Abstract
OBJECTIVE
Successful treatment of osteomyelitis is more likely with accurate diagnosis and identification of the causative pathogens. This typically requires obtaining a specimen of bone, usually by image-guided biopsy. We sought to develop a simpler bedside method for definitively diagnosing osteomyelitis.
RESEARCH DESIGN AND METHODS
Over 2 years, we enrolled consecutive patients presenting to our diabetic foot clinic with a foot ulcer and clinically suspected osteomyelitis but without soft tissue infection. Each underwent hybrid 67Ga single-photon emission computed tomography and X-ray computed tomography (SPECT/CT) imaging; those with a positive scan underwent bedside percutaneous bone puncture. Patients with a positive bone culture received culture-guided antibiotic therapy. Patients with negative 67Ga SPECT/CT imaging or with positive imaging but negative bone culture were not treated with antibiotics. All patients were followed up for ≥1 year.
RESULTS
Among 55 patients who underwent 67Ga SPECT/CT imaging, 13 had negative results and all of their foot ulcers resolved without antibiotic therapy. Among 42 with positive imaging, 2 were excluded (for recent antibiotic therapy) and 40 had bone punctures (3 punctured twice): 19 had negative results, 3 of which were likely false negatives, and 24 had positive results (all gram-positive cocci). At follow-up, 3 patients had died, 3 had undergone amputation, and 47 had no evidence of foot infection. The sensitivity and specificity of this combined method were 88.0 and 93.6%, respectively, and the positive and negative predictive values were 91.7 and 90.7%, respectively.
CONCLUSIONS
Coupling of 67Ga SPECT/CT imaging and bedside percutaneous bone puncture appears to be accurate and safe for diagnosing diabetic foot osteomyelitis in patients without signs of soft tissue infection, obviating the need for antibiotic treatment in 55% of suspected cases.
The worldwide prevalence of diabetes mellitus is expected to exceed 300 million people in 2025, and ~25% will develop a foot ulcer (1). More than half of these ulcers are clinically infected at presentation, and among these infections 20–50% involve bone (2,3). Infection of a diabetic foot ulcer, particularly when accompanied by osteomyelitis, markedly increases the risk of lower-extremity amputation, a highly morbid event (4,5).
The presentation of foot infection in persons with diabetes is often atypical because of the frequent presence of peripheral neuropathy or vascular insufficiency (6). Diagnosis of diabetic foot osteomyelitis is particularly challenging (7), despite the availability of various clinical, serological, and imaging techniques (7–11). Furthermore, cultures of soft tissue specimens do not accurately reflect bone pathogens (12). The criterion standard for diagnosing osteomyelitis is still controversial, but the most conservative (albeit not yet validated) definition is culture of a pathogenic organism from a bone specimen in the presence of characteristic histological changes (13). Bone samples may be obtained at the time of surgical débridement or percutaneously by biopsy or bone marrow aspiration after puncture. A positive culture allows appropriate tailoring of antibiotic treatment, which may improve outcomes in diabetic foot osteomyelitis (14). Nevertheless, in most clinical practices osteomyelitis is diagnosed solely on the basis of clinical and imaging findings (15); bone specimens are infrequently obtained, largely because of the perceived complexity of arranging the procedure or fear of adverse effects of bone biopsy. Bone puncture, although simpler than surgical or radiologically guided bone biopsy, is rarely undertaken.
Bone imaging, especially by plain radiographs, is usually the first step in diagnosing diabetic foot osteomyelitis (11,16). When this is inadequate, magnetic resonance imaging (MRI) is considered the best available advanced imaging study. MRI sensitivity is 90–100% in osteomyelitis cases, but its specificity in most studies is <80% (17). Nuclear medicine imaging, although highly sensitive, is quite nonspecific (18,19). Conventional scintigraphic imaging often cannot identify the precise site of osteomyelitis because of low spatial resolution and a lack of anatomic specificity. The fusion of scintigraphic and morphologic images, using hybrid single-photon emission computed tomography/computed tomography (SPECT/CT), is more accurate than planar scintigraphy alone, correctly differentiating foot osteomyelitis and contiguous soft tissue infection in 97% of cases compared with 59% for planar scintigraphy (18,20,21). 67Ga, a radioactive marker that accumulates in inflammatory and infected tissues (22), appears to be a good radiotracer to localize infection in combination with SPECT/CT (23).
This study investigated the usefulness in diagnosing diabetic foot osteomyelitis by a sequential procedure that uses the results of 67Ga SPECT/CT to exclude infection when it is negative or to localize suspected infection when it is positive, followed by a sterile percutaneous bone puncture to identify the causative pathogens.
RESEARCH DESIGN AND METHODS
Study design
We conducted a single-center prospective study in a university-affiliated hospital (Hôtel Dieu, Paris, France) referral clinic for diabetic foot conditions. We recruited consecutive diabetic inpatients and outpatients with a chronic foot ulcer (present for >3 months) in whom osteomyelitis was suspected on the basis of French consensus criteria (24). The study protocol was approved by the local ethics committee (Comité de Protection des Personnes, Hôtel Dieu, Paris) and classified as a current care proceeding, because SPECT/CT imaging and bone puncture are currently recommended for diagnosing diabetic foot osteomyelitis by the French Infectious Diseases Society (24).
