Tregitopes are capable of inducing tolerance, but lack immunogenicity in vivo, suggesting they are safe for future clinical applications.
Keywords: Treg, IVIg, immunomodulation
Abstract
Tregitopes are a set of epitopes, derived from IgG, that bind to MHCII, activate nTregs, and promote tolerance. We have now confirmed that coadministration of Tregitopes with a range of proteins (autoantigens and nominal antigens, such as OVA) in vitro and in vivo leads to suppression of T cell and antibody responses to the test antigens. In this study, we demonstrate that Tregitopes are not immunogenic in vivo even when emulsified with strong adjuvants, such as IFA or CFA. Moreover, in vivo administration of Tregitopes with IFA or CFA does not induce Th1 or Th2 cytokine expression under restimulation conditions in vitro. We investigated tolerance induction by codelivering Tregitopes with OVA using B cells. When B cells were pulsed with OVA plus Tregitopes and transferred into naïve mice, we found that cellular and humoral immune responses to the OVA were suppressed. As a result of their ability to induce Tregs and the absence of immunogenicity in the context of strong adjuvants, Tregitopes might be considered a novel immunomodulatory approach for the suppression of immune responses to protein therapeutics (such as FVIII and mAb), as well as for treatment of autoimmune diseases.
Introduction
Antigen-specific immunotherapies for tolerance induction are desirable for autoimmune diseases, transplantation, and allergy, as well as for protein therapeutics that have been associated with clinical immunogenicity. Tolerance to self-antigens is controlled through multiple central and peripheral mechanisms. Among them, Tregs play an essential role in controlling peripheral tolerance [1]. CD4+CD25+FoxP3+ nTregs, once activated through the TCR, suppress effector T cells through contact-dependent and -independent mechanisms [2]. Accumulating evidence suggests that nTreg-deficient patients and animals exhibit severe immune deficiencies and sometimes lethal inflammatory conditions [3–5]. Therefore, induction or activation of nTregs may be an ideal approach for therapy of autoimmune diseases. One aspect of nTreg immune responses that remains unresolved is whether their effects are antigen-specific. Much effort has been made to induce or expand antigen-specific nTregs for the purpose of immunotherapy. However, because of the lack of knowledge about the epitopes recognized by nTregs, no effective strategy has been identified to date.
Several lines of evidence suggest that epitopes for nTregs exist in IgG. First, high-dose therapeutic IVIg has been widely used in the clinic to treat multiple autoimmune diseases [6–8]. Whereas several non-Treg-related mechanisms of action of IVIg have been proposed—such as autoantibody neutralization, modulation of expression and function of FcRs, interference with activation of complement and the cytokine network, and modulation of DC maturation and function [9]—additional studies suggest that IVIg also works through Tregs. For example, in an animal model for multiple sclerosis (EAE), prophylactic infusion of IVIg expanded Tregs and prevented disease development in a Treg-dependent manner [10]. Secondly, IgG Fc-containing fusion proteins have been used to induce tolerance in animal models for T1D, EAE, and hemophilia A, via protein or gene therapy [11–14]. In gene therapy animal models of hemophilia A and T1D, we have shown that IgG fusion-induced tolerance depends on nTregs [15, 16].
Tregitopes were first identified when mAb was screened for T cell epitope content. The modulation of the anti-self immune response to antibody hypervariable regions (CDRs) has been the subject of intensive study. To shed light on this phenomenon, we scanned human and murine IgG sequences for T cell epitopes using the EpiMatrix and ClustiMer software algorithms [17]. Several highly conserved, promiscuous T helper epitopes were identified in the Fc and Fab regions of IgG. These were defined as Tregitopes, based on their ability to suppress T cell responses in vitro [17]. hTregitope167 and hTregitope289 contain multiple HLA class II-binding motifs and have been shown to bind to murine and human MHC [17, 18]. In vitro studies demonstrated that these two Tregitopes could activate nTregs and expand inducible Tregs [17]. We have also demonstrated that mTregitope167 and mTregitope289 induced expansion of FoxP3+ Tregs in a transgenic (DO11.10) mouse model and suppressed the antigen-specific response [19, 20]. More importantly, when diabetes-prone NOD mice were treated with mTregitope167 and mTregitope289 at the onset of diabetes, hyperglycemia was reversed. These results suggest that Tregitopes have excellent immunosuppressive properties that are possibly a result of their ability to activate Tregs [20]. Overall, our data indicate that Tregitopes comprise an excellent candidate for immunomodulation in the context of autoimmunity and/or transplantation.
In this study, we investigated the immunogenicity of Tregitopes, focusing on mTregitope167 (stronger H-2b-binding affinity) and mTregitope289 (stronger H-2d-binding affinity). These peptides exhibited limited or no immunogenicity in vivo, even when emulsified with CFA or IFA. We also showed that Tregitopes are capable of suppressing immune-responsiveness to OVA when codelivered with the antigen via B cells. Our results indicate that as an immune-suppression reagent, Tregitopes have very weak immunogenicity that allows for the observed immunomodulatory effect and for their therapeutic potential.
MATERIALS AND METHODS
Mice
C57BL/6 and BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All recipient animals were used at 5–6 weeks of age and housed in pathogen-free microisolator cages in our animal facility.
Peptides and antibodies
mTregitope167, mTregitope289, and OVA323–339 peptides were synthesized by New England Peptide (Gardner, MA, USA). Anti-IFN-γ capture and detection antibodies were purchased from BD Biosciences (San Jose, CA, USA).
