Abstract
OBJECTIVE
The purpose of this study was to examine the psychometric properties of the Diabetes Eating Problem Survey–Revised (DEPS-R) in a large sample of young patients with type 1 diabetes, to establish norms, and to validate it against the Eating Attitudes Test–12 (EAT-12).
RESEARCH DESIGN AND METHODS
A total of 770 children and adolescents aged 11–19 years with type 1 diabetes completed the DEPS-R and the EAT-12. In addition, age- and sex-standardized BMI and HbA1c data were obtained from the Norwegian Childhood Diabetes Registry. In addition to tests of validity, principal axis factoring was conducted to investigate the factor structure of the 16-item DEPS-R.
RESULTS
The DEPS-R demonstrated satisfactory Cronbach α (0.89) and was significantly correlated with the EAT-12 (0.65; P < 0.01), indicating convergent validity. The mean (SD) DEPS-R scores were 11.0 (10.7) for the total sample and 7.7 (7.4) and 14.2 (2.4) for males and females, respectively.
CONCLUSIONS
This study replicates and extends previous research demonstrating the psychometric properties of the abbreviated 16-item DEPS-R. Findings support the utility of this important screening tool to identify disturbed eating in young patients with type 1 diabetes.
Numerous studies indicate that type 1 diabetes is a risk factor for the development of disturbed eating behavior (DEB) (1,2), a term that will be used here to refer to the entire range of clinical and subclinical eating pathologies. DEB is common and persistent among young women with type 1 diabetes, with prevalences being more than double those in nondiabetic populations (3,4). Studies indicate that males with type 1 diabetes may also have an increased risk of developing DEB (5). When DEB and type 1 diabetes occur together, morbidity and mortality are dramatically increased. The study by Nielsen et al. (6) of comorbid type 1 diabetes and anorexia nervosa showed crude mortalities at 10-year follow-up of 2.5% for type 1 diabetes and 6.5% for anorexia nervosa; however, the mortality rose to 34.8% when these conditions occurred together.
The presence of DEB can severely impair metabolic control and advance the onset of long-term complications (7). Insulin restriction is an efficient weight loss strategy uniquely available to patients with type 1 diabetes, and this behavior is reported in as many as 37% of adolescents and young adult females with type 1 diabetes (4,8,9). Insulin restriction is associated with physical complications, and in previous research self-reported insulin restriction at baseline led to a threefold increased risk of mortality during 11 years of follow-up (9).
Given the deleterious effects of comorbidity, routine screening is important to identify DEB in individuals with type 1 diabetes to facilitate early intervention and prevent the development of serious physical complications. In a clinical setting, with limited time and resources, it is important to have access to a valid and sensitive screening instrument that requires few resources and is easy to administer and interpret. There are several screening instruments for the detection of eating pathology, such as the Eating Disorder Examination Questionnaire (10), the SCOFF questionnaire (the acronym was created from the questions) (11), the Eating Attitudes Test (EAT) (12), and the Diabetes Eating Problem Survey–Revised (DEPS-R) (13). In contrast to traditional eating disorders screening measures, such as the Eating Disorder Examination Questionnaire and the EAT, the DEPS-R is designed to assess disturbed eating specific to type 1 diabetes, including insulin restriction to lose weight. Insulin restriction is not likely to be detected by use of generic screening measures of eating pathology, and this represents a serious limitation of those instruments. The Diabetes Eating Problem Survey (DEPS) was first developed in 2001 and consisted of 28 items but was recently revised and shortened to the DEPS-R by Markowitz et al. (13). The original validation study of the DEPS-R included 112 youths (aged 13–19 years) with type 1 diabetes, and results showed that the DEPS-R correlated positively with age, age- and sex-standardized BMI (zBMI), and HbA1c, and that females scored significantly higher than did males. Despite promising initial psychometric properties of the DEPS-R, the instrument has not been validated against an established measure of eating pathology and remains limited by a small sample. We aimed to investigate the internal consistency and construct validity of the DEPS-R, to identify the factor structure, and to establish normative data in a large sample of young patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Design
This was a cross-sectional epidemiological survey of the nationwide, population-based Norwegian Childhood Diabetes Registry (NCDR).