Study population
We evaluated all diabetic patients ≥18 years old in whom active osteomyelitis was clinically suspected on the basis of a foot ulcer with at least one of the following characteristics: presence for >3 months, location over a bony prominence, or relapsing infection or slow healing despite adequate arterial flow and appropriate local care for ≥3 months. There were findings compatible with osteomyelitis on plain radiography in 26 of 42 (60%) of the patients who underwent bone puncture. Thus the enrolled patients were all at high risk for underlying osteomyelitis. We excluded patients who required orthopedic or vascular surgery or who had received any systemic antibiotic therapy during the 14 days preceding imaging and bone puncture. We did enroll patients with a suspicion of osteomyelitis associated with acute soft tissue infection; however, imaging and puncture were delayed until 14 days after the end of antibiotic therapy because urgent treatment of soft tissue infection is mandatory.
Detection of bone inflammation
All enrolled patients underwent hybrid 67Ga SPECT/CT imaging, which provides high-resolution merged 67Ga single-photon emission computed tomographic and computed X-ray transmission tomographic images of the ankles and feet. We obtained images 48 h after the injection of ~150 MBq of [67Ga]gallium citrate and considered patients with no increased uptake on the 67Ga SPECT/CT not to have osteomyelitis. These patients underwent no further diagnostic studies and did not receive antibiotic treatment.
Collection of bone marrow samples
In cases in which imaging revealed a focal accumulation of radioactivity in bone underlying the ulcer, we performed a bedside percutaneous bone puncture with a 16 gauge × 5 cm Mallarmé trocar (Myelo-Gal; Gallini Medical Devices, Mantova, Italy). Punctures were done through intact uninfected skin, not through the ulcer, after first washing the entire foot twice with foaming povidone iodine polyvidone solution (Betadine scrub), rinsing it, then disinfecting the puncture site with Betadine. We inserted the trocar into the presumed site of bone infection, selecting a route according to the results of three-dimensional 67Ga SPECT/CT imaging. We aspirated bone marrow into a sterile 20-mL syringe; the maximum final volume obtained ranged from 0.5 to 3 mL, depending on the size of the bone. The gross appearance of the bone marrow aspirate was like blood with fatty material.
Microbiological assessment
Immediately after puncture, we inoculated the bone marrow sample into a blood culture bottle containing an anaerobic growth medium (BACTEC Lytic/10 Anaerobic/F; Becton Dickinson, Le Pont-de-Claix, France) and another with aerobic medium (BACTEC Plus aerobi/F). We processed the bottles with the BACTEC 9240 system and incubated the bottles for as long as 10 days at 37°C. We identified any isolates by standard procedures, including coagulase tests with rabbit plasma and API 20E strips (bioMérieux, Marcy l’Etoile, France). We identified susceptibility to antibiotics by the disk diffusion method on Mueller-Hinton agar, according to the guidelines of the Committee of the French Society for Microbiology (http://www.sfm.asso.fr), with disks from Bio-Rad (Marnes-la-Coquette, France). We also subcultured any positive blood culture vials on nutrient agar at 37°C and identified pathogens as described above.
Patient outcome
Our primary end point for diagnosis of osteomyelitis was a positive result for both the 67Ga SPECT/CT and bone culture. We used the 67Ga SPECT/CT imaging to determine which patients would undergo bone puncture for culture. If the scan showed focal accumulation of activity in the bone immediately underlying the ulcer, we performed a bone puncture; if not, we assumed the patient did not have osteomyelitis and did not provide antibiotic treatment. Patients who underwent a bone puncture and had a negative culture also did not receive antibiotic treatment. In patients who had a negative bone culture but who had poor wound healing and persistent or worsening clinical local symptoms of inflammation suggestive of osteomyelitis, we performed a second bone puncture. We treated all patients with a positive bone culture with 8 weeks of antibiotic therapy selected according to the susceptibility profile of the isolates and in line with the French diabetic foot infection guidelines (24). We preferred oral antibiotic treatments when possible to avoid the need for a long hospital stay. We evaluated all patients for clinical signs of osteomyelitis and healing of the ulcer after ≥12 months of follow-up treatment. We did not routinely repeat 67Ga SPECT/CT in patients with a positive bone culture; when performed, however, such imaging was carried out at ≥3 months after the start of antibiotic treatment.
Analysis of the results
We assessed end points ≥1 year after a negative 67Ga SPECT/CT result (for those who did not receive antibiotic therapy) or the start of antibiotic treatment (for those who received it). The primary end point was the absence of any clinical evidence of diabetic foot osteomyelitis. The secondary end point was the clinical status of the ulcer, a surrogate marker for cure of infection. Ulcer clinical outcome in those treated with antibiotics was categorized as follows: cure if completely healed, failure if the ulcer persisted or displayed progression in its size or appearance that led to surgical treatment, or improvement if the ulcer had decreased in size by ≥50% but was not completely healed. In those not treated with antibiotics, osteomyelitis was presumed to have resolved if there was no clinical evidence of infection after ≥1 year of follow-up.