B cell purification, Tregitope pulsing, and tolerance induction
B cells were purified from spleens to ∼95% homogeneity with anti-T cell antibody cocktails (anti-Thy1, anti-CD4, and anti-CD8), followed by complement (Low-Tox-M; Cedarlane, Burlington, Ontario, Canada). Purified B cells were cultured with RPMI-1640 medium (Gibco; Invitrogen, Carlsbad, CA, USA), supplemented with 5% FBS, 2 mM L-glutamine, and 2-ME. B cells were prestimulated with 5 μg/ml bacterial LPS (Escherichia coli 055:B5; Sigma, St. Louis, MO, USA) overnight. LPS-stimulated B cells were incubated with 100 μg/ml mTregitope167 and mTregitope289, 1 mg/ml OVA protein, or 100 μg/ml mTregitope167 and mTregitope289 plus 1 mg/ml OVA protein for 2 h at 37°C. Cells were washed three times with PBS before transfer of 107 cells/mouse into syngeneic mice i.p. Seven to 10 days following injection, animals were immunized in a hind footpad and base of the tail with 25 μg OVA323–339 peptide emulsified in CFA. Two weeks later, mice were euthanized, sera were collected, and draining inguinal and popliteal LNs were removed. Cellular and humoral responses were examined by Elispot and ELISA, respectively. Elispot plates were coated with 1 μg/ml anti-IFN-γ capture antibody overnight. LN cells were seeded at 5 × 105/well in a 96-well plate in the presence of the indicated concentration of OVA323–339 peptide. After 48 h, Elispot plates were developed using biotinylated anti-IFN-γ antibody, followed by HRP-conjugated anti-rat IgG. Elispot plates were read on an ImmunoSpot Elispot counter (Cellular Technologies, Shaker Heights, OH, USA). Antibody titers were determined by the endpoint dilution method. Serial 3-fold dilutions were made in PBS + 2% BSA. The endpoint titer represents the highest dilution of sample with an OD450 reading greater than twofold of blank control.
Measurement of immune response
Tregitopes (mixture of mTregitope289 and mTregitope167) were emulsified with IFA or CFA and injected into C57BL/6 or BALB/c mice in one footpad. Each mouse was injected with 12.5 μg mTregitope167 and mTregitope289, with the same amount of OVA323–339 peptide (known binder for MHC IAb and IAd) used as a control. Two weeks later, draining popliteal and inguinal LNs were removed and assayed for cell proliferation by [3H] thymidine incorporation. LN cells were seeded at 5 × 105/well in a 96-well plate in the presence of the indicated concentration of antigen. After 48 h, cultures were pulsed with 1 μCi/well [3H] thymidine and incubated for an additional 16–20 h. Cells were then harvested on glass fiber filters, and incorporated [3H] thymidine was detected via liquid scintillation counting in a Microbeta2 Plate reader (Perkin Elmer, Boston, MA, USA). Data are expressed as Δcpm (by subtraction of the background without antigen). Before pulsing cell cultures with [3H] thymidine, supernatants from a 2-μg/ml antigen culture were collected for a cytokine assay. Production of IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, and IFN-γ was determined by ELISA in the Cytokine Core Laboratory at the University of Maryland (Baltimore, MD, USA).
Real-time PCR
C57BL/6 mice were immunized with a mixture of mTregitope167 and mTregitope289, OVA323–339, or PBS in IFA in a footpad. Two weeks later, draining LNs were isolated, and cells were treated in vitro with mTregitope167 and mTregitope289 or OVA323–339 peptide at 2 μg/ml. Forty-eight hours later, total RNA samples were extracted with a PureLink RNA Mini Kit (Ambion; Life Technologies, Carlsbad, CA, USA), followed by DNAse treatment (Turbo DNA-free; Ambion; Invitrogen, Life Technologies, Carlsbad, CA, USA) and transcription into cDNA using the SuperScript VILO cDNA Synthesis Kit (Life Technologies). FAM probes for detecting the expression of T-bet, GATA-3, RORγt, and Foxp3 were ordered from Life Technologies (TaqMan Gene Expression assays), and quantitative RT-PCR (10 min at 95°C and 45 cycles of 10 s at 95°C, 30 s at 60°C, and 1 s at 72°C) was performed in the LightCycler 480 system (Roche, Basel, Switzerland). Amplification was done in a total volume of 20 μl using TaqMan Gene Expression Master Mix (Applied Biosystems; Life Technologies). For each sample, mRNA expression was normalized to the detected Ct value of GAPDH. The experiment was performed at the Biomedical Instrumentation Center of the Uniformed Services University of the Health Sciences.
Statistics
Paired or unequal variance one-tailed Student's t-test statistics were applied where indicated.
