Abstract
OBJECTIVE
Medical nutrition therapy based on the control of the amount and distribution of carbohydrates (CHO) is the initial treatment for gestational diabetes mellitus (GDM), but there is a need for randomized controlled trials comparing different dietary strategies. The purpose of this study was to test the hypothesis that a low-CHO diet for the treatment of GDM would lead to a lower rate of insulin treatment with similar pregnancy outcomes compared with a control diet.
RESEARCH DESIGN AND METHODS
A total of 152 women with GDM were included in this open, randomized controlled trial and assigned to follow either a diet with low-CHO content (40% of the total diet energy content as CHO) or a control diet (55% of the total diet energy content as CHO). CHO intake was assessed by 3-day food records. The main pregnancy outcomes were also assessed.
RESULTS
The rate of women requiring insulin was not significantly different between the treatment groups (low CHO 54.7% vs. control 54.7%; P = 1). Daily food records confirmed a difference in the amount of CHO consumed between the groups (P = 0.0001). No differences were found in the obstetric and perinatal outcomes between the treatment groups.
CONCLUSIONS
Treatment of women with GDM using a low-CHO diet did not reduce the number of women needing insulin and produced similar pregnancy outcomes. In GDM, CHO amount (40 vs. 55% of calories) did not influence insulin need or pregnancy outcomes.
Gestational diabetes mellitus (GDM) is defined as glucose intolerance with its onset or first recognition during pregnancy. The prevalence of GDM is ∼7% (from 1 to 14%), depending on the population and the diagnostic criteria used (1). In Spain, GDM has an estimated prevalence of 8.8% (2). GDM is associated with an increase in maternal and neonatal complications during pregnancy (3).
Medical nutrition therapy (MNT) is the cornerstone of GDM treatment and is based on the control of the amount and distribution of carbohydrates (CHO) to obtain optimal glycemic control without ketosis. Energy content is also important for appropriate gestational weight gain. For the general population, CHO should represent 45–65% of total daily calories, with a daily consumption of >175 g of CHO for pregnant women (4). This consumption should best be distributed over three meals and two to four snacks (5). Although the control of CHO amount and type is extensively used to optimize blood glucose concentrations, other methods are also important for the management of GDM. This included the introduction of healthy food choices, portion control, cooking practices, and regular physical activity (6).
In Spain, clinical guidelines state that if blood glucose levels are not controlled with MNT, then insulin treatment should be initiated. MNT is a tool to lower postprandial blood glucose values, either by modifying CHO distribution or by modifying any of the components of the glycemic load (GL) (the product of the CHO content of a serving of food and its glycemic index [GI]). In recent years, two randomized controlled trials (RCT) have been published concerning the effect of low-GI diets in GDM. Moses et al. (7) demonstrated a significantly lower proportion of women meeting the criteria for starting insulin treatment when assigned to a low-GI diet, without any differences in key obstetric and fetal outcomes. Louie et al. (8) did not find any differences in the need for insulin treatment, with similar pregnancy outcomes, in women following a modestly lower-GI diet than a control group treated with a high-fiber moderate-GI diet.
In our setting, MNT for GDM is primarily based on the control of the amount and distribution of CHO, rather than control of GI. To our knowledge, there are no RCTs that demonstrate an advantage of a diet with a prespecified amount of CHO. The American Diabetes Association recommended in the Fifth International Workshop Conference on Gestational Diabetes Mellitus that research trials should be conducted in this area (6).
We conducted an RCT to assess whether a diet with a low content of CHO (40% of calories) compared with a control diet (55% of calories) could reduce the need for insulin treatment in women with GDM without increasing adverse pregnancy outcomes.
RESEARCH DESIGN AND METHODS
The study was a two-arm, open, parallel, randomized controlled trial comparing two dietary interventions designed to treat GDM. Participation in the trial was offered to all women diagnosed with GDM in the only diabetes and pregnancy outpatient clinic of the reference hospital of the Public Health System of the province of Lleida (Catalonia, northeastern Spain) between November 2008 and July 2011.
Subject recruitment and randomization
Inclusion criteria were women aged 18–45 years (inclusive) diagnosed with GDM with singleton pregnancies and a gestational age ≤35 weeks. Exclusion criteria were an unwillingness to follow a prescribed diet, inability to understand the Spanish language, and pregnancy comorbidities other than obesity, hypertension, and/or dyslipidemia.