We considered the patient not to have had osteomyelitis if the baseline 67Ga SPECT/CT result was negative or if 67Ga SPECT/CT result was positive but the bone culture was negative, and if no clinical signs of osteomyelitis were present after ≥1 year of follow-up. Patients were considered to have had osteomyelitis if 67Ga SPECT/CT and bone culture results were both positive at baseline. For patients with a negative initial bone culture who underwent a second bone puncture at the same location (because of a poor clinical response, as explained) that yielded a positive culture, we considered the first culture to have been a false negative.
Statistics
We summarized continuous variables with descriptive statistics and calculated the sensitivity and specificity of our evaluation procedure (25). We determined the comparability of groups for categorical variables by the χ2 test with a significance level set at P < 0.05. We used the Youden index (J = sensitivity + specificity − 1) to evaluate the performance of the diagnostic test; it has minimum and maximum values of −1 and +1, respectively, with a value of +1 representing the optimal value for an algorithm.
RESULTS
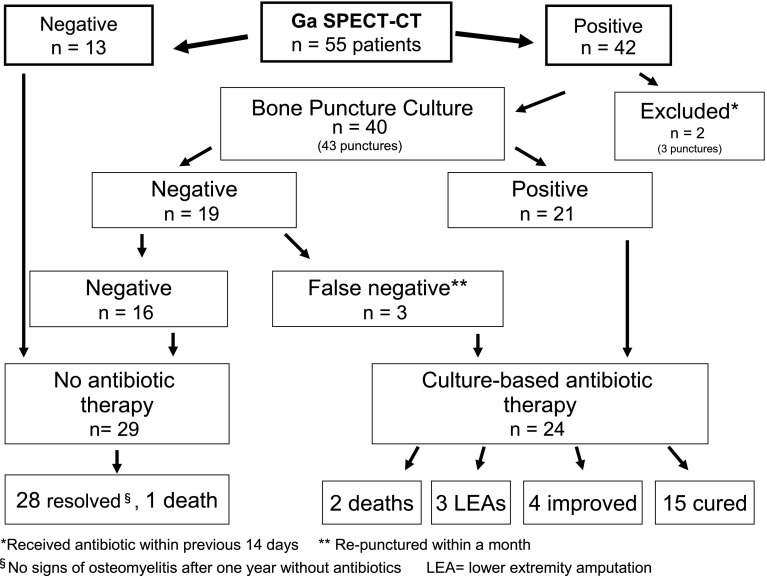
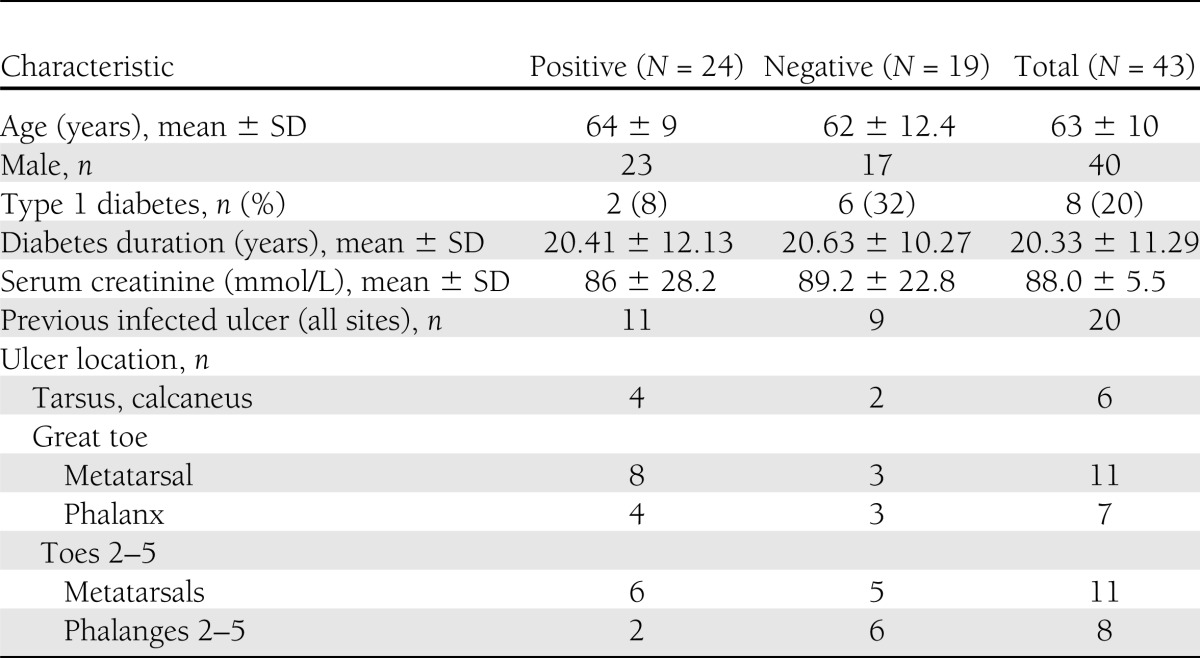
From 1 May 2007 to 1 May 2009, we enrolled 55 diabetic patients with suspected foot osteomyelitis in this study (Fig. 1). 67Ga SPECT/CT scans in 13 patients were negative, showing no increased 67Ga uptake in the bone underlying the ulcer. None of these patients received antibiotic therapy, and after ≥1 year of follow-up all their ulcers were healed without any signs of soft tissue or bone infection. In 42 patients 67Ga SPECT/CT showed markedly increased bone uptake, consistent with a diagnosis of osteomyelitis. All the included patients with positive imaging were men (93%), with a mean ± SD age of 63 ± 10 years and duration of diabetes of 20.3 ± 11.3 years, and most (80%) had type 2 diabetes (Table 1). All patients had evidence of peripheral neuropathy (loss of protective sensation) of the feet, 60% had lower-limb arterial insufficiency (ankle brachial pressure index <0.9), and 42% had already had a distal limb amputation. During the year of follow-up, 3 (5.5%) of the patients died and 3 (all of whom had a positive bone puncture culture) underwent lower-extremity amputation of the affected limb.