RESULTS
mTregitope167 and mTregitope289 delivered by B cells suppress immune response to OVA protein in vivo
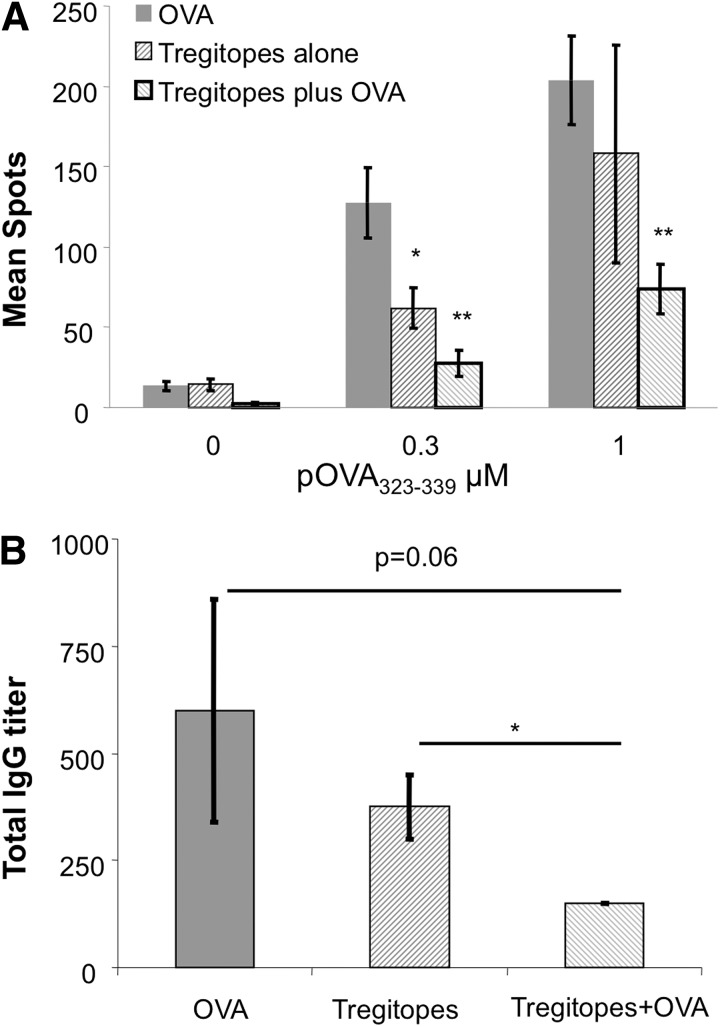
We showed previously that Tregitopes induce Treg expansion in a TCR transgenic mouse model and suppress disease progression in a T1D model [20]. In the latter model, we treated NOD mice with Tregitopes emulsified in IFA when the mice became hyperglycemic and found that Tregitope treatment could reverse new onset diabetes in the majority of the mice. As IFA is not an ideal adjuvant for the clinic, in this study, we first asked whether Tregitopes could be delivered by other routes for immune suppression. We have used a protocol for tolerance induction that uses B cells as APCs [21, 22]. This method of inducing tolerance by retrovirally transduced B cells with IgG-antigen fusions has been used in a variety of disease models, such as hemophilia A [15], T1D [16, 23], EAE [23–25], and experimental autoimmune uveoretinitis [26]. Thus, we hypothesized that antigen-pulsed B cells might also deliver Tregitopes to effectively induce tolerance in vivo. Splenic B cells were activated overnight with the TLR agonist LPS and pulsed with Tregitopes (mTregitope167 and mTregitope289), OVA protein, or Tregitopes plus OVA protein the following day. These B cells were then adoptively transferred into naïve, syngeneic C57BL/6 mice. One week later, recipients were immunized with OVA323–339 peptide in CFA in the footpad. Two weeks later, T cells from draining popliteal and inguinal LNs were cultured with OVA323–339 peptide for evaluation of cellular response by IFN-γ Elispot. In parallel, serum samples were assayed for anti-OVA IgG titer by ELISA. As shown in Fig. 1A compared with OVA protein-pulsed B cells, treating mice with Tregitope-pulsed B cells led to a significant reduction in IFN-γ production in the ex vivo restimulation assay. T cells from mice treated with Tregitopes and OVA protein-pulsed B cells responded with lower levels of IFN-γ production than T cells from mice treated with Tregitope-pulsed B cells alone, suggesting that coadministration of Tregitopes with the target antigen promotes antigen-specific hyporesponsiveness. The antibody responses showed the same trend; lower antibody responses were observed in mice treated with Tregitopes plus OVA protein (Fig. 1B) compared with mice treated with Tregitopes alone. Collectively, these data indicate that Tregitope peptides, delivered by B cells, with and without target antigen, are capable of inducing hyporesponsiveness.
Figure 1. Tregitopes/OVA-pulsed B cells reduce anti-OVA response in vivo.
(A) LPS-activated C57BL/6 B cells were pulsed with Tregitopes, OVA protein, or Tregitopes plus OVA protein in vitro, and then 107-treated B cells were transferred into naïve C57BL/6 recipients. On Day 7 postinjection, animals were immunized in a hind footpad and the base of tail with 25 μg OVA323–339 peptide (pOVA323–339) emulsified in CFA. Two weeks postimmunization, animals were killed, and draining LNs were removed. T cell proliferation was assayed by measuring IFN-γ production by Elispot. Cells were incubated for 48 h with indicated concentrations of OVA323–339 peptide. Data represent mean spots ± se for four animals. This is a representative of two independent experiments (**P<0.01; *P≤0.05). (B) Treatment is same as A. Sera were collected 2 weeks after immunization and analyzed by ELISA, and ELISA plates were coated with 10 μg/ml OVA protein. Data represent mean anti-IgG titers ± se for four animals.