GDM diagnosis was made following the 2006 National Diabetes and Pregnancy Clinical Guidelines. In Spain, all women are screened for GDM at 24–28 weeks of gestation with a 50-g glucose challenge test. If GDM risk factors are present, the screening test is performed in the first trimester. If the 1-h glucose value of the screening test is ≥7.8 mmol/L, the patient is scheduled for a 100-g glucose challenge test, and the diagnosis of GDM is made following the National Diabetes Data Group criteria (2,9).
Participation in the trial was offered to all women at the first outpatient appointment. Between November 2008 and July 2011, 152 patients were randomized to one of the two treatment groups. Group allocation was performed using a sealed envelope. Of the 313 patients assessed for eligibility, 161 were excluded because they did not meet the inclusion criteria (n = 128) or declined to participate (n = 33) (Supplementary Fig. 1).
The local ethics committee approved the protocol, and all of the patients signed the written informed consent form.
Patient care and follow-up
Participants received routine care by the treatment team following the institutional protocol, which was based on local and national guidelines. Patients were seen 1 week after study allocation, which were followed by visits every 1 to 3 weeks, depending on clinical judgment.
All of the women were provided with a glucose meter (Accu-Chek Aviva; Roche Diagnostics SL, Barcelona, Spain) and instructed to perform self-monitoring of blood glucose (SMBG). Patients were also instructed to record the SMBG results using the following pattern: six controls a day during the first week (before and 1 h after the three main meals) and, in the event the glucose values were on target, four controls a day for the remaining follow-up (fasting and 1 h after the three main meals). Ketonuria was self-monitored every day before breakfast and once a week before lunch and dinner using a semiquantitative method (Keto-Diastix; Quimica Farmaceutica Bayer SL, Barcelona, Spain). The results of urine ketone assessments were reported as absent, mild (1+), moderate (2+), or high (3+) according to the manufacturer. Except for the different diet treatments, all other management strategies were the same for both groups.
Dietary interventions
The energy content of the diet for each patient was calculated on the basis of pregestational weight (Supplementary Table 1) with a minimum of 1,800 kcal/day. The two study diets had similar protein content (20% of the total daily calorie amount) but a different amount of CHO (40% in the low-CHO diet and 55% in the control diet) and fat (40% in the low-CHO diet and 25% in the control diet, mainly at the expense of increased olive oil intake). Specific diets were designed manually by the team of dietitians for the purpose of the trial (Supplementary Table 2). The diets were divided into three principal meals and three snacks, all with a prespecified number of CHO servings. No changes in the CHO distribution were allowed while the patient was under dietary therapy alone. Once insulin was started, changes in the CHO distribution could be made to optimize insulin treatment because the patients had already reached the primary outcome of the study.
Nutritional analysis and assessment of compliance
CHO intake was evaluated using the estimated food record method over 3 nonconsecutive days, including a weekend or a holiday (10). All women were asked to record their initial intake of foods containing CHO with this method. The first dietary assessment was made after the initial study diet prescription, and a second assessment occurred after the following appointment at which the dietary plan for the patient was revised for adherence. This method was used for its technical simplicity and cost. A book with graphic representations of food portions was used at the dietary interviews (11). One nutritionist who was blinded to the patient group allocation documented the food records in a separate database. Two food composition tables were used to estimate the total CHO, starch, and sugar intake (12,13).
Insulin therapy
The decision to initiate insulin treatment was made by the endocrinologist according to institutional protocol and followed strict criteria: insulin treatment was started if at least two SMBG values at the same time point of the day in a 1-week time interval were higher than the target. The glycemic targets were as follows: fasting and preprandial glycemia ≤5.3 mmol/L and 1-h postprandial glycemia ≤7.8 mmol/L. Regular human insulin was used to treat postprandial glucose excursions (Actrapid Innolet; Novo Nordisk AS, Bagsværd, Denmark). Bedtime NPH insulin was prescribed to correct fasting hyperglycemia (Insulatard Flexpen; Novo Nordisk AS). Initial insulin doses were calculated according to Supplementary Table 1.
Data collection
The following data were assessed and recorded at each follow-up visit: weight, blood pressure, number of SMBG results outside the target values, insulin dose, and ketonuria. The predelivery weight was the last weight registered in the medical record during any of the 4 weeks preceding delivery. All of the deliveries took place in our hospital. Pregnancy complications, ultrasound follow-up data, and pregnancy outcomes, including newborn weight and length adjusted for gestational age, occurrence of newborn hypoglycemia (glycemia <2.2 mmol/L), and the type of delivery, were obtained from the electronic medical record system. Gestational age was calculated based on the last menstrual period and corrected, if indicated, by an early ultrasound scan. Newborns were categorized into small-for-gestational age (birth weight < 10th centile), normal, or large-for-gestational-age (LGA) (birth weight > 90th centile) using the Spanish tables of neonatal weight adjusted for sex and gestational age (14). Macrosomia was defined as birth weight ≥4 kg.