Figure 1.
Flowchart.
Table 1.
Baseline demographic and clinical characteristics of patients who underwent bone puncture for culture by culture results
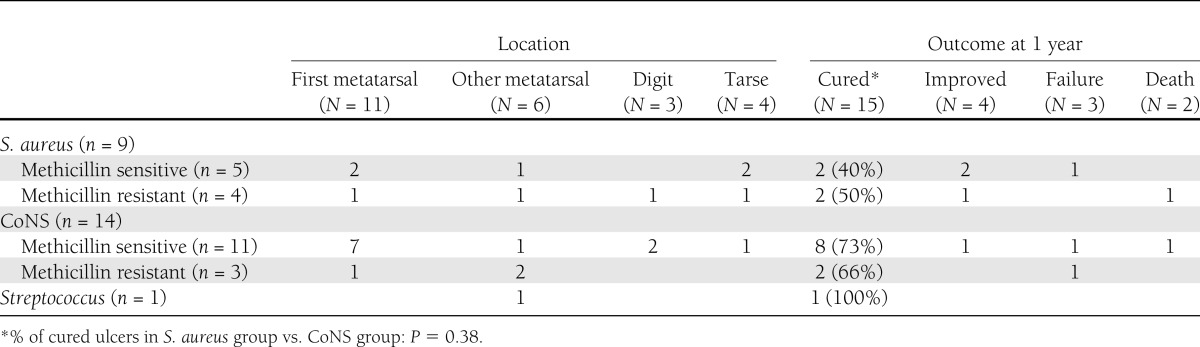
We performed bone puncture on the 42 patients with positive 67Ga SPECT/CT imaging; 4 patients underwent bone puncture twice for a total of 46 bone punctures. Only one patient required local anesthesia for the bone puncture. We excluded the results of three bone punctures in two different patients from the analysis because these patients had received antibiotic therapy in the previous 14 days. Among the 40 patients whose bone punctures we included, 19 were culture negative. We deemed three of these cultures to be false negatives because in each case a second bone puncture carried out within a month grew pathogens. In total, 24 patients had bone cultures that were positive: the isolates were coagulase-negative Staphylococcus species (CoNS) in 14, Staphylococcus aureus in 9, and a nutritionally deficient Streptococcus in 1. CoNS species were isolated from bone cultures after a mean growth period of 1.5 ± 0.82 days, and 9 of the 14 grew within 24 h, as did all the S. aureus isolates. Only one culture was polymicrobial, containing two methicillin-resistant S. aureus strains. We found antibiotic-resistant strains in 8 of the 24 isolates (33%): three methicillin-resistant CoNS species (21% of the CoNS isolates), 1 fluoroquinolone-resistant CoNS species, and 5 methicillin- and fluoroquinolone-resistant S. aureus strains (44% of the S. aureus isolates).
All the culture-positive patients were treated with antibiotic therapy that consisted of a combination of ofloxacin (200 mg twice daily) and rifampicin (10 mg/kg twice daily) for fluoroquinolone-sensitive isolates in 18 patients, linezolid alone (600 mg twice daily) for the fluoroquinolone-resistant isolates in 6 patients, and amoxicillin (1 g three times a day) plus rifampicin (10 mg/kg twice daily) for the 1 patient with a Streptococcus isolate.