mTregitope167 and mTregitope289 have no immunogenicity in vivo
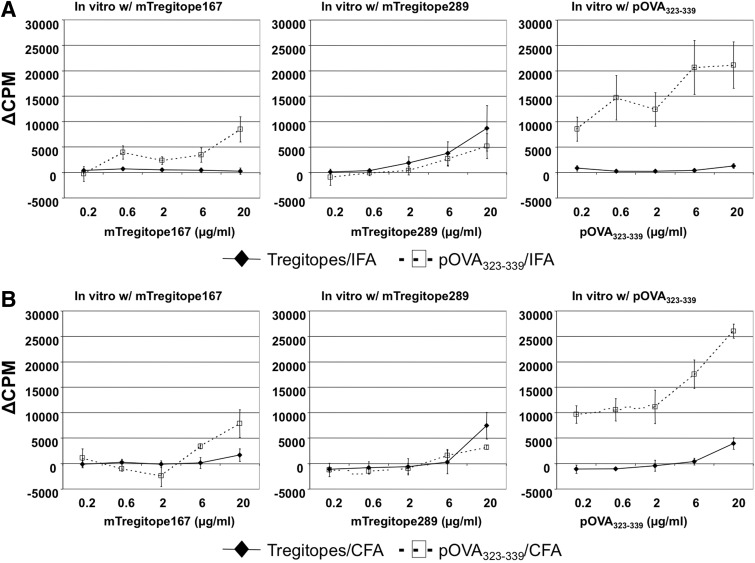
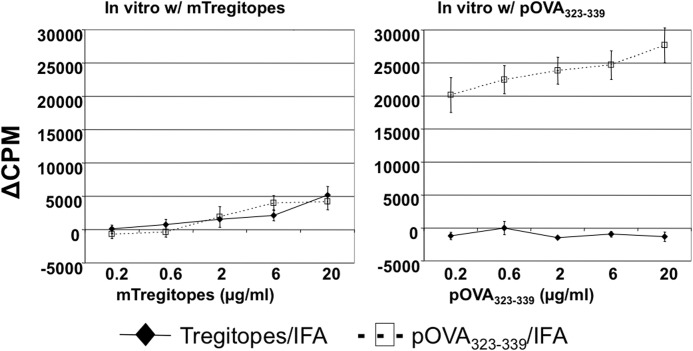
One of the major hurdles preventing some therapeutic proteins from being used in the clinic is their immunogenicity. For example, recombinant clotting FVIII is used routinely as replacement therapy in hemophilia A patients. However, up to 30% of patients develop anti-FVIII inhibitory antibodies [27]. The conventional treatment of patients with inhibitory antibodies is high-dose and frequent administration of FVIII, a costly and inconvenient procedure. Thus, before applying Tregitopes in future clinical studies, we wished to evaluate their immunogenicity. We emulsified a mixture of mTregitope167 and mTregitope289 with IFA or CFA and immunized C57BL/6 mice. After 2 weeks, draining inguinal and popliteal LNs were removed and assayed for T cell responses to individual Tregitopes. OVA323–339 peptide was used as a positive, MHC-binding control. In vitro T cell proliferation against individual peptides (mTregitope167, mTregitope289, and OVA323–339) is summarized in Fig. 2. As shown, mice immunized with Tregitopes in IFA or CFA did not respond to mTregitope167, mTregitope289, or OVA323–339 peptide in vitro. In contrast, OVA323–339 peptide-immunized mice exhibited a robust response to only OVA323–339 peptide in vitro after immunization with either adjuvant. These data indicate that Tregitopes have low or no immunogenicity in vivo, even when coadministered with the strong adjuvant CFA. These findings were also validated in BALB/c mice; similar results were obtained (Fig. 3).
Figure 2. Tregitopes elicit a low or nonexistent immune response in vivo.
Tregitopes (mixture of mTregitope167 and mTregitope289) or OVA323–339 peptide were emulsified with IFA (A) or CFA (B) and then injected in one footpad of C57BL/6 mice. Two weeks later, draining popliteal and inguinal LNs were removed and assayed for T cell proliferation by [3H] thymidine incorporation. Cells (5×105) were seeded/well in 96-well plates in the presence of mTregitope167, mTregitope289, or OVA323–339 peptide. Forty-eight hours later, cells were pulsed with 1 μg/ml [3H] thymidine and cultured for another 16–20 h. Data represent mean Δcpm ± se for four animals. This is a representative of two independent experiments.
Figure 3. Tregitopes have a low or no immunogenicity in BALB/c mice.
BALB/c mice were treated with IFA-emulsified Tregitopes (mixture of mTregitope167 and mTregitope289) or OVA323–339 peptide. Two weeks later, T cell proliferation assay was performed (same as in Fig. 2). Cells (5×105) were seeded/well in 96-well plates in the presence of mTregitope167/mTregitope289 or OVA323–339 peptide. Forty-eight hours later, cells were pulsed with 1 μg/ml [3H] thymidine and cultured for another 16–20 h. Data represent mean Δcpm ± se for four animals. This is a representative of two independent experiments.
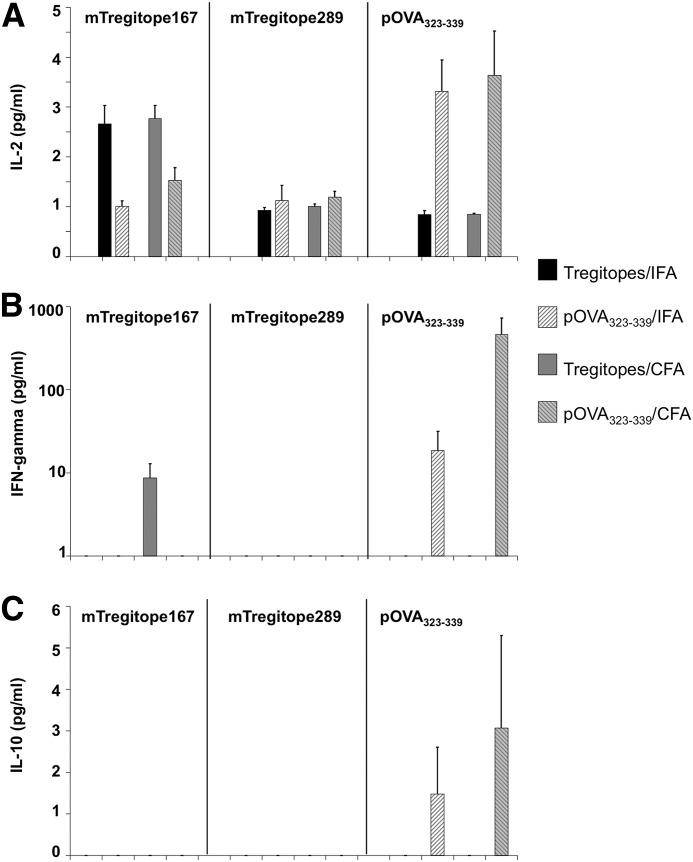
To further study the immunogenicity of Tregitopes, culture supernatants from the above experiments were collected and assayed for cytokine production, including IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, and IFN-γ. We observed similar levels of IL-2 production (1 pg/ml) in samples cultured with mTregitope289 (Fig. 4A, middle panel). Samples from Tregitopes/IFA- or Tregitopes/CFA-immunized mice did not show higher IL-2 production than OVA/IFA or OVA/CFA controls. When cultured with mTregitope167 in vitro, samples from Tregitopes/IFA- and Tregitopes/CFA-immunized mice exhibited a twofold increase in IL-2 production compared with the OVA/IFA or OVA/CFA controls exposed to mTregitope167, respectively (Fig. 4A, left panel). This was not unexpected, as we used C57BL/6 mice as recipients, whose H-2b MHC background better matches the binding restriction of mTregitope167. On the other hand, when cultured with OVA323–339 peptide, Tregitopes/IFA and Tregitopes/CFA produced much less IL-2 than the OVA/IFA and OVA/CFA controls, suggesting that the observed IL-2 production in vitro was antigen-specific (Fig. 4A, right panel).
