Abstract
There is a growing body of evidence that soluble oligomeric forms of amyloid β (Aβ) play a critical role in Alzheimer's disease (AD). Despite the importance of soluble Aβ oligomers as a therapeutic target for AD, the dynamic metabolism of these Aβ species in vivo has not been elucidated because of the difficulty in monitoring brain Aβ oligomers in living animals. Here, using a unique large pore-sized membrane microdialysis, we characterized soluble Aβ oligomers in brain interstitial fluid (ISF) of awake, freely moving APP/PS1 transgenic and control WT mice. We could detect high-molecular-weight (HMW) and low-molecular-weight (LMW) Aβ oligomers in the brain ISF of living animals, which increased dramatically in an age-dependent manner (5- to 8-fold increase, 4 vs. 17–18 mo). Notably, HMW Aβ decreased more slowly than other forms of Aβ after acute γ-secretase inhibition [% decrease from the baseline (HMW vs. LMW) was 36.9 vs. 74.1% (Aβ40, P<0.05) and 25.4 vs. 88.0% (Aβ42, P<0.01)], suggesting that HMW Aβ oligomers clear more slowly than other forms from the brain. These data reveal the dynamic metabolism of neurotoxic Aβ oligomers in AD brain and could provide new insights into Aβ-targeted therapies for AD.—Takeda, S., Hashimoto, T., Roe, A. D., Hori, Y., Spires-Jones, T. L., and Hyman, B. T. Brain interstitial oligomeric amyloid β increases with age and is resistant to clearance from brain in a mouse model of Alzheimer's disease.
Keywords: in vivo microdialysis, apolipoprotein E
Amyloid β (Aβ) is the main component of senile plaques in the brain in Alzheimer's disease (AD) and plays a pivotal role in the pathogenesis of AD (1–3). Many disease-modifying therapies for AD that are currently being developed target the reduction of Aβ, as this is thought to possibly both treat and prevent AD (1). Aβ is a 38- to 43-aa peptide produced from amyloid precursor protein (APP) by proteolytic processing (4–7). There is a growing body of evidence that soluble oligomeric forms, not fibrillar deposits, of Aβ are pathologically important for the synaptic dysfunction of AD (8–14). However, the dynamic metabolism of soluble oligomeric forms of Aβ, particularly in the brain interstitial fluid (ISF), which reflects biochemical changes around neurons and is closely associated with neuronal activity, has not been fully elucidated because of the difficulty in monitoring soluble Aβ oligomers in the brains of living animals. Characterizing the in vivo metabolism of Aβ oligomers in the AD brain is essential to understanding the significance of these Aβ species. Microdialysis is a powerful in vivo technique for the continuous sampling of various molecules within the brain extracellular fluid space. The most common technique used to measure Aβ uses a 35- to 38-kDa molecular weight cutoff (MWCO) membrane probe (15–18), but high-molecular-weight (HMW) Aβ oligomers are difficult to collect with a conventional membrane having a small pore size (16). The extracellular accumulation of HMW Aβ has been suggested as being neurotoxic with the potential to induce cognitive dysfunction (9). In addition, Aβ could be physiologically found in a HMW complex with its carrier proteins, such as apolipoprotein E (apoE), which has been strongly associated with AD synaptotoxicity (14, 19). Because apoE protein modulates Aβ metabolism in the brain (19–22), understanding the interaction between Aβ and apoE is also important for understanding the dynamics of ISF Aβ.
In this study, we used a unique large-pore-sized (1000-kDa-MWCO) membrane microdialysis probe to obtain ISF samples from Alzheimer model APP/PS1 Tg mice at 3 different age stages of AD-like amyloid plaque development: young (4 mo), middle-aged (11–12 mo), and old (17–18 mo). By using size-exclusion chromatography (SEC) and Aβ-specific ELISAs, we confirmed that HMW and low-molecular-weight (LMW) Aβ oligomers are present in brain ISF samples and that levels of ISF Aβ oligomers become elevated with age in the brain of APP/PS1 Tg mice. Notably, HMW Aβ oligomers cleared more slowly from the brain than LMW Aβ oligomers after acute inhibition of γ-secretase activity to stop Aβ synthesis, suggesting different clearance kinetics of Aβ oligomers from the brain. We also demonstrated that this technique could be used simultaneously to measure brain ISF apoE. These results clarify the dynamics of ISF Aβ oligomers and demonstrate that microdialysis using a large pore-sized membrane is a useful method for analyzing large-molecular weight species in the ISF.