Among the patients who had died at follow-up (two of myocardial infarction and one of multiple organ failure), one was from the group with a negative bone culture and two were from the group with a positive bone culture (one methicillin-resistant S. aureus and one methicillin-sensitive CoNS). At follow-up, none of the 13 patients who had negative results on 67Ga SPECT/CT (who had no antibiotic treatment) showed clinical evidence of infection, and all their ulcers had resolved. Similarly, among the 16 patients with a true-negative bone culture, 15 had no evidence of infection and a resolved ulcer at follow-up; 1 had died of a myocardial infarction. In the positive bone culture group, 19 of the 24 patients had no clinical signs of infection at follow-up (15 ulcers were completely cured and 4 had improved), 3 patients had undergone amputation surgery of an infected metatarsal (first, third, and fifth metatarsal head), and 2 patients had died (Table 2).
We observed no adverse events in any patient after either 67Ga SPECT/CT imaging or bone puncture. All patients with positive bone cultures completed their antibiotic treatments; however, 13 had the following antibiotic-associated adverse effects: gastrointestinal disturbances in 6 patients receiving the ofloxacin plus rifampicin regimen (resulting in the discontinuation of rifampicin in 2 cases) and anemia in 7 patients receiving linezolid (which was not severe enough to require treatment discontinuation).
CONCLUSIONS
Accurate diagnosis of diabetic foot osteomyelitis and identification of the causative pathogens should lead to better outcomes for this highly morbid infection. Because all currently available diagnostic tests have some deficiencies, newer diagnostic schemes are needed. The coupling of 67Ga SPECT/CT imaging with subsequent bedside bone puncture for those with a positive result has many potential advantages. This approach uses a highly accurate imaging test to help determine which patients should undergo bone sampling and then adds a simple bedside bone puncture that does not require concomitant imaging because the 67Ga SPECT/CT very precisely marks the bony part suspected of infection, even in the forefoot. In this prospective study of 55 patients with suspected diabetic foot osteomyelitis, we found this approach to be highly effective.
None of the 13 patients with negative 67Ga SPECT/CT imaging demonstrated any clinical sign of bone infection after ≥1 year of follow-up, giving a sensitivity for this imaging test alone of 100%. Among the 29 patients whom we presumed not to have osteomyelitis (the 13 who had negative 67Ga SPECT/CT imaging and no osteomyelitis on follow-up and the 16 who had a negative bone culture), 13 had negative imaging, for a specificity of 45% for imaging alone. The negative predictive value for 67Ga SPECT/CT imaging was 100%, similar to that of MRI (90–100%) (26). Use of 67Ga SPECT/CT made it possible to avoid bone puncture in 13 of 55 cases (23.6%). Thus this imaging technique appears to be at least as accurate as other relatively new imaging methods, such as [18F]fludeoxyglucose positron emission tomography and 99mTc-labeled monoclonal antigranulocyte antibody scintigraphy (27). Furthermore, the combined imaging and bone puncture technique enabled us to avoid antibiotic therapy in 55% of our patients with suspected osteomyelitis (29 of 55).
For diabetic patients with suspected osteomyelitis, virtually all guidelines recommend plain radiography as the first line of imaging investigation. Although these tests may be useful in patients at high risk for bone infection, their overall sensitivity and specificity have been relatively low in most studies (28–30). Thus in special subgroups of patients with normal or indeterminate radiographic results, advanced imaging methods may be needed (24,29,31,32). Among these, MRI has been the preferred technique; however, its specificity is also limited by other entities that give a similar appearance, especially neuro-osteoarthropathy (Charcot foot). Radionuclide bone scans have good diagnostic sensitivity (generally >90%) (33) but rather poor specificity (∼40%) related to increased uptake with any form of inflammation. Leukocyte scans are more specific but have poor resolution and are difficult to perform (33).
The hybrid imaging SPECT/CT procedure provides the clinician with two types of information: functional (scintigraphic uptake) and anatomic (low-dose X-ray tomographic) definition. When the radiotracer used is specific for activated macrophages, as is the case for 67Ga, this method can determine the precise location of inflammation within a bone, even in the forefoot. In a general population, 67Ga SPECT/CT was found to be as useful for the diagnosis and localization of osteomyelitis as the best nuclear test, 111In-labeled leukocyte scintigraphy (specificities of 55 and 48% respectively; P > 0.05) in a series of 12 suspected cases (23). More recently, Heiba et al. (18) demonstrated the ability of SPECT/CT imaging to discriminate soft tissue from bone infection specifically in patients with a diabetic foot infection. Moreover, Gotthardt et al. (34) suggested SPECT/CT as an alternative to positron emission tomography or MRI for diagnosing peripheral osteomyelitis, although no previous study has assessed SPECT/CT in the distal forefoot. In our patients, 67Ga SPECT/CT was sufficiently accurate for precise localization of the affected bone part, even in the proximal phalanx of toes. Thus, 67Ga SPECT/CT may be better than MRI for marking the precise site of inflammation in suspected osteomyelitis, helping to plan the bedside bone puncture.
Bone puncture has advantages relative to standard bone biopsy: it is a bedside procedure that does not require concomitant imaging; it uses a trocar smaller than the type used for bone biopsy; and bone marrow aspiration is probably less traumatic than bone biopsy. Although bone biopsy is considered to be the criterion standard for diagnosing osteomyelitis, it is rarely performed in most clinical settings. This is probably because it generally requires specialized surgical or radiological facilities, it is seen as time-consuming and expensive, and some fear adverse effects (15). Our experience suggests that a bone puncture with bone aspiration may be as useful as a bone biopsy, at least for culture although not for histopathological studies. It may be especially useful compared with biopsy for small bones in the forefoot.