Figure 4. Tregitopes elicit low or no Th1 and Th2 cytokine production.
Mice were treated as described in Fig. 2. Supernatants were collected from 2 μg/ml antigen-treated cells after 48 h cell culture. Samples were examined for the production of IL-2 (A), IFN-γ (B), and IL-10 (C) and IL-4, IL-5, IL-6, IL-12p40, and TGF-β (data not shown). Data represent mean concentration ± se for four animals. This is a representative of two similar experiments.
When cultured in the presence of OVA323–339 peptide, lymphocytes from OVA/IFA- and OVA/CFA-immunized mice secreted IFN-γ at 25 and 600 pg/ml, respectively. In contrast, neither the Tregitopes/IFA nor Tregitopes/CFA groups produced IFN-γ under these conditions (Fig. 4B, right panel). When cultured with mTregitope167, cells from Tregitopes/IFA-immunized mice did not produce IFN-γ, and even samples from mice immunized with Tregitopes/CFA only produced IFN-γ at <10 pg/ml (Fig. 4B, left panel), suggesting that Tregitopes are weak antigens and do not induce a Th1 response in vivo. None of the samples produced IFN-γ when cultured in vitro in the presence of mTregitope289 (Fig. 4B, middle panel). As shown in Fig. 4C, neither Tregitopes/IFA nor Tregitopes/CFA treatment induced IL-10 production under in vitro culture conditions, suggesting that no Th2 response was present. In addition, no IL-4, IL-5, IL-6, or IL-12p40 was detected from supernatants in any groups (data not shown).
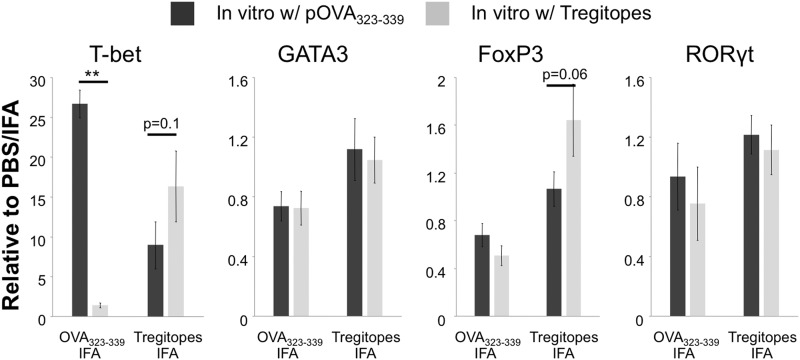
Finally, we validated the immunogenicity of Tregitopes at the transcription factor level. C57BL/6 mice were immunized with a mixture of mTregitope167 and mTregitope289 or OVA323–339 in IFA; PBS/IFA-immunized mice were used as a normalization control. Two weeks later, lymphocytes from the draining LNs were cultured with a mixture of mTregitope167 and mTregitope289 or OVA323–339, respectively. After 48 h, cells were collected, and RNA samples were extracted for assay by real-time PCR. We evaluated the expression of T-bet, GATA3, RORγt, and Foxp3, representing Th1, Th2, and Th17 cells and Tregs, respectively. As shown in Fig. 5, lymphocytes from mice immunized with Tregitopes only exhibited a modest increase in expression of T-bet when in vitro-stimulated with Tregitopes, compared with those stimulated with OVA323–339 peptide. In contrast, cells from OVA323–339 peptide-immunized mice exhibited a robust up-regulation of T-bet expression under the condition of OVA323–339 stimulation. These results are consistent with the IL-2 and IFN-γ production and the cell proliferation data. We also detected an up-regulated expression of FoxP3 in LN cells from Tregitope-treated mice when cultured in vitro with Tregitopes compared with the OVA323–339 condition (P=0.06). No difference has been found in the expression of GATA3 and RORγt under our experimental conditions. Together, these data confirmed that Tregitopes have limited, if any, immunogenicity.
Figure 5. Tregitopes have a modest effect on the expression of T-bet mRNA.
C57BL/6 mice were immunized with Tregitopes (mixture of mTregitope167 and mTregitope289; n=5), OVA323–339 peptide (n=4), or PBS (n=1) in IFA. Two weeks later, draining popliteal and inguinal LNs were removed, and cells were cultured with a mixture of Tregitopes or OVA323–339 peptide. Forty-eight hours later, RNA samples were extracted for detecting the expression of T-bet, GATA-3, RORγt, and Foxp3. Individual samples were normalized to the detected Ct values of control gene GADPH, and data were presented as the relative expression to the PBS/IFA control. Data represent mean ± se. This is a representative of two similar experiments (**P<0.01).
