk in men and women aged at least 50 years at stage 2 of the NWAHS
CONCLUSIONS
In our representative adult population, compared with MHNW subjects, the MHO phenotype was significantly associated with disadvantaged SES, physical inactivity, and a smoking history—factors that expose them to additional poor health outcomes. We would argue that caution is warranted when using the term “healthy obesity” (24), because the relatively young MHO demonstrated impaired health-related quality-of-life and a significant risk of developing metabolic risk factors during 5 to 10 years of follow-up. The development of cardiometabolic abnormalities in one-third of MHO subjects conferred a significantly increased risk of diabetes.
The MHO phenotype was relatively unstable over time, and this was an important influence on cardiometabolic outcomes for this group. When outcomes were assessed in relation only to baseline status, the MHO experienced elevated but nonsignificant risks (P = 0.09) of developing diabetes and no increased CVD risk during our 5- to 10-year follow-up period compared with MHNW subjects. This is similar to previous studies where metabolic change was not considered (2,6,12). The increased diabetes risk that we observed was attributable to those who progressed over time from MHO to MRO. A novel finding of our study is that longitudinally, youth and lower central adiposity drove the maintenance of metabolic health, and obese subjects who maintained their metabolic health experienced risks for diabetes and CVD similar to MHNW subjects. Thus, consistent with previous findings in relation to CVD (12,13), the concomitant presence of metabolic risk factors was associated with the development of diabetes in our obese subjects.
Compared with obese adults with metabolic abnormalities (MRO), the MHO were younger, with less central adiposity, a finding consistent with previous cross-sectional studies (3,7). The MHO phenotype was also associated with favorable social and behavioral factors, being characterized by younger age (militating against CVD events), physical activity, and being resident in high SES neighborhoods, suggesting protection by these factors in the context of socioeconomic advantage.
Exposure to potentially hazardous adipokines and poor health behaviors in the MHO may have played a role in the switch in phenotype from MHO to MRO in 32% of subjects, suggesting that many MHO subjects are at an intermediate stage of progression toward significant metabolic abnormalities and disease. Therefore, targeted intervention opportunities may exist for the maintenance of metabolic health in younger people who are obese without metabolic abnormalities by promotion of healthier waist circumferences.
The mechanisms underlying metabolic health in obese subjects are unclear, but the MHO may be characterized by the presence of less secretory adipose tissue or reduced responsiveness to the effects of adipokines (3). Our findings suggest the possibility that fat distribution and, by inference, the differences in adipocytokine secretory properties of those fat depots (25) may play an important role in the outcomes associated with the MHO phenotypes. Further investigation of the potential protective properties of increased femoral-gluteal fat in the presence of reduced central fat in MHO women (despite similar total adiposity) is required given that accumulation of fat peripherally in the legs has been shown to be protective against disturbed glucose metabolism (26). MHO men also demonstrated significantly lower levels of waist circumference, but not increased leg fat, which may be related to sexual dimorphism in fat distribution (27). Studies in generally small selected samples of obese adults reveal other important biochemical insights into the MHO, including lower levels of hepatic enzymes (15), ectopic fat (24), carotid intima-media thickness (24), insulin disposition index (28), C-reactive protein (16), and visceral fat accumulation (29), as well as enhanced esterification of fatty acids into triglycerides and sequestration of those triglycerides (30).
Studies suggest that the MHO are also physically active (3,6). Ortega et al. (6) recently demonstrated that the MHO had better objectively assessed fitness and lower CVD mortality and morbidity risks compared with the MRO in a large cohort of high SES adults with low baseline levels of obesity. The contribution of social advantage to CVD outcomes (31) was not considered by Ortega et al. Cross-sectionally, metabolic health in our obese subjects was also associated with physical activity, albeit low-level activity such as walking. However, maintenance of MHO status was not related to recreational physical activity at baseline or follow-up, and contrary to our hypothesis, lean mass and grip strength were not greater in MHO compared with MRO participants. The independent contributions of strength and cardiorespiratory fitness to the development of metabolic risk (32,33) and CVD (34) are unclear, and the significantly higher levels of lean/FFM in our MRO women may represent infiltration of fatty tissue into skeletal muscle and organs that cannot be discriminated as fat by DXA. Given the conflicting evidence of the hazards of diet-induced weight loss in the MHO (35,36), physical activity has been proposed as an intervention for MHO individuals (37). Randomized controlled trial evidence to support this is required, and these findings will have significant clinical implications for interventions to reduce obesity.