Participants
The participants were recruited from the NCDR between 1 April 2010 and 31 March 2011. In our study, a total of 1816 individuals with type 1 diabetes aged 11–19 years in the NCDR were invited to participate in the study. The NCDR, a nationwide, population-based registry established in 2006, includes all newly diagnosed children with diabetes. All the pediatric departments in Norway perform an annual examination of all their patients with diabetes and report the results to the NCDR. The final sample consisted of 770 children and adolescents with type 1 diabetes aged 11–19 years (42.40% response rate). There were 380 (49.4%) males and 390 (50.6%) females. Participants were somewhat younger than nonparticipants (14.6 vs. 15.1 years; P ≤ 0.001), had slightly lower HbA1c (8.5 vs. 8.7%; P ≤ 0.01), and had somewhat shorter duration of type 1 diabetes (5.3 vs. 6.1 years; P ≤ 0.001) than nonparticipants; however, the effect sizes were very small (−0.2, −0.1, and −0.2, respectively). These groups did not differ with respect to zBMI or age at onset of type 1 diabetes.
Procedure
The Regional Ethics Committee approved the study. Written consent was obtained from participants and their parents, if the participant was below the age of 16 years. Questionnaires were distributed to the participants at their regularly scheduled appointments at their local outpatient diabetes clinics.
Measures
The DEPS (14) was the first measure designed to screen for DEB in type 1 diabetes, such as insulin restriction to lose weight. The original instrument consisted of 28 items but has recently been revised to create the DEPS-R, a brief 16-item version that can be completed in less than 10 min and has demonstrated good psychometric properties (13). Responses are scored on a 6-point Likert scale ranging from 0 to 5, and higher scores indicate greater pathology.
The EAT (12) is a generic screening measure of eating pathology used internationally to detect pathologic eating attitudes and behaviors. A 12-item Norwegian version, EAT-12 has been developed (15) and has demonstrated adequate psychometric properties (16). Answers are ranked on a 4-point scale, and higher scores indicate greater pathology.
Somatic data were obtained from NCDR. HbA1c was determined for all participants by high-performance liquid chromatography (Tosoh G7; Tosoh Europe N.V., Tessenderlo, Belgium) at the same central Diabetes Control and Complications Trial–standardized laboratory. The reference range was 4.0–6.0%, and the analytical coefficient of variation was 0.8%. BMI was calculated from weight and height (kg/m2) and standardized to a z-score (zBMI) according to age and sex because the participants were primarily younger than 18 years using the Centers for Disease Control and Prevention (CDC) growth charts for 2000 (17). Weight was categorized in four groups according to the World Health Organization (18): underweight (BMI <18.5 kg/m2), normal weight (BMI ≥18.5–24.9 kg/m2), overweight (BMI ≥25–29.9 kg/m2), and obese (BMI ≥30 kg/m2). BMIs for all participants were adjusted for age and sex to generate these groups. Data were assessed as part of the annual extended diabetes examination at the local diabetes outpatient clinic.
Statistical analyses
Data are given as mean (SD). Nonparametric tests were used for skewed data. For the DEPS-R and the EAT-12, internal consistency was assessed by Cronbach α coefficients. Convergent validity between the DEPS-R and the EAT-12 was investigated with Spearman population correlation coefficient (ρ). In line with Cohen (19), correlations of 0.10–0.29 were interpreted as small, 0.30–0.49 as medium, and 0.50–1.0 as large. Correlations were also carried out to explore relationships with other constructs hypothesized to covary with DEPS-R scores, such as HbA1c, zBMI, age, and sex. P < 0.05 indicates statistical significance. Sex differences in DEPS-R scores were investigated with t tests. Pearson χ2 was used for categorical data. A principal axis factoring (PAF) was used to explore the factor structure of DEPS-R. The data were considered suitable for PAF because the Kaiser-Meyer-Olkin measure of sampling adequacy value was >0.6 and the Bartlett test of sphericity value was significant. Effect sizes were calculated by means of Cohen d value, and the guidelines used for interpreting this value were as follows: 0.20, small effect; 0.50, moderate effect; and 0.80, large effect (19). Statistical analyses were conducted with SPSS version 18 (IBM Corporation, Armonk, NY).