DISCUSSION
Here, we have demonstrated that Tregitopes, which have promising potential as immune-regulatory therapeutics, have little or no immunogenicity in vivo. We also studied the administration route of Tregitopes and found that B cell delivery of Tregitopes very effectively suppressed the immune response to the accompanying antigen in vivo. This study suggests that effective antigen presentation by professional APCs, such as B cells (or DCs), in the presence of immune-activating adjuvants, such as IFA or CFA, may be an effective means of delivering Tregitopes in future research applications. As neither IFA nor CFA is approved for human therapeutic use, and peptide therapies are notoriously difficult to formulate, other means of delivering the Tregitopes to APCs will be necessary to accelerate their path to the clinic.
A wide range of strategies that reduce the activity of autoreactive effector CD4+ T cells and increase Tregs in number or potency have been examined. Some have been applied as therapeutics and are undergoing clinical trials [28]. As Tregs play an essential role in immune regulation, induction or expansion of antigen-specific Tregs is one of the major directions under consideration for establishing antigen-specific tolerance. However, because of the lack of knowledge regarding the epitope specificity of Tregs, no effective means of achieving this goal has been developed thus far.
We reported previously that several Tregitopes, which are promiscuous MHCII epitopes for T cells, were able to activate CD4+FoxP3+ nTregs in human PBMCs in vitro and in vivo [17, 20]. Since that time, the potential applications of Tregitopes have been investigated in most of the animal models for allergy and autoimmune diseases. In a T1D model, we demonstrated that when NOD female mice were treated s.c. with Tregitopes in IFA at disease onset, Tregitope treatment reversed the hyperglycemia significantly [20]. Elyaman and colleagues [29] found that Tregitopes suppress EAE in a mouse model. In an allergy model, coadministration of OVA protein with Tregitopes reduced the allergic response to OVA and induced Tregs [Mazer and Massoud, personal communication, June, 2011]. Other collaborators have found that Tregitopes suppressed CD8+ T cell responses or reduced clinical scores in hemophilia B gene therapy [Mingozzi et al. (unpublished results)] and were effective in a model for Crohn's disease [30]. Based on these preliminary studies, which have been summarized recently [19], we believe that Tregitopes have significant potential to translate into clinical use as an immuno-regulatory therapeutic.
The immunogenicity of protein therapeutics, including humanized mAb, reduces their efficacy in clinical applications [31]. Some of these protein therapeutics are immune-activating; thus, we were curious as to whether Tregitopes would be immunogenic under inflammatory circumstances. Several intrinsic and extrinsic factors determine the immunogenicity of a protein therapeutic, including its origin (self vs. nonself), T cell epitope content, formulation, aggregation, post-translational modification, route of delivery, and patient HLA [31]. As Tregitopes are short peptides, the intrinsic factors (such as post-translational modification) that contribute to immunogenicity are less likely to be influential. Two common delivery routes of therapeutics in patients are i.v. and i.m. injection. The former favors tolerance, and the latter is prone to inducing immune responses. Therefore, we immunized mice with Tregitopes s.c. in the presence of CFA or IFA as a stringent test for immunogenicity, as both are well-known adjuvants for inducing immune responses in mice. However, even under such conditions, Tregitopes elicited little to no T cell response. In addition, we did not find significant Th1 and Th2 cytokine production, with the exception of Tregitopes/CFA-immunized mice, in which T cells produced minimal IFN-γ. To our surprise, we did not find any IL-10 production from T cells isolated from Tregitope-treated animals, as opposed to OVA peptide-immunized mice. We could not exclude the role of IL-10 played in Tregitope-induced immune suppression, as Tregitopes might stimulate the IL-10 production by APCs, such as B cells. However, our current data suggest that production of Th2 cytokines by T cells may not be a major mechanism by which Tregitopes function under this protocol. Indeed, we did not find any increase in GATA3 expression as a result of Tregitope treatment. Whether Tregitopes treatment increases the IL-10 production by APCs requires further verification, as it has been established that gene therapy with IgG fusion proteins requires host IL-10 production [32].
Our goals are to deliver Tregitopes effectively and to induce Tregs specifically. To achieve them, we have tested different protocols, including IFA emulsification, liposome delivery, multiple injections, different sites of injection, and coadministration or fusion with a target antigen [19]. In this study, we investigated whether B cells could effectively deliver Tregitopes to induce tolerance. B cells were chosen as Tregitope carriers, as our group has a successful record using them as tolerogenic APCs. When retrovirally transduced with an antigen-IgG fusion, B cells are able to induce antigen-specific tolerance in vivo [13, 14]. This gene therapy strategy has shown efficacy in multiple models of autoimmune diseases and hemophilia A inhibitor formation [15, 23, 24]. We have also shown that when pulsed in vitro with a target antigen fused with a TAT peptide, B cells are also capable of inducing tolerance to the target antigen. This protein therapy strategy has shown efficacy in EAE and T1D [33]. Therefore, we proposed that Tregitopes can be delivered with an accompanying antigen by B cells for tolerance induction. Indeed, our results have shown that B cells pulsed in vitro with Tregitopes alone or Tregitopes combined with OVA protein were able to reduce anti-OVA T cell and antibody responses in vivo, with codelivery exhibiting a stronger effect. Thus, our findings indicate that B cell delivery may be an effective delivery route for Tregitopes, although alternative routes may be more feasible for translation to the clinic. Moreover, our data could provide insights into the mechanisms of tolerance induction by gene therapy and/or IVIg infusion. We have shown previously that B cell presentation of peptide-IgG by the MHCII pathway is critical for tolerance induction and that the tolerogenic properties of peptide-IgG-transduced B cells depend on Tregs [15, 34, 35]. More importantly, although it is not essential for tolerance induction in our gene therapy system, IgG significantly enhances the degree of tolerance and prolongs the hyporesponsive state [36, 37]. Thus, we propose that the presence of Tregitopes in the IgG facilitates the induction of Tregs in vivo. As a result, Tregs induced by peptide-IgG gene therapy or therapeutic IVIg might be a result of the presence of Tregitopes in the Fc region. We will examine this hypothesis by generating a fusion protein containing target antigens linked to Tregitopes and transferring this fusion construct in vivo to test tolerance induction to the target moiety.