MATERIALS AND METHODS
Animals
All experiments were performed under national (U.S. National Institutes of Health) and institutional (Massachusetts General Hospital Subcommittee for Research Animal Care and the Institutional Animal Care and Use Committee at Harvard Medical School) guidelines. APP/PS1 Tg mice [strain B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/J; ref. 23) and wild-type (WT) littermate mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). ApoE-deficient mice [apoE-knockout (KO); Jackson Laboratory] were also used (24). Both male and female mice were used in this study.
In vivo microdialysis
In vivo microdialysis sampling of brain ISF Aβ and apoE was performed as described previously (25). The microdialysis probe had a 4-mm shaft with a 3.0-mm, 1000-kDa-MWCO polyethylene membrane (PEP-4-03; Eicom, Kyoto, Japan). This probe contains a ventilation hole near the top, which serves to produce a reservoir of fluid within the probe that is open to the atmosphere. This structure minimizes pressure, which would otherwise cause a net flow of perfusate out through the large pore membrane (25). Before use, the probe was conditioned by briefly dipping it in ethanol, and then washed with an artificial cerebrospinal fluid (aCSF) perfusion buffer (in mM: 122 NaCl, 1.3 CaCl2, 1.2 MgCl2, 3.0 KH2PO4, and 25.0 NaHCO3) that was filtered through a 0.2-μm-pore membrane. The preconditioned probe's outlet and inlet were connected to a peristaltic pump (ERP-10; Eicom) and a microsyringe pump (ESP-32; Eicom), respectively, using fluorinated ethylene propylene (FEP) tubing (φ 250 μm i.d.).
Probe implantation was performed as described previously (15, 25), with slight modifications. Briefly, the animals were anesthetized with isoflurane, while a guide cannula (PEG-4; Eicom) was stereotactically implanted in the hippocampus (bregma −3.1 mm, −2.5 mm lateral to midline, −1.2 mm ventral to dura). The guide was fixed using binary dental cement.
At 3 d after the implantation of the guide cannula, the mice were placed in a standard microdialysis cage, and a probe was inserted through the guide. After insertion of the probe, in order to obtain stable recordings, the probe and connecting tubes were perfused with aCSF for 240 min at a flow rate of 10 μl/min before sample collection. Samples were collected at a flow rate of 0.1–1.0 μl/min and stored at 4°C in polypropylene tubes. During microdialysis sample collection, mice were awake and freely moving in the microdialysis cage, designed to allow unrestricted movement of the animals without applying pressure on the probe assembly (AtmosLM microdialysis system; Eicom).
In vitro microdialysis
Membranes of 2 different pore sizes (35- and 1000-kDa MWCO; Eicom) were used for the in vitro microdialysis study. Probes were connected to the perfusion system for the in vivo microdialysis experiment. After equilibration, the probes were submerged in a polypropylene tube (1.5 ml) containing a solution of Aβ oligomer mixture. Sampling was carried out at a flow rate of 1.0 μl/min. All experiments were performed on ice to prevent further oligomerization of Aβ. Samples were collected from the pull site collection tubing and stored at 4°C in polypropylene tubes.
Preparation of Aβ oligomer mixtures and ELISA quantification
Aβ oligomer mixtures were prepared from synthetic human Aβ1–42 (Peptide Institute, Osaka, Japan), as described previously (26). First, synthetic Aβ1–42 was dissolved in hexafluoro-2-propanol (HFIP). The HFIP solvent was then removed by evaporation under a vacuum, and the aliquot was dissolved in PBS with 2% dimethyl sulfoxide (DMSO) to yield a 0.1 mg/ml concentration, followed by filtration through a 0.2-μm-pore membrane (preincubation sample). This solution was incubated for 3 h at 37°C to make a solution of Aβ oligomer mixture (postincubation sample). The existence of small protofibrils in this solution was confirmed by electron microscopy (Supplemental Fig. S1). Samples were incubated with and without 8 M guanidine HCl (final concentration of guanidine HCl in the sample was 4 M) for 30 min at room temperature to dissociate oligomerized Aβ. Concentrations of Aβ in preincubation and postincubation samples were measured by 82E1-82E1 Aβ oligomer-specific and BAN50-BC05 ELISA (Supplemental Fig. S2).