A bone aspirate sample can be cultured in blood culture bottles. The volume of bone marrow obtained is dependent on the size of the bone sampled. We found that negative bone culture results were more frequent for phalanges than for the first metatarsal or tarsal bones (Table 2), but the number of patients in our study was small. Studies of the microbiological etiology of foot osteomyelitis in diabetic patients have mostly revealed polymicrobial infection, with a mean of 1.35–1.54 isolates per sample noted in recent series (35,36). All but one of our positive cultures yielded a single gram-positive microorganism, almost all staphylococci. CoNS species are well documented as primary pathogens in diabetic foot osteomyelitis (35,37,38). In diabetic foot infection, monomicrobial staphylococcal culture is associated with a lower risk of amputation than other microbial causes (39). In our patients, the amputation rate was low (3 of the 24 cases of osteomyelitis), but about three quarters of our patients were outpatients and therefore probably had relatively less severely infections.
Table 2.
Microorganisms isolated on bone puncture and outcome after ≥1 year of follow-up
The bone puncture procedure has two potential limitations. First, it is essentially blind, unlike surgical or radiologically guided bone biopsy. The high quality of 67Ga SPECT/CT images makes it possible to localize the infection very precisely, however, even in the forefoot (Figs. 2 and 3). Furthermore, because the bones of the foot are located just under the skin, they can be reached percutaneously without the need for imaging guidance techniques. Second, it uses broth cultures rather than solid agar; however, recent studies of bone samples for hip and knee prosthetic infections have shown that the use of blood culture bottles was associated with good specificity (40).
Figure 2.
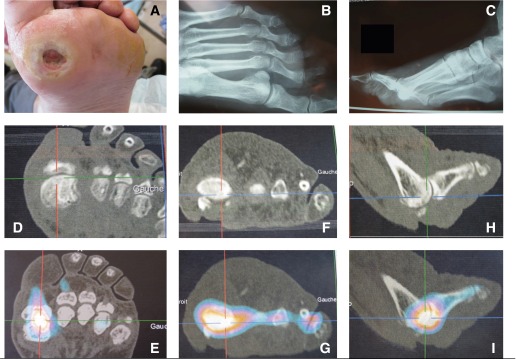
Chronic ulcer of the first metatarsal head. A: Photograph. B and C: Plain radiographs. D, F, and H: Three-dimensional X-ray tomographic images. E, G, and I: Corresponding 67Ga SPECT/CT images. The gallium accumulation marks the best puncture place.
Figure 3.
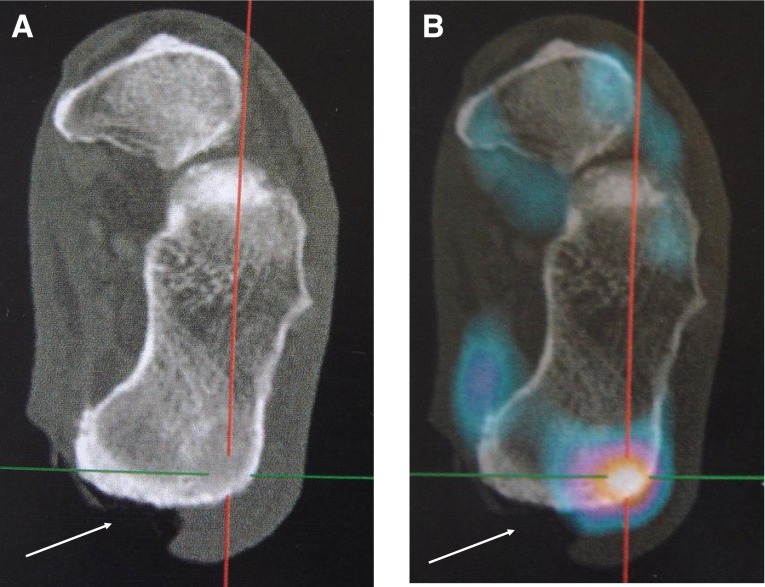
Example of a large calcaneus ulcer (white arrow) with a marked 67Ga uptake near but not under the ulcer. The precise site of puncture could not have been determined without 67Ga spotting. A: Three-dimensional X-ray tomographic image. B: Corresponding 67Ga SPECT/CT image.