RESULTS
Participant characteristics
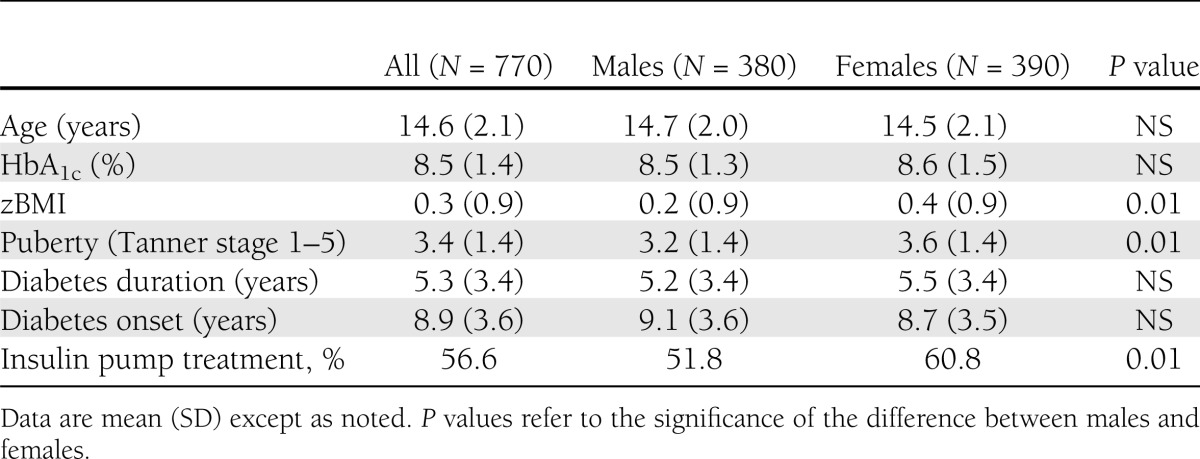
Table 1 illustrates sample characteristics. As shown below, mean age of the 770 participants was 14.6 (2.1) years and age at onset of type 1 diabetes was 8.9 (3.6) years. Mean type 1 diabetes duration was 5.3 (3.4) years, mean zBMI was 0.3 (0.9), and mean HbA1c was 8.5% (1.4%). Pubertal state was categorized by Tanner stage 1–5 as prepubertal (Tanner stage 1) pubertal (Tanner stage 2–4), and postpubertal (Tanner stage 5).
Table 1.
Participant characteristics
Internal consistency
The Cronbach α coefficients for the DEPS-R were 0.89, 0.81, and 0.90 for the entire sample, males, and females, respectively. For the EAT-12, coefficients were 0.74 for the whole sample, 0.63 for males, and 0.76 for females.
Construct validity
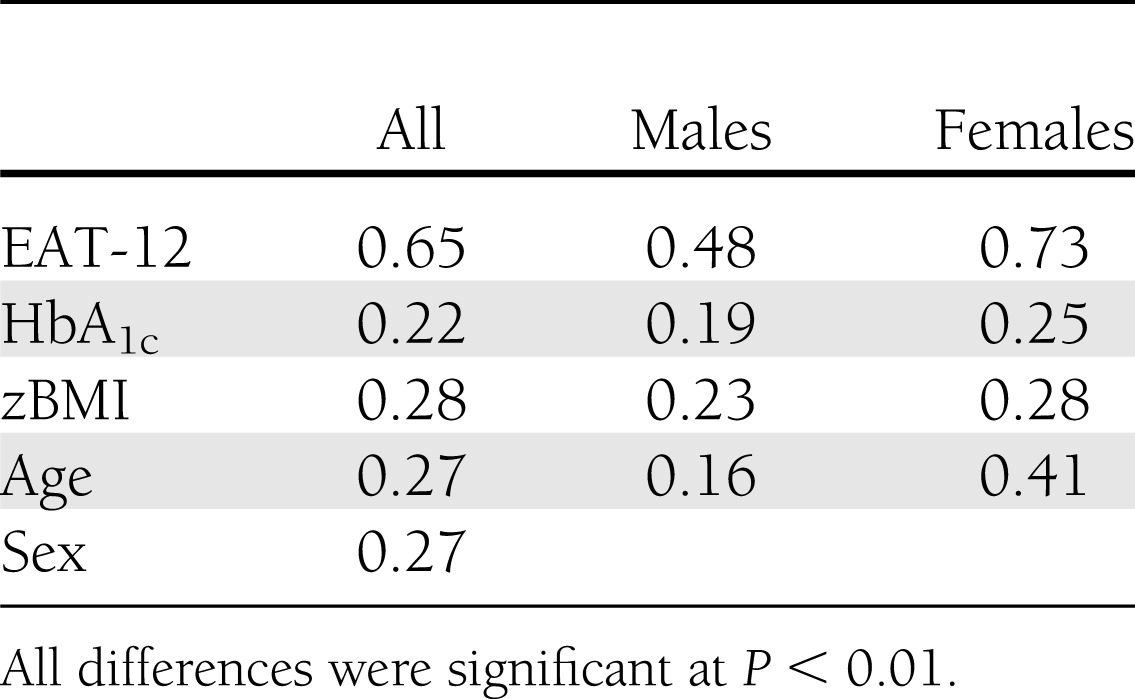