Brain Aβ quantification
The concentrations of Aβ40 and Aβ42 in the brain TBS-soluble and formic acid (FA) fractions and ISF samples were determined by BNT77-BA27 (for Aβ40) and BNT77-BC05 (for Aβ42) sandwich ELISA (Wako Pure Chemicals Industries, Osaka, Japan), according to the manufacturer's instructions. The amount of oligomer Aβ in each sample was determined by 82E1-82E1 sandwich ELISA (Immuno-Biological Laboratories, Hamburg, Germany), in which the same N-terminal (residues 1–16) antibody was used for both capture and detection. For guanidine treatment, each ISF sample was incubated with and without 500 mM guanidine HCl for 30 min at room temperature.
Brain protein extraction
Mice were perfused with cold PBS containing protease inhibitors (protease inhibitor mixture; Roche, Indianapolis, IN, USA), and the brain was rapidly excised and immediately frozen in liquid nitrogen, then stored at −80°C before use. For ELISA analysis, brain tissue was homogenized in 10 vol (w/v) of cold TBS containing protease inhibitors using a Teflon-glass homogenizer. The homogenate was centrifuged at 45,000 g for 60 min at 4°C, and the supernatant was used to determine brain TBS-soluble fraction Aβ levels. The pellets were resuspended in 2% SDS in TBS (containing protease inhibitor), homogenized and incubated for 30 min at 37°C, and then centrifuged at 45,000 g for 30 min at 20°C. After collecting supernatant, the pellets were resuspended in 70% FA, sonicated for 20 s, and centrifuged at 45,000 g for 30 min at 4°C. The supernatant was used to determine brain FA fraction Aβ levels.
Immunoblot analysis
Brain TBS-soluble fractions and microdialysates were electrophoresed on 4–20% Novex Tris-glycine gels (Invitrogen, Grand Island, NY, USA) in Tris-glycine SDS running buffer for SDS-PAGE (Invitrogen). Gels were transferred to PVDF membranes, and membranes were blocked for 60 min at room temperature in 5% BSA/TBS-T, and then probed with goat anti-apoE antibody (1:1000, AB947; Millipore, Billerica, MA, USA), followed by HRP-conjugated anti-goat IgG antibody (Vector, Burlingame, CA, USA). Anti-actin antibody (1:1000, 4968; Cell Signaling, Beverly, MA, USA) and anti-albumin antibody (1:1000, SAB3500217; Sigma-Aldrich, St. Louis, MO, USA) were also used as primary antibodies. Immunoreactive proteins were developed using an ECL kit (Western Lightning; PerkinElmer, Waltham, MA, USA) and detected on Hyperfilm ECL (GE Healthcare, Pittsburgh, PA, USA).
Compound E treatment
During in vivo microdialysis sampling, after baseline sample collection for 8 h, the potent γ-secretase inhibitor compound E (200 nM; Alexis Biochemicals, San Diego, CA, USA) was added to the microdialysis perfusion buffer to rapidly inhibit Aβ production near the probe (27). The IC50 for this compound to inhibit γ-secretase activity is 0.3 nM.
SEC
Brain TBS-soluble fractions and ISF samples were separated by SEC on single Superdex75 columns (GE Healthcare) in 50 mM ammonium acetate (pH 8.5), at a flow rate of 0.5 ml/min, with an AKTA purifier 10 (GE Healthcare; ref. 28). The individual fractions separated by SEC were analyzed by immunoblotting and Aβ-specific ELISAs.
Statistical analysis
All data are expressed as means ± se. Two-group comparisons were performed by Student's t test. Comparison of means among ≥3 groups was performed by ANOVA followed by Tukey-Kramer multiple-range test. Values of P < 0.05 were considered significant.