The primary end point for our study was the absence of clinical signs of osteomyelitis after ≥1 year of follow-up. Because patients with a negative 67Ga SPECT/CT scan did not undergo bone puncture, we cannot be certain that there were no false-negative scans. The long-term follow-up of all cases, however, makes this highly unlikely. Because all patients with positive bone cultures received antibiotic treatment, it was not possible to exclude false-positive cases in this study. It is not impossible that some patients have osteomyelitis that resolves and wounds that heal without antibiotic therapy, especially in lesser toes. From the frequency of CoNS as a cause of false-positive blood cultures during the same period in our laboratory (3.3%), we can extrapolate to obtain the rate of false-positive cultures as 0.033 × 43 positive bone cultures, giving 1.4 cases (2 in our count to evaluate specificity). Applying these data, the sensitivity of the procedure should be no worse than 88%, its specificity 93.6%, and its positive and negative predictive values 91.7 and 90.7%, respectively. The Youden index was 0.82 for the imaging plus puncture combined vs. 0.45 for 67Ga SPECT/CT alone, supporting the superiority of the coupled proceedings.
This coupled technique has several important advantages. The SPECT/CT scan allows a substantial minority of patients to avoid bone puncture, although MRI would probably give similar results because the two methods have the same sensitivity. 67Ga SPECT/CT has a similar cost to MRI and is widely available in France, both in public and private hospitals. The bone puncture procedure can be carried out at the patient’s bedside, by a clinician, without the need for an orthopedic surgeon or radiologist and imaging equipment. It is also less expensive than surgically or radiologically guided procedures. We therefore believe that coupling 67Ga SPECT/CT with bedside bone puncture is an efficient procedure for the diagnosis of foot osteomyelitis in diabetic patients without signs of soft tissue infection. This method is simple, safe, and ambulatory, and it avoided the unnecessary use of antibiotics in more than half of the suspected osteomyelitis cases in our study.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
E.A. and B.A.L. wrote the manuscript. J.M., N.C.-V., and S.C. contributed to data collection and analysis. E.L. and C.B. contributed to discussion and reviewed the manuscript. E.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Claire Le Jeunne, Université Paris Descartes, Paris, France, for help during all stages of the work.
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 2.Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract 2009;83:347–352 [DOI] [PubMed] [Google Scholar]
- 3.Richard JL, Lavigne JP, Got I, et al. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab 2011;37:208–215 [DOI] [PubMed] [Google Scholar]
- 4.Carmona GA, Hoffmeyer P, Herrmann FR, et al. Major lower limb amputations in the elderly observed over ten years: the role of diabetes and peripheral arterial disease. Diabetes Metab 2005;31:449–454 [DOI] [PubMed] [Google Scholar]
- 5.Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–1293 [DOI] [PubMed] [Google Scholar]
- 6.Edmonds M. Double trouble: infection and ischemia in the diabetic foot. Int J Low Extrem Wounds 2009;8:62–63 [DOI] [PubMed] [Google Scholar]
- 7.Berendt AR, Peters EJ, Bakker K, et al. Specific guidelines for treatment of diabetic foot osteomyelitis. Diabetes Metab Res Rev 2008;24(Suppl. 1):S190–S191 [DOI] [PubMed] [Google Scholar]
- 8.Dinh T, Snyder G, Veves A. Current techniques to detect foot infection in the diabetic patient. Int J Low Extrem Wounds 2010;9:24–30 [DOI] [PubMed] [Google Scholar]
- 9.Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg 2009;48:39–46 [DOI] [PubMed] [Google Scholar]
- 10.Lipsky BA. Bone of contention: diagnosing diabetic foot osteomyelitis. Clin Infect Dis 2008;47:528–530 [DOI] [PubMed] [Google Scholar]
- 11.Teh J, Berendt T, Lipsky BA. Rational Imaging. Investigating suspected bone infection in the diabetic foot. BMJ 2009;339:b4690. [DOI] [PubMed] [Google Scholar]
- 12.Mutluoglu M, Uzun G, Turhan V, Gorenek L, Ay H, Lipsky BA. How reliable are cultures of specimens from superficial swabs compared with those of deep tissue in patients with diabetic foot ulcers? J Diabetes Complications 2012;26:225–229 [DOI] [PubMed] [Google Scholar]
- 13.Berendt AR, Peters EJ, Bakker K, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev 2008;24(Suppl. 1):S145–S161 [DOI] [PubMed] [Google Scholar]
- 14.Senneville E, Lombart A, Beltrand E, et al. Outcome of diabetic foot osteomyelitis treated nonsurgically: a retrospective cohort study. Diabetes Care 2008;31:637–642 [DOI] [PubMed] [Google Scholar]
- 15.Powlson AS, Coll AP. The treatment of diabetic foot infections. J Antimicrob Chemother 2010;65(Suppl. 3):iii3–iii9 [DOI] [PubMed] [Google Scholar]