ACKNOWLEDGMENTS
This project is supported, in part, by the U.S. National Institutes of Health (R01 AI035622 to D.W.S. and R43DK081261-01 to A.S.D.).
We thank Patrick Adair and Drs. Ai-Hong Zhang, Yong Chan Kim, Ryan Tassone, and Leslie Cousens for critical reading of this manuscript. We thank Dr. Zhaozhang Li for her assistance with the real-time PCR.
Footnotes
- Δcpm
- change in cpm
- Ct
- comparative threshold
- EAE
- experimental autoimmune encephalomyelitis
- FVIII
- Factor VIII
- FoxP3
- forkhead box P3
- hTregitope167/289
- human regulatory T cell epitope 167/289
- IVIg
- i.v. IgG
- mTregitope167/289
- murine regulatory T cell epitope 167/289
- nTreg
- natural regulatory T cell
- RORγt
- retinoic acid-related orphan receptor γt
- T1D
- type 1 diabetes
- T-bet
- T-box transcription factor
- Treg
- regulatory T cell
- Tregitope
- regulatory T cell epitope
AUTHORSHIP
Y.S., A.S.D., and D.W.S. designed research. Y.S. and R.R. performed research. Y.S., A.S.D., and D.W.S. analyzed data and wrote the paper.
DISCLOSURES
D.W.S. is on the Scientific Advisory Board of EpiVax. A.S.D. is majority owner of EpiVax and chief executive officer/chief security officer of the company, where the Tregitope technology intellectual property is held. They both acknowledge the inherent conflict of interest and attest that this manuscript is free of bias to the best of their knowledge.
REFERENCES
- 1. Bluestone J. A., Abbas A. K. (2003) Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3, 253–257 [DOI] [PubMed] [Google Scholar]
- 2. Tang Q., Bluestone J. A. (2008) The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9, 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gambineri E., Torgerson T. R., Ochs H. D. (2003) Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 15, 430–435 [DOI] [PubMed] [Google Scholar]
- 4. Malek T. R., Yu A., Vincek V., Scibelli P., Kong L. (2002) CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17, 167–178 [DOI] [PubMed] [Google Scholar]
- 5. Kim J. M., Rasmussen J. P., Rudensky A. Y. (2007) Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 [DOI] [PubMed] [Google Scholar]
- 6. Achiron A., Gabbay U., Gilad R., Hassin-Baer S., Barak Y., Gornish M., Elizur A., Goldhammer Y., Sarova-Pinhas I. (1998) Intravenous immunoglobulin treatment in multiple sclerosis. Effect on relapses. Neurology 50, 398–402 [DOI] [PubMed] [Google Scholar]
- 7. Bayry J., Lacroix-Desmazes S., Kazatchkine M. D., Kaveri S. V. (2007) Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: rationale and mechanisms of action. Nature clinical practice. Rheumatology 3, 262–272 [DOI] [PubMed] [Google Scholar]
- 8. Negi V. S., Elluru S., Siberil S., Graff-Dubois S., Mouthon L., Kazatchkine M. D., Lacroix-Desmazes S., Bayry J., Kaveri S. V. (2007) Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J. Clin. Immunol. 27, 233–245 [DOI] [PubMed] [Google Scholar]
- 9. Nimmerjahn F., Ravetch J. V. (2008) Anti-inflammatory actions of intravenous immunoglobulin. Annu. Rev. Immunol. 26, 513–533 [DOI] [PubMed] [Google Scholar]
- 10. Ephrem A., Chamat S., Miquel C., Fisson S., Mouthon L., Caligiuri G., Delignat S., Elluru S., Bayry J., Lacroix-Desmazes S., Cohen J. L., Salomon B. L., Kazatchkine M. D., Kaveri S. V., Misra N. (2008) Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood 111, 715–722 [DOI] [PubMed] [Google Scholar]
- 11. Jain R., Tartar D. M., Gregg R. K., Divekar R. D., Bell J. J., Lee H. H., Yu P., Ellis J. S., Hoeman C. M., Franklin C. L., Zaghouani H. (2008) Innocuous IFNγ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 205, 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Divekar R. D., Haymaker C. L., Cascio J. A., Guloglu B. F., Ellis J. S., Tartar D. M., Hoeman C. M., Franklin C. L., Zinselmeyer B. H., Lynch J. N., Miller M. J., Zaghouani H. (2011) T cell dynamics during induction of tolerance and suppression of experimental allergic encephalomyelitis. J. Immunol. 187, 3979–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skupsky J., Su Y., Lei T. C., Scott D. W. (2007) Tolerance induction by gene transfer to lymphocytes. Curr. Gene. Ther. 7, 369–380 [DOI] [PubMed] [Google Scholar]
- 14. Scott D. W., Zhang A. H., Su Y. (2012) B-cell based gene therapy for autoimmune diseases. Infect. Dis. Drug Targets 12, 241–247 [DOI] [PubMed] [Google Scholar]
- 15. Lei T. C., Scott D. W. (2005) Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood 105, 4865–4870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soukhareva N., Jiang Y., Scott D. W. (2006) Treatment of diabetes in NOD mice by gene transfer of Ig-fusion proteins into B cells: role of T regulatory cells. Cell. Immunol. 240, 41–46 [DOI] [PubMed] [Google Scholar]
- 17. De Groot A. S., Moise L., McMurry J. A., Wambre E., Van Overtvelt L., Moingeon P., Scott D. W., Martin W. (2008) Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood 112, 3303–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cousens L. P., Tassone R., Mazer B. D., Ramachandiran V., Scott D. W., De Groot A. S. (2013) Tregitope update: mechanism of action parallels IVIg. Autoimmun. Rev. 12, 436–443 [DOI] [PubMed] [Google Scholar]