Variability in criteria to identify MHO and differences in study sample populations make direct comparisons between studies difficult. The lack of an operational MHO definition is therefore a limitation of all studies examining the phenomenon of “healthy obesity,” and an expert consensus to standardize the identification of MHO individuals is warranted (14). Fewer NWAHS normal-weight subjects were metabolically at risk (12%) than the 23% reported by Wildman et al. (3) using NHANES data. The NWAHS did not measure two of six NHANES risk factors (insulin sensitivity and C-reactive protein) and might account for the lower levels of metabolic abnormalities we reported.
There are limitations associated with our study. This study reports ORs for incident diabetes and CVD rather than relative risks; however, the incidence of these conditions in the population was low and less than 10%, and in this case, the OR closely approximates the relative risk. When the equation of Zhang and Yu (38) was used to correct the adjusted ORs to better represent the relative risk, very minor changes to the estimates reported in Table 3 were observed (generally first decimal place increases or decreases), and no changes were seen in the statistical significance of the associations. The number of incident cases of diabetes (n = 112) and CVD/stroke (n = 167) were small, as reflected by the very wide CIs around the ORs, and thus, our results should be interpreted with caution.
Furthermore, the use of self-reported CVD and physical activity might be considered a limitation of this study. However, self-reports of cardiac and stroke events have been reported to be accurate (39,40), and the assessment of recreational physical activity by self-report only, without identification of the type of activity (endurance/resistance training), is similar to other studies. Our survey was limited to households with telephones, but because 97% of the households in the region have telephones (18) and the demographic characteristics were representative of the population of profile of Adelaide overall, the extent of any bias is likely to be small.
The strengths of our study, however, include our large randomly recruited representative population sample with biomedical and SES measures at several time points, which permitted an examination of the effects of change in metabolic status on diabetes and CVD outcomes. To our knowledge, this is the first study to report DXA-assessed body composition in the MHO in a large representative sample of older community-dwelling men and women.
In conclusion, our data are consistent with an MHO phenotype where younger age and smaller waistlines may maintain the metabolic health of this group over time, and this state is associated with low diabetes and CVD risks. A potential protective effect of peripheral fat distribution against the development of metabolic complications in metabolically healthy but obese women requires further investigation. However, the MHO is temporary for a sizeable proportion of obese adults; thus, further longitudinal study of the MHO phenotype is also important to avoid inappropriate health messages about the risks of obesity.
Acknowledgments
The study was funded by the University of Adelaide and a Health Services Research Improvement Projects Grant from the South Australian Department of Health.
In relation to the submitted work, the salary of S.L.A. is provided by program grant funding from The Hospital Research Foundation to The Health Observatory. T.K.G. is currently a National Health and Medical Research Council Early Career Fellow (Australian Public Health, ID 1013552). R.J.A. received funding from the South Australian Department of Health and the Premier’s Science fund, paid to the University of Adelaide for the conduct of the first and second follow-up of the cohort, respectively.
In relation to financial activities outside the submitted work, R.V. received grant funding, an honorarium for advisory group membership (Malnutrition in the Elderly), and an educational grant to cover meeting travel expenses from Nestlé. No other potential conflicts of interest relevant to this article were reported.
S.L.A. researched the data, contributed to discussion, and wrote, reviewed, edited, and revised the manuscript. C.J.S. contributed to the writing, revision, and editing of the manuscript. R.V., C.L.H., and T.K.G. contributed to discussion and reviewed and edited the manuscript. A.W.T. reviewed and edited the manuscript. R.J.A. contributed to discussion and wrote, reviewed, edited, and revised the manuscript. S.L.A. is the guarantor of this work and, as such, had full access to the data and takes responsibility for the accuracy of data analysis.
This study was presented at the International Congress on Abdominal Obesity, Hong Kong, 28–30 January 2010, and published in abstract form in CMR eJournal 2010;3:9.
Dr. Pat Phillips (Endocrinology Unit, The Queen Elizabeth Hospital Campus) facilitated the conduct of DXA scans.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1971/-/DC1.