The DEPS-R was significantly and positively correlated with the EAT-12 (ρ = 0.65; P ≤ 0.01) (Table 2). Additional significant, although small, correlations were found between the DEPS-R and zBMI (ρ = 0.28; P ≤ 0.01), HbA1c (ρ = 0.22; P ≤ 0.01), age (ρ = 0.27; P ≤ 0.01), and sex (ρ = 0.27; P ≤ 0.01). The EAT-12 was also significantly correlated with HbA1c; however, this was an even smaller correlation (ρ = 0.08; P ≤ 0.05).
Table 2.
Correlations between DEPS-R and EAT-12, HbA1c, zBMI, age, and sex
Normative data
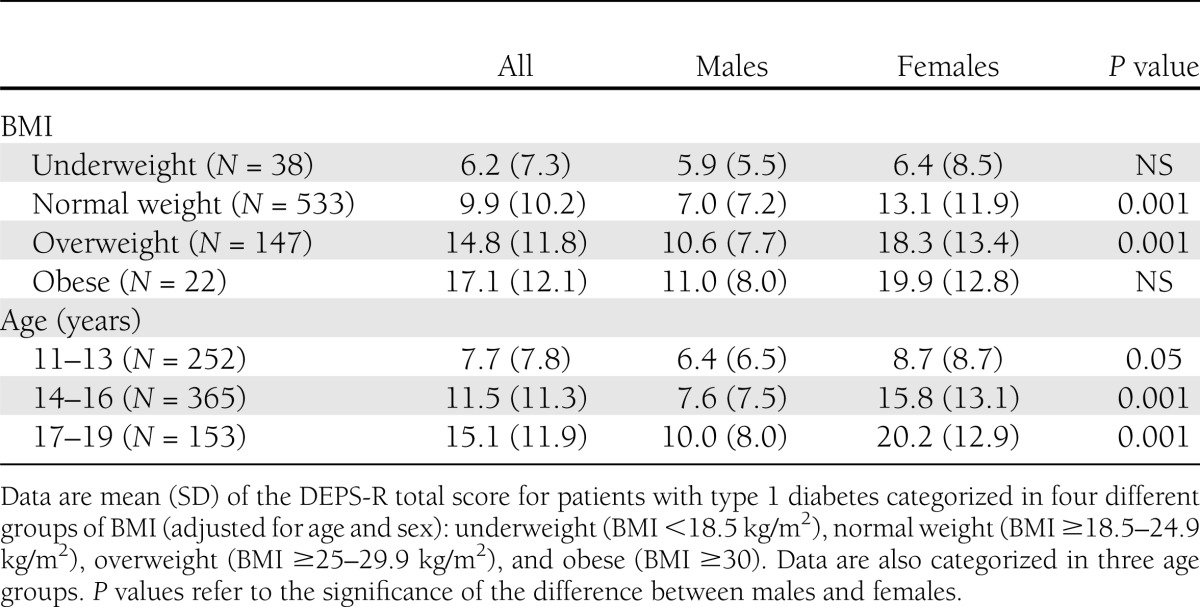
The DEPS-R norms (SD) for the total sample, males, and females were as follows: 11.0 (10.7), 7.7 (7.4) and 14.2 (12.4), respectively. Females scored significantly higher than did males (P ≤ 0.001).
Mean DEPS-R score was more than three times higher in the obese group than in the underweight group, and two times higher in the oldest than in the youngest age group. Table 3 presents the DEPS-R scores according to sex, age, and zBMI category.