Sample size calculation
Based on previous clinical data from the local clinic, we determined that 40–50% of the patients with GDM do not achieve the desired blood glucose concentrations despite following dietary treatment and subsequently require insulin therapy. We designed the study to provide 80% statistical power (bilateral confidence of 95%) to find a minimum difference of 22% on the risk of insulinization (45% was the expected rate of insulin-treated women in the control group). We anticipated that 10% of the patients would be lost to follow-up. The calculated number of patients for inclusion was 152 (76 per group).
Statistical analyses
A biostatistician blinded to the diet allocation of participants performed the statistical analyses. Prior to conducting the main analyses, baseline characteristics were compared between the intervention and control groups to identify potential confounders. The Fisher exact test was applied to estimate differences between both diets in the distribution of qualitative variables. The log-rank test was used to estimate differences in the distribution of time until insulin treatment administration or the delivery date. An estimation of the median time to insulin use, together with its 95% CI, was computed separately for each group if the test showed a statistically significant difference. To estimate differences in quantitative variables between the two groups, such as predelivery insulin dose, maternal weight gain in reference to the first visit, and newborn glycemia, the Mann–Whitney test was used. The Mann–Whitney test was also used to estimate differences between both groups in CHO intake derived from the 3-day food records (total CHO, as well as starches and sugars individually).
An intention-to-treat analysis was performed with a 95% CI and a significance level of 0.05. All statistical analyses were performed with R software (version 2.15.1) (15).
RESULTS
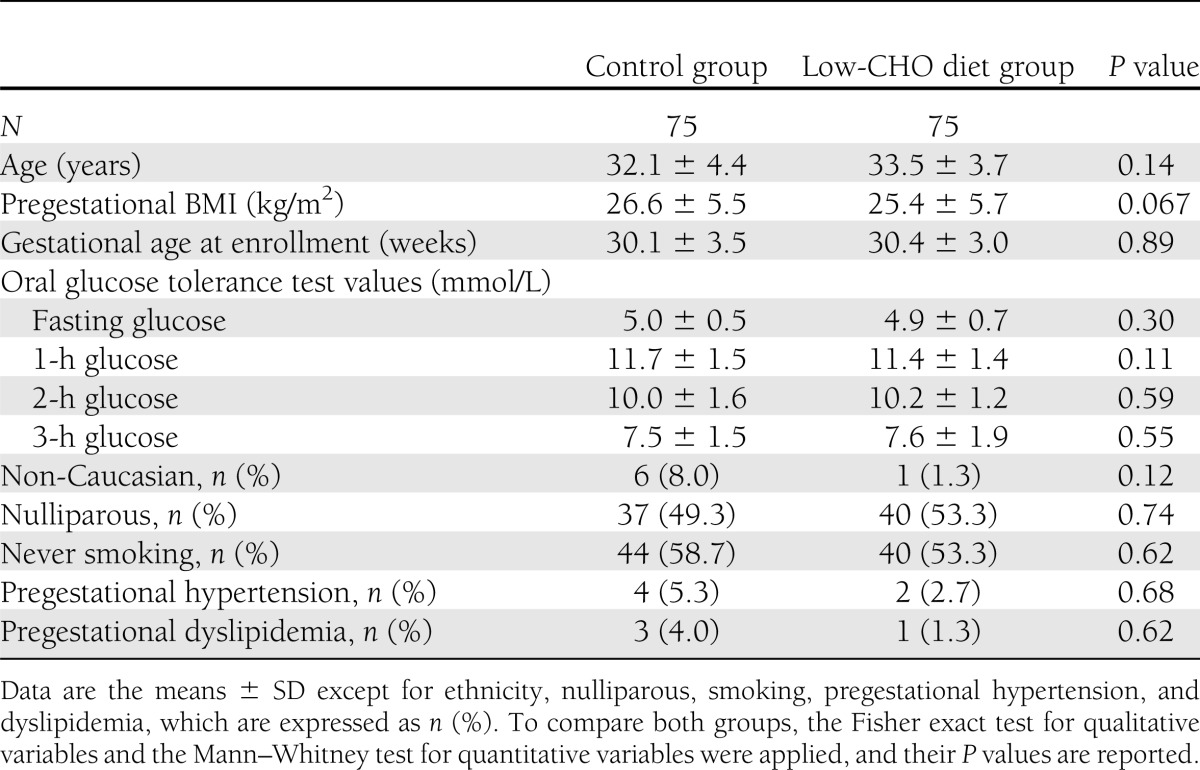
The overall design and subject flow through the study is illustrated in Supplementary Fig. 1. A total of 152 women were randomly assigned to one of the two diets, 76 in each group. However, one patient randomized to the control group withdrew the consent before receiving any dietary intervention. After randomization, another patient in the low-CHO diet group was excluded at the second appointment due to major violation of the protocol (inclusion/exclusion criteria: twin pregnancy). A total of 130 patients finished the trial. There were no significant differences in the baseline characteristics between the two groups, as shown in Table 1.