- 19. Cousens L., Mingozzi F., van der Marel S., Su Y., Garman R., Ferreira V., Martin W., Scott D. W., De Groot A. S. (2012) Teaching tolerance: new approaches to enzyme replacement therapy for Pompe disease. Hum. Vaccin. Immunother. 8, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cousens L. P., Su Y., McClainea E., Li X., Terry F., Smith R., Leed J., Martina W., Scott D. W., De Groot A. S. (2013) Application of IgG-derived natural Treg epitopes (IgG Tregitopes) to antigen-specific tolerance induction in a murine model of type 1 diabetes. J. Diabetes Res. doi: http://dx.doi.org/10.1155/2013/621693 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 21. Zambidis E. T., Scott D. W. (1996) Epitope-specific tolerance induction with an engineered immunoglobulin. Proc. Natl. Acad. Sci. USA 93, 5019–5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zambidis E. T., Barth R. K., Scott D. W. (1997) Both resting and activated B lymphocytes expressing engineered peptide-Ig molecules serve as highly efficient tolerogenic vehicles in immunocompetent adult recipients. J. Immunol. 158, 2174–2182 [PubMed] [Google Scholar]
- 23. Melo M. E., Qian J., El-Amine M., Agarwal R. K., Soukhareva N., Kang Y., Scott D. W. (2002) Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J. Immunol. 168, 4788–4795 [DOI] [PubMed] [Google Scholar]
- 24. Xu B., Scott D. W. (2004) A novel retroviral gene therapy approach to inhibit specific antibody production and suppress experimental autoimmune encephalomyelitis induced by MOG and MBP. Clin. Immunol. 111, 47–52 [DOI] [PubMed] [Google Scholar]
- 25. Zhang A. H., Li X., Onabajo O. O., Su Y., Skupsky J., Thomas J. W., Scott D. W. (2010) B-cell delivered gene therapy for tolerance induction: role of autoantigen-specific B cells. J. Autoimmun. 35, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agarwal R. K., Kang Y., Zambidis E., Scott D. W., Chan C. C., Caspi R. R. (2000) Retroviral gene therapy with an immunoglobulin-antigen fusion construct protects from experimental autoimmune uveitis. J. Clin. Invest. 106, 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lacroix-Desmazes S., Misra N., Bayry J., Artaud C., Drayton B., Kaveri S. V., Kazatchkine M. D. (2002) Pathophysiology of inhibitors to factor VIII in patients with haemophilia A. Haemophilia 8, 273–279 [DOI] [PubMed] [Google Scholar]
- 28. Getts D. R., Shankar S., Chastain E. M., Martin A., Getts M. T., Wood K., Miller S. D. (2011) Current landscape for T-cell targeting in autoimmunity and transplantation. Immunotherapy 3, 853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elyaman W., Khoury S. J., Scott D. W., De Groot A. S. (2011) Potential application of Tregitopes as immunomodulating agents in multiple sclerosis. Neurol. Res. Int. 2011, 256460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Marel S., Majowicz A., Kwikkers K., van Logtenstein R., te Velde A. A., De Groot A. S., Meijer S. L., van Deventer S. J., Petry H., Hommes D. W., Ferreira V. (2012) Adeno-associated virus mediated delivery of Tregitope 167 ameliorates experimental colitis. World J. Gastroenterol. 18, 4288–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scott D. W., De Groot A. S. (2010) Can we prevent immunogenicity of human protein drugs? Ann. Rheum. Dis. 69 (Suppl. 1), i72–i76 [DOI] [PubMed] [Google Scholar]
- 32. Su Y., Zhang A. H., Noben-Trauth N., Scott D. W. (2011) B-cell gene therapy for tolerance induction: host but not donor B-cell derived IL-10 is necessary for tolerance. Front. Microbiol. 2, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Su Y., Zhang A. H., Li X., Owusu-Boaitey N., Skupsky J., Scott D. W. (2011) B cells “transduced” with TAT-fusion proteins can induce tolerance and protect mice from diabetes and EAE. Clin. Immunol. 140, 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su Y., Carey G., Maric M., Scott D. W. (2008) B cells induce tolerance by presenting endogenous peptide-IgG on MHC class II molecules via an IFN-γ-inducible lysosomal thiol reductase-dependent pathway. J. Immunol. 181, 1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Litzinger M. T., Su Y., Lei T. C., Soukhareva N., Scott D. W. (2005) Mechanisms of gene therapy for tolerance: B7 signaling is required for peptide-IgG gene-transferred tolerance induction. J. Immunol. 175, 780–787 [DOI] [PubMed] [Google Scholar]
- 36. Lei T. C., Su Y., Scott D. W. (2005) Tolerance induction via a B-cell delivered gene therapy-based protocol: optimization and role of the Ig scaffold. Cell. Immunol. 235, 12–20 [DOI] [PubMed] [Google Scholar]
- 37. Kang Y., Melo M., Deng E., Tisch R., El-Amine M., Scott D. W. (1999) Induction of hyporesponsiveness to intact foreign protein via retroviral-mediated gene expression: the IgG scaffold is important for induction and maintenance of immune hyporesponsiveness. Proc. Natl. Acad. Sci. USA 96, 8609–8614 [DOI] [PMC free article] [PubMed] [Google Scholar]