Table 3.
Norms according to different categories of age and weight
Factor analysis
After the suitability of data for factor analysis was assessed, PAF was performed on the 16 items of the DEPS-R. The Kaiser-Meyer-Olkin value was 0.92, and the Bartlett test of sphericity reached statistical significance, supporting the factorability of the correlation matrix.
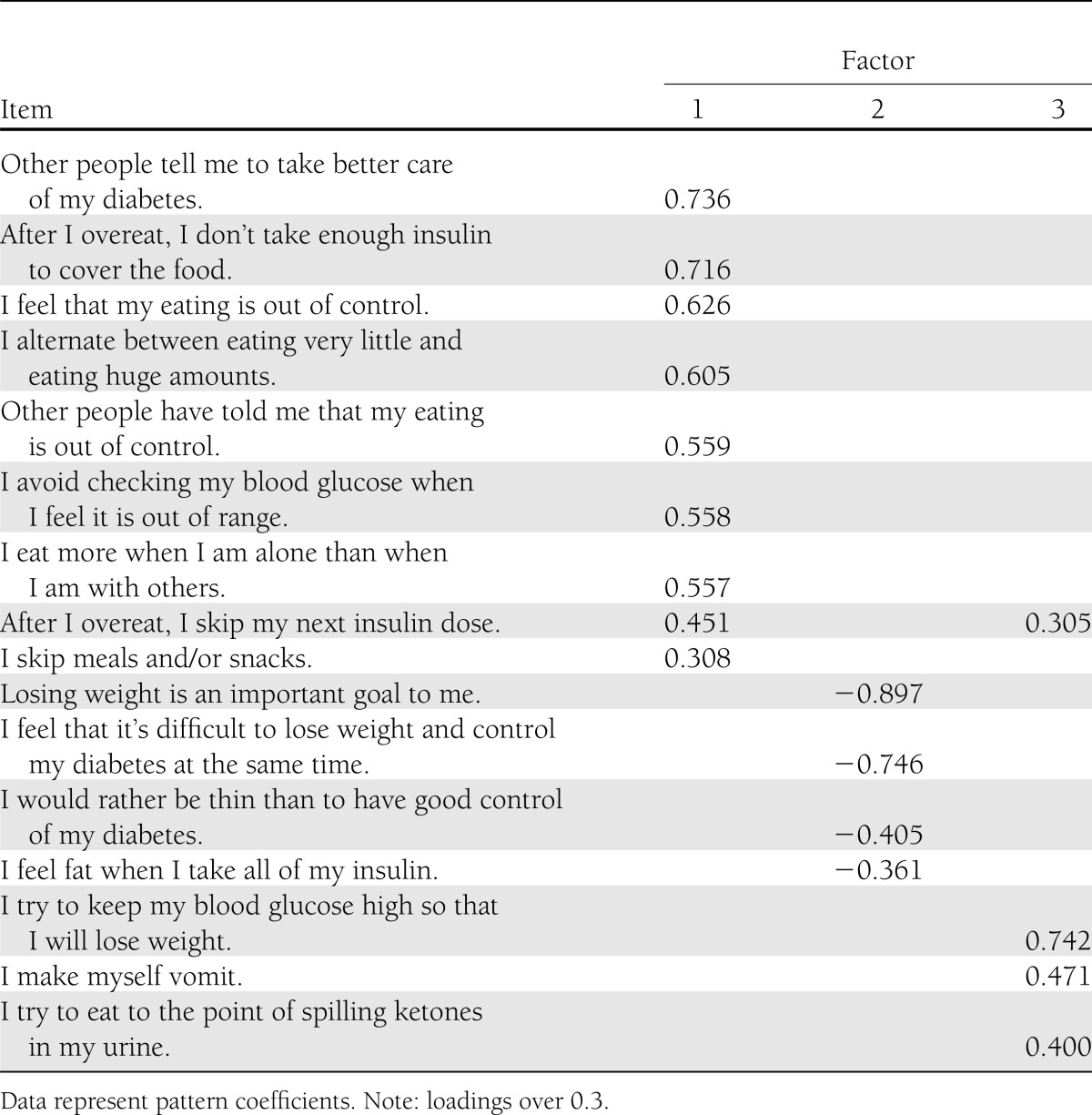
PAF revealed three components with eigenvalues >1, explaining 38.7, 8.5, and 7.4% of the variance, respectively, accounting for a total of 54.6% of the variance. On the basis of eigenvalues and inspection of the scree plot, three components were chosen for further investigation. To assist in the interpretation of these three components, oblimin rotation was performed. All three components showed relatively strong loadings and items loaded exclusively on one component, with the exception of one item (item 13). Further examination of the three factors was conducted by running correlations with the DEPS-R total score and HbA1c. Factor 1 correlated more strongly with both the DEPS-R total score (0.91; P < 0.001) and HbA1c (0.27; P < 0.001) than did factors 2 (0.88; P < 0.001 and 0.16; P < 0.001, respectively) and 3 (0.47; P < 0.001 and 0.09; P < 0.05, respectively). The pattern matrix is presented in Table 4.