Table 1.
Baseline characteristics of the study group participants
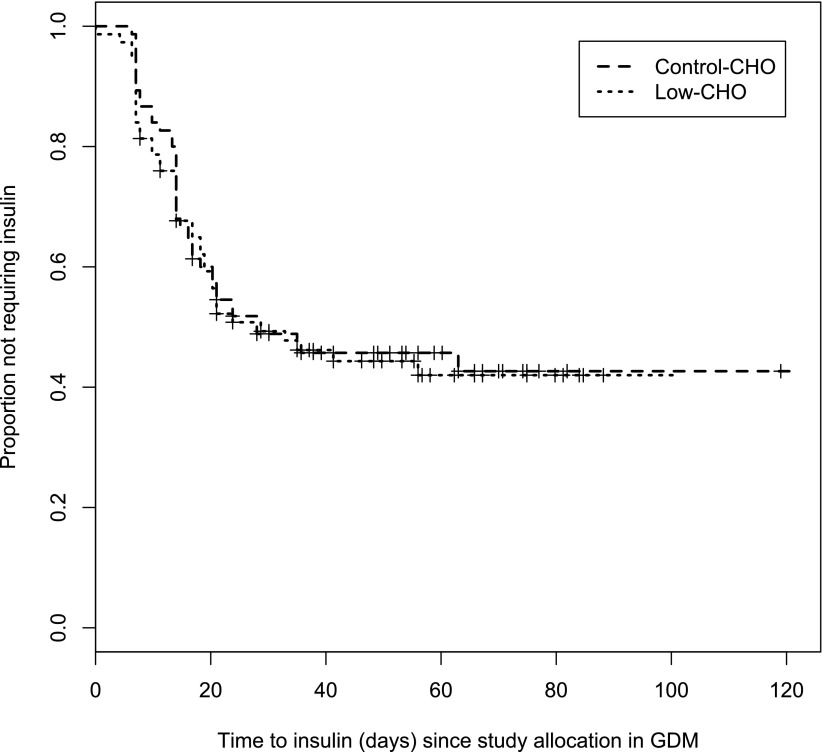
Regarding the main outcome of the study, the intention-to-treat analysis showed that the cumulative rate of insulin treatment was 54.7% (41 women of 75) in the control group and 54.7% (41 women of 75) in the low-CHO diet group, with no significant differences between groups (P = 1). This result remained unchanged even after adjusting for gestational age (incidence density ratio of 1.06 [95% CI 0.69–1.63]; P = 0.792). The analysis of the main outcome in the per-protocol population showed that there were no differences in the cumulative rate of insulin treatment between women who completed the study in the low-CHO diet (38 women of 70; 54.3%) and the control group (33 women of 60; 55%) (P = 1). There were no significant differences between the two groups with respect to insulin dose or time to insulin treatment at the first visit or at the time of the last menstrual period (Fig. 1).
Figure 1.
Time to insulin treatment from study allocation in the two study groups.