RESULTS
In vitro recovery of HMW Aβ oligomers using large-pore-sized (1000-kDa-MWCO) membrane microdialysis
To characterize the oligomeric Aβ species collected by the 1000-kDa-MWCO membrane microdialysis probe, we first performed in vitro microdialysis sampling from a solution of Aβ oligomer mixture. An Aβ oligomer mixture was prepared by incubating synthetic Aβ1–42 peptide for 3 h at 37°C (Fig. 1A–C and Supplemental Fig. S1), with the concentrations of Aβ in preincubation and postincubation samples being quantified by 2 different ELISA systems. 82E1-82E1 sandwich ELISA, in which the same N-terminal-specific anti-Aβ monoclonal antibody (82E1; Supplemental Fig. S2) was used for both antigen capture and detection, is specific for Aβ oligomers with multiple epitopes, and theoretically cannot detect monomer Aβ with a single binding site (29). Indeed, a significantly high signal level was detected from the solution (postincubation sample) in this single-antibody sandwich ELISA, and dissociation of Aβ oligomers by treating the sample with guanidine HCl markedly reduced the signal (Fig. 1A), demonstrating the specificity of this ELISA for Aβ oligomers. On the other hand, the amount of Aβ1–42 detected by BAN50-BC05 ELISA (Supplemental Fig. S2) was decreased after incubation (Fig. 1B), and the signal was recovered to baseline level (preincubation) by dissociating Aβ oligomers with guanidine HCl treatment, which indicates that this ELISA preferentially detects monomer or LMW Aβ species (since dissociation is not necessarily complete). The decrease in Aβ oligomer signal in 82E1-82E1 ELISA and increase in Aβ1–42 signal in BAN50-BC05 ELISA after guanidine HCl treatment of the preincubation sample indicate that a small amount of Aβ oligomer is already present even before incubation (Fig. 1A, B). We separated preincubation and postincubation Aβ solutions by SEC, followed by quantification of Aβ levels in each SEC fraction by BAN50-BC05 ELISA, and found that a large portion of the mixture consisted of HMW Aβ oligomers (SEC fractions 4–11, ∼40–160 kDa) and small amounts of LMW Aβ oligomers (fractions 14–19) after incubation (Fig. 1C).
Figure 1.
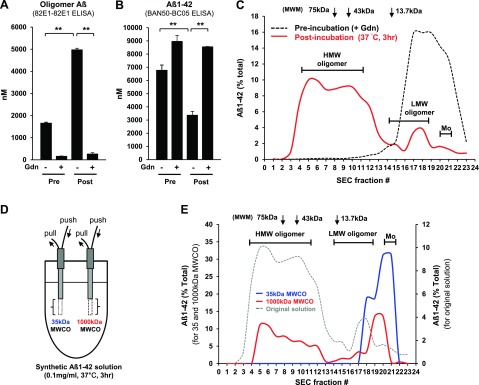
In vitro microdialysis sampling of HMW Aβ oligomers by using a large-pore-sized (1000-kDa-MWCO) membrane probe. A–C) An Aβ oligomer mixture was prepared by incubating synthetic human Aβ1–42 (0.1 mg/ml) for 3 h at 37°C. Concentrations of Aβ in preincubation and postincubation samples were measured by 82E1-82E1 ELISA for Aβ oligomers (A) and BAN50-BC05 ELISA for Aβ1–42 (B). Samples were treated with and without guanidine HCl before ELISA measurement (n=3/group). **P < 0.01. C) SEC of the preincubation and postincubation Aβ1–42 solutions was performed on a Superdex75 column. Aβ levels in each SEC-separated sample were measured by ELISA (BAN50-BC05). The preincubation sample was treated with guanidine HCl to completely dissociate Aβ oligomers. D, E) In vitro microdialysis with small- and large-pore-sized (35- and 1000-kDa-MWCO) membranes from Aβ oligomer mixtures. D) Microdialysis samples were collected from the same Aβ oligomer mixture solution by using 35- and 1000-kDa-MWCO membranes at a flow rate of 1.0 μl/min. E) Microdialysis samples and the original solution (Aβ oligomer mixtures) were separated by SEC, followed by Aβ quantification of each SEC fraction (BAN50-BC05 ELISA). Mo, monomer; MWM, molecular weight marker.
Then, we performed in vitro microdialysis sampling using membranes of 2 different pore sizes (35- and 1000-kDa MWCO) from the same Aβ oligomer mixture solution in order to clarify the molecular size of the Aβ species collected using each membrane (Fig. 1D). SEC separation of microdialysate revealed that a 1000-kDa-MWCO membrane probe collected HMW Aβ oligomers in addition to LMW oligomer and monomer Aβ (fractions 20–22), although only monomer and LMW (dimer) Aβ were detected in the dialysate of a small-pore-sized (35-kDa-MWCO) probe (Fig. 1E). These data clearly demonstrate that a large-pore-sized membrane is required to collect HMW Aβ oligomers.