Table 4.
Pattern matrix for PAF with oblimin rotation of three-factor solution of the DEPS-R items
To examine sex differences in factor structure, the PAF was also conducted separately for males and females. Data confirmed the three-factor solution in the female sample. When investigating males only, however, the intercorrelations among the items were not strong enough (20), and consequently the data were not considered appropriate for factor analysis.
CONCLUSIONS
This study replicates and extends previous research demonstrating the psychometric properties of the DEPS-R for children and adolescents. Internal consistency was found to be very good (0.89) and consistent with the original validation study of DEPS-R (13), which reported a Cronbach α of 0.86. In contrast, the Cronbach α for EAT-12 was 0.74 in our study. Data also supported the construct validity of the DEPS-R, as indicated by significant and positive correlations with the EAT-12. Additional positive, although relatively weak, correlations were found with HbA1c, zBMI, and age, constructs previously found to be associated with DEB (13,21).
The relatively small correlations between the DEPS-R and other variables are in line with earlier studies (13). This underscores the importance of screening for DEB in young patients with type 1 diabetes, because clinical variables such as zBMI and HbA1c seem to be poor indicators of DEB in this population.
When comparing the DEPS-R with the EAT-12, the DEPS-R generally appears to be a better screening tool than the EAT-12 for DEB in young patients with type 1 diabetes. In addition to convincing internal consistency, the DEPS-R was more strongly correlated with HbA1c than was the EAT-12, although both correlations were relatively weak. HbA1c is commonly reported to be associated with DEB (7). Also, previous research comparing the DEPS with the 26-item EAT found that DEPS scores were more strongly correlated with formally diagnosed eating problems than were EAT scores. These findings might be explained by the fact that the DEPS and the DEPS-R can identify diabetes-specific DEB, such as insulin omission to lose weight, which is a core feature of DEB in type 1 diabetes. Such diabetes-specific behaviors are not likely to be detected by the use of generic screening tools, indicating the risk of false negatives associated with these measures.
Norms of the DEPS-R were established in this study, and significant differences were found between males and females. Overall, male adolescents reported fewer DEBs than did female adolescents. Our results are similar to those reported in the previous DEPS-R validation study (13). The norms were additionally categorized according to zBMI and age and demonstrated a threefold increase in DEBs in the obese group relative to the underweight group and a twofold increase in the oldest age group (17–19 years) relative to the youngest age group (11–13 years). Determining fixed norms is challenging because of the variability in mean scores between different weight and age categories. In a clinical setting, we therefore recommend interpreting the DEPS-R score in relation to the patients zBMI and age. Both higher zBMI and higher age appear to be risk factors for the development of DEBs among adolescents and children with type 1 diabetes. This finding is consistent with studies of eating pathology in the general population (22,23).
Because the factor structure of the DEPS-R has not been previously reported, PAF was conducted. Three components were identified, explaining 54.6% of the total variation. Factor 1 was the most dominant factor, explaining 38.7% of the variance. Factors 2 and 3 explained 8.5% and 7.4% of the variance, respectively. Although our model identified three factors, it is difficult at this point to establish obvious subscales related to these three factors. One possible interpretation is that factor 1 appears to address maladaptive eating habits, factor 2 the preoccupation with thinness or weight, and factor 3 the concept of maintaining high blood glucose values to lose weight. Further examination of the three factors showed that factor 1 correlated more strongly with HbA1c than did factors 2 and 3. Even though three factors were identified, it is unclear at this time whether the DEPS-R would benefit from using this factor structure for scoring purposes. The ease of administration and short administration time already make the DEPS-R clinically useful as a screening tool in busy clinical settings. Nonetheless, the factor structure could be worth considering for further psychometric work on the DEPS-R. It is possible that scores on each factor could facilitate treatment recommendations according to which factors the individual scores highest.