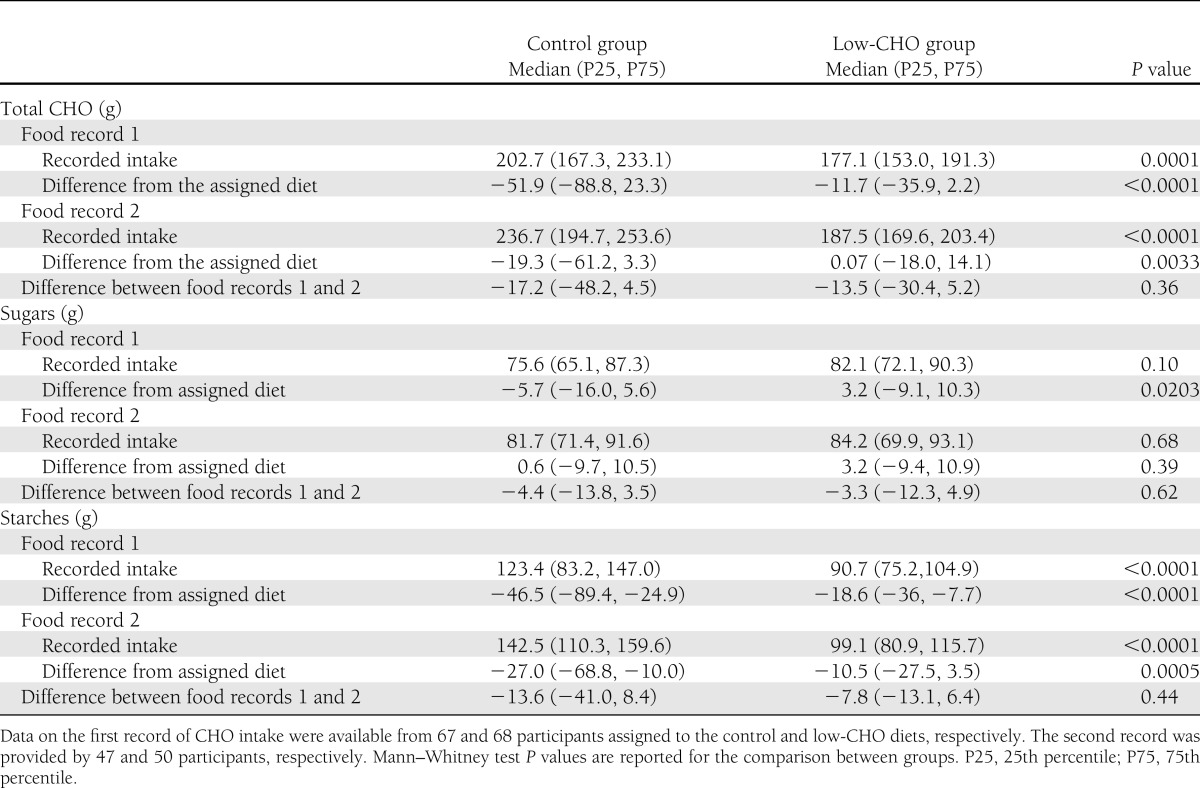
The first 3-day food record was completed by 135 patients (67 in the control group and 68 in the low-CHO diet group), and 97 women returned the second one (47 in the control group and 50 in the low-CHO diet group). Table 2 shows the detailed total CHO, starch, and sugar daily intake, as reported. The total CHO and starch intake was always significantly different between the groups as per protocol (P = 0.0001 and P < 0.0001 for the first and second records, respectively). The intake was lower than the amount prescribed, except in the low-CHO group in the second record (Table 2). The intake of sugar was not different between the groups. The total CHO, sugar, and starch intake increased between the first and second record and approached the prescribed diet after patients received a second dietary advice.
Table 2.
Reported intake of CHOs (total CHO, sugars, and starches) assessed by adapted 3-day estimated food records
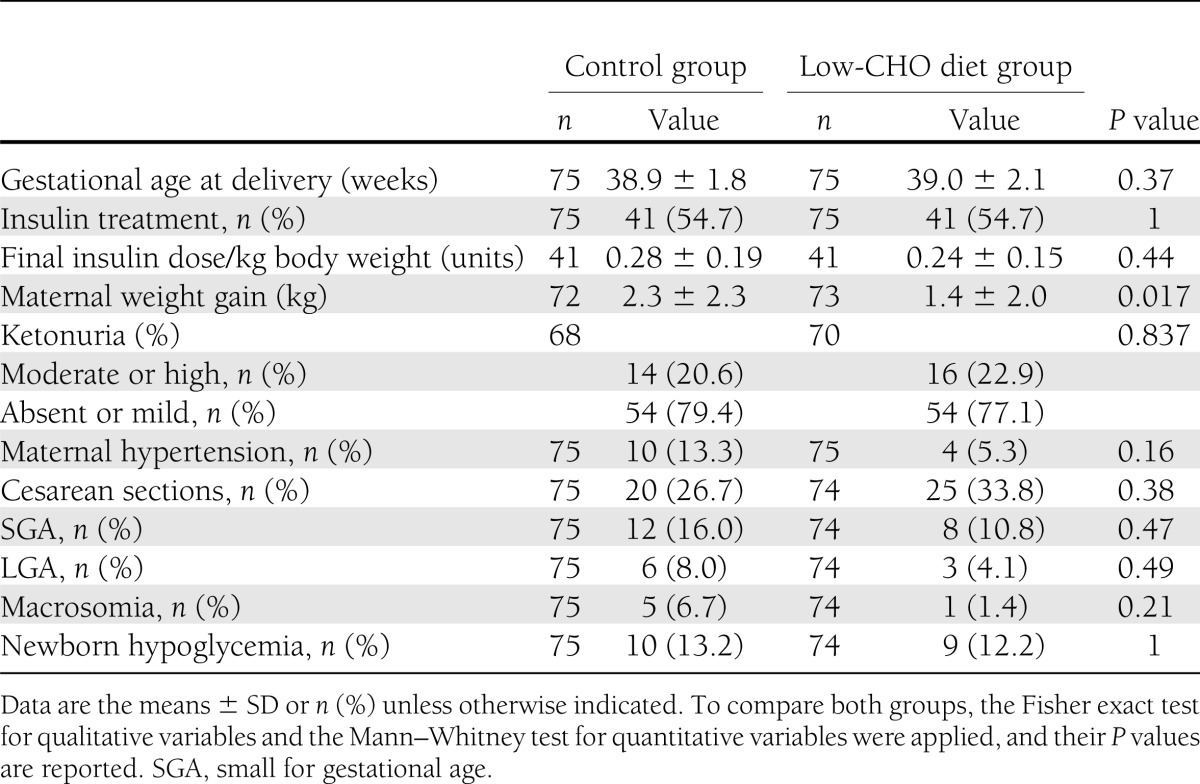
The main pregnancy outcomes are shown in Table 3. There was a case of unexplained stillbirth at week 35 of pregnancy in a woman in the low-CHO group. The rate of moderate ketonuria was similar in both groups, and only one case with a high value of ketonuria occurred in the control group. Maternal weight gain, from study allocation until delivery, was higher in the control group {median 2.1 kg [interquartile range (IQR) 0.6–3.5]} than in the low-CHO group (median 1.1 kg [IQR 0.3–2.5]; P = 0.017), but this difference disappeared after correction for time to follow-up: 0.27 kg/week (IQR 0.11–0.52) versus 0.23 kg/week (IQR 0.05–0.35) (P = 0.054). No differences were found in other maternal and neonatal outcomes.
Table 3.
Pregnancy outcomes of the two study groups
CONCLUSIONS
This RCT was designed to determine whether a diet with low CHO content compared with a control CHO-content diet could prevent insulin treatment in women with GDM without deleterious effects with respect to main pregnancy outcomes. Contrary to our hypothesis, the low-CHO diet did not result in a significantly lower rate of women requiring insulin treatment. Additionally, we did not find any differences between the groups in terms of insulin dose or the time to start of insulin treatment.