- 16.Loredo R, Rahal A, Garcia G, Metter D. Imaging of the diabetic foot diagnostic dilemmas. Foot Ankle Spec 2010;3(Spec):249–264 [DOI] [PubMed] [Google Scholar]
- 17.Ertugrul MB, Baktiroglu S, Salman S, et al. The diagnosis of osteomyelitis of the foot in diabetes: microbiological examination vs. magnetic resonance imaging and labelled leucocyte scanning. Diabet Med 2006;23:649–653 [DOI] [PubMed] [Google Scholar]
- 18.Heiba SI, Kolker D, Mocherla B, et al. The optimized evaluation of diabetic foot infection by dual isotope SPECT/CT imaging protocol. J Foot Ankle Surg 2010;49:529–536 [DOI] [PubMed] [Google Scholar]
- 19.Papanas N, Zissimopoulos A, Maltezos E. The role of nuclear medicine in the diagnosis of common and specific diabetic infections. Hell J Nucl Med 2010;13:150–157 [PubMed] [Google Scholar]
- 20.Filippi L, Uccioli L, Giurato L, Schillaci O. Diabetic foot infection: usefulness of SPECT/CT for 99mTc-HMPAO-labeled leukocyte imaging. J Nucl Med 2009;50:1042–1046 [DOI] [PubMed] [Google Scholar]
- 21.Horger M, Eschmann SM, Pfannenberg C, et al. The value of SPET/CT in chronic osteomyelitis. Eur J Nucl Med Mol Imaging 2003;30:1665–1673 [DOI] [PubMed] [Google Scholar]
- 22.Ando A, Nitta K, Ando I, et al. Mechanism of gallium 67 accumulation in inflammatory tissue. Eur J Nucl Med 1990;17:21–27 [DOI] [PubMed] [Google Scholar]
- 23.Bar-Shalom R, Yefremov N, Guralnik L, et al. SPECT/CT using 67Ga and 111In-labeled leukocyte scintigraphy for diagnosis of infection. J Nucl Med 2006;47:587–594 [PubMed] [Google Scholar]
- 24.Société de Pathologie Infectieuse de Langue Française Management of diabetic foot infections. Long text. Société de Pathologie Infectieuse de Langue Française. Med Mal Infect 2007;37:26–50 [in French] [DOI] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Regression towards the mean. BMJ 1994;308:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig JG, Amin MB, Wu K, et al. Osteomyelitis of the diabetic foot: MR imaging-pathologic correlation. Radiology 1997;203:849–855 [DOI] [PubMed] [Google Scholar]
- 27.Schwegler B, Stumpe KD, Weishaupt D, et al. Unsuspected osteomyelitis is frequent in persistent diabetic foot ulcer and better diagnosed by MRI than by 18F-FDG PET or 99mTc-MOAB. J Intern Med 2008;263:99–106 [DOI] [PubMed] [Google Scholar]
- 28.Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:1679–1684 [DOI] [PubMed] [Google Scholar]
- 29.Lipsky BA, Peters EJ, Senneville E, et al. Expert opinion on the management of infections in the diabetic foot. Diabetes Metab Res Rev 2012;28(Suppl. 1):163–178 [DOI] [PubMed] [Google Scholar]
- 30.Tan T, Shaw EJ, Siddiqui F, Kandaswamy P, Barry PW, Baker M, Guideline Development Group Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ 2011;342:d1280. [DOI] [PubMed] [Google Scholar]
- 31.Apelqvist J, Bakker K, van Houtum WH, Schaper NC, International Working Group on the Diabetic Foot (IWGDF) Editorial Board The development of global consensus guidelines on the management of the diabetic foot. Diabetes Metab Res Rev 2008;24(Suppl. 1):S116–S118 [DOI] [PubMed] [Google Scholar]
- 32.Apelqvist J, Bakker K, van Houtum WH, Schaper NC, International Working Group on the Diabetic Foot (IWGDF) Editorial Board Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) Prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev 2008;24(Suppl. 1):S181–S187 [DOI] [PubMed] [Google Scholar]
- 33.Palestro CJ, Love C. Nuclear medicine and diabetic foot infections. Semin Nucl Med 2009;39:52–65 [DOI] [PubMed] [Google Scholar]
- 34.Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med 2010;51:1937–1949 [DOI] [PubMed] [Google Scholar]
- 35.Senneville E, Melliez H, Beltrand E, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: concordance with ulcer swab cultures. Clin Infect Dis 2006;42:57–62 [DOI] [PubMed] [Google Scholar]
- 36.Senneville E, Morant H, Descamps D, et al. Needle puncture and transcutaneous bone biopsy cultures are inconsistent in patients with diabetes and suspected osteomyelitis of the foot. Clin Infect Dis 2009;48:888–893 [DOI] [PubMed] [Google Scholar]
- 37.Armstrong DG, Lanthier J, Lelievre P, Edelson GW. Methicillin-resistant coagulase-negative staphylococcal osteomyelitis and its relationship to broad-spectrum oral antibiosis in a predominantly diabetic population. J Foot Ankle Surg 1995;34:563–566 [DOI] [PubMed] [Google Scholar]
- 38.Lavery LA, Sariaya M, Ashry H, Harkless LB. Microbiology of osteomyelitis in diabetic foot infections. J Foot Ankle Surg 1995;34:61–64 [DOI] [PubMed] [Google Scholar]
- 39.Lipsky BA, Weigelt JA, Sun X, Johannes RS, Derby KG, Tabak YP. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care 2011;34:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothman MDA 2011. Presented at the 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), 7–10 May 2011, Milan, Italy [Google Scholar]