References
- 1.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism 2001;50:1499–1504 [DOI] [PubMed] [Google Scholar]
- 2.Meigs JB, Wilson PWF, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006;91:2906–2912 [DOI] [PubMed] [Google Scholar]
- 3.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008;168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 4.Lee K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: characteristics and health behaviors. Asia Pac J Clin Nutr 2009;18:280–284 [PubMed] [Google Scholar]
- 5.Wildman RP. Healthy obesity. Curr Opin Clin Nutr Metab Care 2009;12:438–443 [DOI] [PubMed] [Google Scholar]
- 6.Ortega FB, Lee DC, Katzmarzyk PT, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J 2013;34:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calori G, Lattuada G, Piemonti L, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 2011;34:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 2009;32:2297–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010;121:230–236 [DOI] [PubMed] [Google Scholar]
- 10.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 2012;97:2482–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 2012;20:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St-Pierre AC, Cantin B, Mauriège P, et al. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ 2005;172:1301–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzmarzyk PT, Janssen I, Ross R, Church TS, Blair SN. The importance of waist circumference in the definition of metabolic syndrome: prospective analyses of mortality in men. Diabetes Care 2006;29:404–409 [DOI] [PubMed] [Google Scholar]
- 14.Messier V, Karelis AD, Prud’homme D, Primeau V, Brochu M, Rabasa-Lhoret R. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity (Silver Spring) 2010;18:911–917 [DOI] [PubMed] [Google Scholar]
- 15.Messier V, Karelis AD, Robillard ME, et al. Metabolically healthy but obese individuals: relationship with hepatic enzymes. Metabolism 2010;59:20–24 [DOI] [PubMed] [Google Scholar]
- 16.Karelis AD, Faraj M, Bastard J-P, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005;90:4145–4150 [DOI] [PubMed] [Google Scholar]
- 17.Grant JF, Chittleborough CR, Taylor AW, et al. North West Adelaide Health Study Team The North West Adelaide Health Study: detailed methods and baseline segmentation of a cohort for selected chronic diseases. Epidemiol Perspect Innov 2006;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor AW, Wilson DH, Wakefield M. Differences in health estimates using telephone and door-to-door survey methods—a hypothetical exercise. Aust N Z J Public Health 1998;22:223–226 [DOI] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome [article online]; 2005. Available from http://wwwidforg/metabolic-syndrome Accessed 26 September 2012
- 20.World Health Organization Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. Geneva, Switzerland, World Health Organization, 1997 [PubMed] [Google Scholar]
- 21.Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA) 2001, South Australia-2033.4.55.001 Canberra, Australia, Australian Bureau of Statistics, 2001
- 22.Australian Bureau of Statistics Census of Population and Housing Selected Social and Housing Characteristics for Statistical Local Areas South Australia, 2001. Canberra, Australia, Australian Bureau of Statistics, 2002 [Google Scholar]
- 23.Australian Bureau of Statistics Estimated Residential Population by Age and Sex. Canberra, Australia, Australian Bureau of Statistics, 1999 [Google Scholar]
- 24.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008;168:1609–1616 [DOI] [PubMed] [Google Scholar]
- 25.Arner P. Regional differences in protein production by human adipose tissue. Biochem Soc Trans 2001;29:72–75 [DOI] [PubMed] [Google Scholar]
- 26.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol 2006;35:83–92 [DOI] [PubMed] [Google Scholar]
- 27.Stevens J, Katz EG, Huxley RR. Associations between gender, age and waist circumference. Eur J Clin Nutr 2010;64:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Succurro E, Marini MA, Frontoni S, et al. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring) 2008;16:1881–1886 [DOI] [PubMed] [Google Scholar]
- 29.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab 2001;86:1020–1025 [DOI] [PubMed] [Google Scholar]
- 30.Puri V, Ranjit S, Konda S, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U S A 2008;105:7833–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marmot MG, Bosma H, Hemingway H, Brunner E, Stansfeld S. Contribution of job control and other risk factors to social variations in coronary heart disease incidence. Lancet 1997;350:235–239 [DOI] [PubMed] [Google Scholar]
- 32.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 2005;112:505–512 [DOI] [PubMed] [Google Scholar]
- 33.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc 2005;37:1849–1855 [DOI] [PubMed] [Google Scholar]
- 34.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr 1999;69:373–380 [DOI] [PubMed] [Google Scholar]
- 35.Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia 2008;51:1752–1754 [DOI] [PubMed] [Google Scholar]
- 36.Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 2010;33:1957–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perseghin G. Is a nutritional therapeutic approach unsuitable for metabolically healthy but obese women? Diabetologia 2008;51:1567–1569 [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1691 [DOI] [PubMed] [Google Scholar]
- 39.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol 1998;147:969–977 [DOI] [PubMed] [Google Scholar]
- 40.Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients’ self-reports and on determinants of inaccuracy. J Clin Epidemiol 1996;49:1407–1417 [DOI] [PubMed] [Google Scholar]