Insulin treatment was started in ∼55% of the patients. This percentage is slightly higher than the 48% reported in a recent large retrospective survey (1981–2007) of 2,299 women with GDM in a population similar to ours using the same diagnostic criteria for GDM (16). The higher BMI (26.5 vs. 23.3) of our study population and the strict criteria applied to make changes in the prescribed diet until the primary objective of the study was reached could explain these differences.
Differences in daily CHO consumption were observed between the control and intervention groups throughout the study. Initial CHO intake was lower than that prescribed in both groups as assessed in the first dietary record, mainly as a result of lower intake of complex CHOs. After receiving additional dietary advice, the CHO intake approached the prescribed diets in both groups. The daily amount of CHO intake is consistent with the results of the 2009–2010 National Dietary Survey in Spain (17), in which the 50th percentile of CHO intake in women aged 25–44 years old was 202 g/day, which coincides with the mean initial intake by women in the control group.
CHO is the main nutrient that affects postprandial glucose concentrations. With MNT, we can manipulate the distribution, total amount, and type of CHO. Currently, there is a lack of evidence from RCTs on what is the ideal amount of CHO for the treatment of GDM, with the only recommendation advocating a minimum consumption of 175 g/day (4). In a small nonrandomized study, Major et al. (18) compared groups of women with GDM allocated to two diets, one containing <42% CHO and one containing 45–50% CHO. They found that fewer patients in the low-CHO diet group needed insulin (relative risk 0.14 [95% CI 0.02–1]; P = 0.047). Additionally, the low-CHO diet group had a lower rate of LGA newborns and cesarean deliveries. Cypryk et al. (19) randomized 30 patients with GDM to follow diets with 45 or 60% of daily calories from CHO, but no relevant pregnancy outcomes were reported.