Significant sex differences were demonstrated in this study. As expected, and in line with other studies of DEB (13,22), females scored significantly higher than males on both the DEPS-R and the EAT-12. This is consistent with previous research demonstrating that females are at higher risk of developing DEB than males, both among patients with type 1 diabetes and in the general population (24). It is also possible that males have underreported their symptoms of eating pathology compared with females. This has also been suggested in studies of other types of pathology (25,26). Further, when males and females were analyzed separately, internal consistency and construct validity of the DEPS-R were stronger among females. For example, the Cronbach α coefficient was lower among males (0.81) than among females (0.90). Weaker validity among males has been previously demonstrated in studies conducted with other measures of disordered eating (21,27). Because of sex disparities in prevalence rates of disordered eating (28), assessment tools have largely been developed with female populations; however, some studies suggest that traditional assessments may fail to provide a comprehensive assessment of male-specific eating, weight, and shape concerns (29). Items focused predominantly on restricted eating and a desire for thinness may not reflect the dual presence of a drive for thinness and a drive for muscularity that characterizes the lean, mesomorphic body ideal unique to boys and men. This may contribute to the lower validity coefficients for the DEPS-R among males in this study and raises the general issue of whether existing screening tools provide an adequate assessment of DEB among men.
In Norway, all patients with type 1 diabetes are offered the same modern and intensive insulin treatment independent of social status. The strengths of this study include the high number of participants, the population-based national registry inclusive of >95% of all eligible children and adolescents, and the validation of DEPS-R against an established measure of disturbed eating and somatic data. This study also has limitations. First, the response rate was relatively low (42%), and participants were on average 6 months younger with a shorter duration of illness; however, effect sizes were small. Second, it fails to compare the DEPS-R with a diagnostic interview such as the Eating Disorder Examination (30). Screening tools are generally not sufficient to determine eating disorder diagnoses. Comparison with the Eating Disorder Examination would allow an investigation of the ability of the DEPS-R to identify clinical eating disorder diagnoses. Future research is warranted to validate the DEPS-R against a diagnostic interview.
In conclusion, because of the significant risks of morbidity and mortality associated with the occurrence of disordered eating and type 1 diabetes together in youth, screening and early intervention for DEB should be routinely included in standard diabetes care (3). The DEPS-R is a valid screening tool for DEB in type 1 diabetes, is easy to administer, and is a potentially important addition to clinical practice. There were significant differences in DEPS-R scores according to sex, age, and zBMI, and we therefore recommend that these aspects be considered when interpreting DEPS-R scores.
Acknowledgments
The Research Council of Norway funded this work. The Norwegian Childhood Diabetes (NCDR) Registry is funded by Health South-East.
No potential conflicts of interest relevant to this article were reported.
L.W. contributed to the planning of the study, analyzed the data, and wrote the manuscript. D.H.F. planned the study, collected data for DEBs and contributed to data analyses and to the manuscript. T.S., the leader of the NCDR, contributed to the planning of the study, collected somatic data with the NCDR, and contributed to the manuscript. K.D.-J., one of the initiators of the NCDR, supervised D.H.F., contributed to the planning of the study, and contributed to the manuscript. Ø.R. supervised L.W, contributed to the data analyses, and contributed to the writing of the manuscript. L.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the International Eating Disorders Conference, London, 19–21 March 2013.
The authors thank the staff and patients from the outpatient clinics around Norway for participating in the study, and the NCDR and Regional Eating Disorders Service, Oslo University Hospital, for contributing to the study.