In recent years, MNT for GDM has mainly focused on modifications of the GI to ameliorate postprandial glucose levels. Brand-Miller et al. (20) performed an analysis to determine how much of the variation in GL values was explained by the amount of CHO versus the corresponding GI values in 1,058 nominal portions of food. The linear regression analysis showed that the CHO content alone explained 68% of the variance in the GL, whereas the GI value alone explained 49% of the variance in GL values.
Recently, three RCTs have been conducted in women with GDM to evaluate the effect of a low GI on pregnancy outcomes (7,8,21). No differences were found regarding pregnancy outcomes between low and high-GI diets in all three trials. Only Moses et al. (7) found a lower need for insulin treatment in the low-GI group. It must be stressed that the study populations in these trials were different. Moses et al. (7) studied Caucasian women with a mean BMI of 32, and Louie et al. (8) and Grant et al. (21) studied predominantly non-Caucasian women with a mean BMI of 24 and 26 to 27, respectively. As suggested by Louie et al. (8), a low-GI diet may be more effective in overweight and obese women. There were also differences between the trials in the diagnostic criteria for GDM and in the target, frequency, and timing of postprandial glucose values. All of these factors may explain the observed differences between the trials, including ours.
A low-GI or a low-GL diet could be associated with a lower weight gain, as has been described in a recent RCT conducted in healthy pregnant women (22). Maternal weight gain was lower in the low-CHO diet group in the primary analysis of our trial. However, this difference disappeared after adjusting for time since randomization. Unfortunately, we do not know whether women on a low-CHO diet had also a lower total daily energy intake as we did not assess this parameter. Additionally, this trial was not properly powered to answer the question of whether a low-CHO diet can reduce weight gain in women with GDM. Therefore, we cannot conclude that a low-CHO diet is associated with a lower maternal weight gain in women with GDM in our population. However, the difference in weight gain between groups may be of clinical relevance. This finding raises the question of whether a low-CHO diet may reduce gestational weight gain and the potential pregnancy complications associated with overweight in obese women with GDM. This issue should be addressed in a randomized clinical trial.
No differences were found in any of the main pregnancy outcomes. This fact reinforces that a diet with a lower CHO content is safe for the treatment of GDM. The different CHO amount of the two diets tested in this trial did not influence the need of insulin or other relevant pregnancy outcomes. Therefore, the amount of CHO of the diet may not be a key issue in future clinical recommendations on MNT of GDM.
Despite being considered as the cornerstone of GDM treatment, there is still relatively little evidence-based information regarding specific nutritional approaches to the management of this condition. Data from observational studies show that certain dietary patterns are associated to important maternal and pregnancy outcomes (23). For instance, women with a vegetarian diet pattern show a lower prevalence of GDM (24) and also less gestational weight gain (23). Therefore, there are still several nutritional strategies that deserve the design and conduct of adequate clinical trials.
There are several limitations of our study. First, dietary food records were not obtained from all of the patients (the first record was obtained from 91% of the low-CHO group and 89% of the control-CHO group). In addition, the study was not offered to women who were not able to speak and understand the Spanish language; therefore, the results cannot be applied to the entire local population. Finally, the dropout rate in the control CHO group was clearly higher than expected. However, the method chosen to analyze the trial results reinforces the final conclusion of the trial.
We can conclude that a diet with a CHO content of 40% does not reduce the need for insulin treatment in women with GDM compared with a high-CHO diet (55% CHO) in our population. A low-CHO diet produces similar pregnancy outcomes. Additional randomized intervention studies that consider different populations and different strategies to modify GL are warranted to assess the optimal MNT for GDM.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
C.M.-C. was involved in the conception and design of the study, designed the study diets, provided dietary education to the study participants, collected data, drafted the manuscript, and was involved in the interpretation of data and the subsequent edits to the manuscript. M.H. and D.M. were involved in the conception and design of the study, provided medical management to the study participants, and were involved in the interpretation of data and subsequent edits to the manuscript. M.B., M.D.S., L.R.P., and Y.B. provided medical management to the study participants, collected data, and were involved in the interpretation of the data. M.C.A., M.A.A., M.I., M.M., and N.B. provided nurse management to the study participants and collected data. K.R. and N.A. researched the data and contributed to the interpretation of the data. M.M.-A. researched the data, contributed to the interpretation of the data, and was involved in the subsequent edits to the manuscript. E.M. provided dietary education to the study participants and collected data. All of the authors contributed to the discussion and read and approved the final manuscript. D.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Prof. V. Arija (Unitat de Salut Pública i Nutrició, Universitat Rovira i Virgili, Institut d’Investigació Sanitària Pere Virgili, Reus, Spain and Institut d’Investigació en Atenció Primària, Jordi Gol i Gorina, Catalunya, Spain) for allowing the use of the 3-day food record database and all of the women who agreed to participate in this clinical trial.
Footnotes
Clinical trial reg. no. NCT00911404, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2714/-/DC1.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricart W, Lopez J, Mozas J, et al. Spanish Group for the Study of the Impact of Carpenter and Coustan GDM thresholds Potential impact of American Diabetes Association (2000) for diagnosis of gestational diabetes mellitus in Spain. Diabetologia 2005;48:1135–1441 [DOI] [PubMed] [Google Scholar]
- 3.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med 1999;341:1749–1756 [DOI] [PubMed] [Google Scholar]
- 4.National Academy of Sciences , Ed. Dietary Reference Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty acids, Cholesterol, Protein, and Aminoacids (macronutrients). Washington, D.C., National Academy Press, 2005 [Google Scholar]
- 5.American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 6.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care 2007;30(Suppl. 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 7.Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009;32:996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie JCY, Markovic TP, Perera N, et al. A randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 2011;34:2341–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger BE. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes 1991;40(Suppl. 2):197–201 [DOI] [PubMed] [Google Scholar]
- 10.Nelson M, Bingham S. Assessment of food consumption and nutrient intake. In Design Concepts in Nutritional Epidemiology. 2nd ed. Margetts B, Nelson M, Eds. New York, Oxford University Press, 1997, p. 123–169 [Google Scholar]
- 11.Porta A, Bergua M. Documentación Gráfica para la Valoración Nutricional: Alimentos y su Cocción. Barcelona, Laboratorios LifeScan, 2002 [Google Scholar]
- 12.Favier JC, Ireland-Ripert J, Toque C, Feinberg M. Répertoire General des Aliments. Table de Composition. Paris, TEC & DOC Lavoisier-INRA, 1997 [Google Scholar]
- 13.Mataix J. Tabla de Composición de Alimentos Española. 2nd ed. Granada, Servicio de Publicaciones Universidad de Granada, 1995 [Google Scholar]
- 14.Santamaria R, Verdu LI, Martin C, Garcia G. Tablas Españolas de Pesos Neonatales Según edad Gestacional. Barcelona, Master-Graf SL, 1998 [Google Scholar]
- 15.R Core Team R: A language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2012 [Google Scholar]
- 16.Tundidor D, García-Patterson A, María MA, et al. Perinatal, maternal and neonatal outcomes in women with gestational diabetes mellitus according to fetal sex. Gend Med 2012;9:411–417 [DOI] [PubMed] [Google Scholar]
- 17.Ministerio de Sanidad Servicios Sociales e Igualdad. Evaluación nutricional de la dieta española. I. Energía y macronutrientes. Sobre datos de la Encuesta Nacional de Ingesta Dietética (ENIDE). Madrid, Agencia Española de Seguridad Alimentaria y Nutrición, 2011 [Google Scholar]
- 18.Major CA, Henry MJ, De Veciana M, Morgan MA. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet Gynecol 1998;91:600–604 [DOI] [PubMed] [Google Scholar]
- 19.Cypryk K, Kamińska P, Kosiński M, Pertyńska-Marczewska M, Lewiński A. A comparison of the effectiveness, tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol 2007;58:314–319 [PubMed] [Google Scholar]
- 20.Brand-Miller J, Holt SH, Petocz P. Reply to R. Mendosa (Letter). Am J Clin Nutr 2003;77:994–995 [DOI] [PubMed] [Google Scholar]
- 21.Grant SM, Wolever TM, O’Connor DL, Nisenbaum R, Josse RG. Effect of a low glycaemic index diet on blood glucose in women with gestational hyperglycaemia. Diabetes Res Clin Pract 2011;91:15–22 [DOI] [PubMed] [Google Scholar]
- 22.Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 2012;345:e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streuling I, Beyerlein A, Rosenfeld E, Schukat B, von Kries R. Weight gain and dietary intake during pregnancy in industrialized countries—a systematic review of observational studies. J Perinat Med 2011;39:123–129 [DOI] [PubMed] [Google Scholar]
- 24.Jali MV, Desai BR, Gowda S, Kambar S, Jali SM. A hospital based study of prevalence of gestational diabetes mellitus in an urban population of India. Eur Rev Med Pharmacol Sci 2011;15:1306–1310 [PubMed] [Google Scholar]