References
- 1.Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005;28:417–419 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S. Eating disorders in females with type 1 diabetes: an update of a meta-analysis. Eur Eat Disord Rev 2002;10:241–254 [Google Scholar]
- 3.Colton PA, Olmsted MP, Daneman D, Rydall AC, Rodin GM. Five-year prevalence and persistence of disturbed eating behavior and eating disorders in girls with type 1 diabetes. Diabetes Care 2007;30:2861–2862 [DOI] [PubMed] [Google Scholar]
- 4.Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005;28:84–88 [DOI] [PubMed] [Google Scholar]
- 5.Svensson M, Engström I, Aman J. Higher drive for thinness in adolescent males with insulin-dependent diabetes mellitus compared with healthy controls. Acta Paediatr 2003;92:114–117 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Emborg C, Mølbak AG. Mortality in concurrent type 1 diabetes and anorexia nervosa. Diabetes Care 2002;25:309–312 [DOI] [PubMed] [Google Scholar]
- 7.Jones JM, Lawson ML, Daneman D, Olmsted MP, Rodin G. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ 2000;320:1563–1566 [PMC free article] [PubMed] [Google Scholar]
- 8.Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HA. Poor prognosis of young adults with type 1 diabetes: a longitudinal study. Diabetes Care 2003;26:1052–1057 [DOI] [PubMed] [Google Scholar]
- 9.Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care 2008;31:415–419 [DOI] [PubMed] [Google Scholar]
- 10.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord 1994;16:363–370 [PubMed] [Google Scholar]
- 11.Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ 1999;319:1467–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med 1982;12:871–878 [DOI] [PubMed] [Google Scholar]
- 13.Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care 2010;33:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antisdel JE LLAB: improved detection of eating problems in women with type 1 diabetes using a newly developed survey (Abstract). Diabetes 2001;50(Suppl. 1):A47
- 15.Lavik NJ, Clausen SE, Pedersen W. Eating behaviour, drug use, psychopathology and parental bonding in adolescents in Norway. Acta Psychiatr Scand 1991;84:387–390 [DOI] [PubMed] [Google Scholar]
- 16.Engelsen BK, Hagtvet KA. The dimensionality of the 12-item version of the Eating Attitudes Test. Confirmatory factor analyses. Scand J Psychol 1999;40:293–300 [DOI] [PubMed] [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;(314):1–27 [PubMed] [Google Scholar]
- 18.World Health Organization. BMI classification, 2012. Available from http://www.who.int/features/factfiles/obesity/facts/en/ Accessed 27 February 2013
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 20.Tabachnick B, Fidell L. Using Multivariate Statistics 4th ed. Boston, Allyn & Bacon, 2001
- 21.Reas, D. L, Øverås, M, and Rø, Ø. Norms for the Eating Disorder Examination Questionnaire (EDE-Q) among high school and university men. Eat Disord 2012;20:437–443 [DOI] [PubMed]
- 22.Rø O, Reas DL, Rosenvinge J. The impact of age and BMI on Eating Disorder Examination Questionnaire (EDE-Q) scores in a community sample. Eat Behav 2012;13:158–161 [DOI] [PubMed] [Google Scholar]
- 23.Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep 2012;14:406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant-Waugh R, Lask B. Overview of the eating disorders. In Eating Disorders in Childhood and Adolescence 3rd ed. Lask B, Bryant-Waugh R, Eds. London, Routledge, 2007, p. 35–50 [Google Scholar]
- 25.Cougle JR, Fitch KE, Fincham FD, Riccardi CJ, Keough ME, Timpano KR. Excessive reassurance seeking and anxiety pathology: tests of incremental associations and directionality. J Anxiety Disord 2012;26:117–125 [DOI] [PubMed] [Google Scholar]
- 26.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17:748–757 [DOI] [PubMed] [Google Scholar]
- 27.Darcy AM, Doyle AC, Lock J, Peebles R, Doyle P, Le Grange D. The Eating Disorders Examination in adolescent males with anorexia nervosa: how does it compare to adolescent females? Int J Eat Disord 2012;45:110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34:383–396 [DOI] [PubMed] [Google Scholar]
- 29.Anderson CB, Bulik CM. Gender differences in compensatory behaviors, weight and shape salience, and drive for thinness. Eat Behav 2004;5:1–11 [DOI] [PubMed] [Google Scholar]
- 30.Fairburn CG, Cooper Z. The Eating Disorder Examination. In Binge Eating: Nature, Assessment, and Treatment. Fairburn CG, Wilson GT, Eds. New York, Guilford Press, 1993, p. 317–360 [Google Scholar]