Brain ISF Aβ oligomers increase with age in Alzheimer APP/PS1 Tg mice
Next, we evaluated the large-pore-sized membrane microdialysis in vivo using an Alzheimer APP/PS1 Tg mouse model. APP/PS1 Tg mice develop Alzheimer-like amyloid plaque in an age-dependent manner (30). Levels of soluble Aβ40 and Aβ42 in brain TBS-soluble fractions from young (4 mo), middle-aged (11–12 mo), and old (17–18 mo) APP/PS1 Tg mice elevated with age (Fig. 2A, B). Furthermore, the amount of Aβ oligomers (detected by 82E1-82E1 ELISA) in the brain TBS-soluble fractions also increased with age (Fig. 2C). Brain FA fraction Aβ40 and Aβ42 increased with age, as well (Fig. 2D, E).
Figure 2.
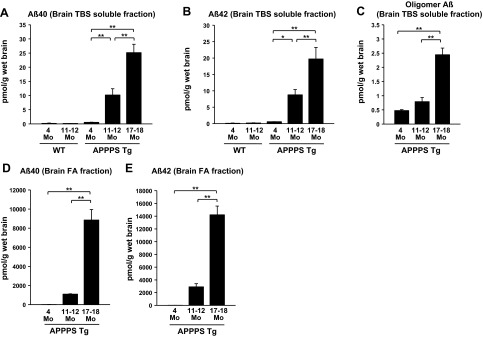
Age-dependent increase in brain TBS-soluble Aβ levels, including Aβ oligomers. A–C) Brains from WT and APP/PS1 Tg mice were homogenized in TBS, and after ultracentrifugation, TBS-soluble Aβ levels were quantified by ELISA (BNT77-BA27 ELISA for Aβ40, BNT77-BC05 ELISA for Aβ42, and 82E1-82E1 ELISA for Aβ oligomers). Brain TBS-soluble Aβ40 (A), Aβ42 (B), and Aβ oligomers (C) increased with age in APP/PS1 Tg mice. D, E) Brain FA fraction Aβ40 (BNT77-BA27 ELISA, D) and Aβ42 (BNT77-BC05 ELISA, E) also increased with age. (n=3–7). *P < 0.05, **P < 0.01.
In agreement with these age-dependent elevations in brain TBS-soluble Aβ levels in APP/PS1 Tg mice, there was a significant increase in Aβ levels in brain ISF samples collected by the large-pore-sized (1000-kDa-MWCO) membrane probe (Fig. 3). The Aβ40 and Aβ42 levels were measured by BNT77-BA27 ELISA (for Aβ40) and BNT77-BC05 ELISA (for Aβ42), which also preferentially detect monomer (or LMW) Aβ (Supplemental Fig. S3). Interestingly, the amount of Aβ detected by these ELISAs was increased by guanidine HCl treatment (Fig. 3A, B), suggesting that highly oligomerized or complexed Aβ, which could inhibit the interaction between Aβ and antibodies, was dissociated into monomer/LMW Aβ, which, in turn, increased the reactivity of the ELISAs. We also found a similar increase in Aβ levels after guanidine HCl treatment in ISF samples from WT mice (Fig. 3A, B), indicating the presence of some amount of Aβ oligomers in the WT mouse brain or unidentified interacting molecules with Aβ that may interfere with the detection of Aβ by anti-Aβ antibodies in the ELISA system. We also confirmed the presence of Aβ oligomers in ISF samples of APP/PS1 mice using the 82E1-82E1 oligomer ELISA (Fig. 3C). We could detect significant signal with the same-site ELISA assay in ISF samples from young and old APP/PS1 Tg mice. Notably, the level of oligomeric Aβ increased with age. Disappearance of the Aβ oligomer signal after guanidine HCl treatment demonstrates the specificity of this ELISA for oligomeric Aβ (Fig. 3C). The levels of brain ISF and TBS soluble Aβ oligomers positively correlated with brain FA fraction Aβ40 (Supplemental Fig. S4A, C) and Aβ42 (Supplemental Fig. S4B, D) levels. This correlation might indicate a presence of equilibration between ISF Aβ oligomers and amyloid plaque deposits (14).
Figure 3.
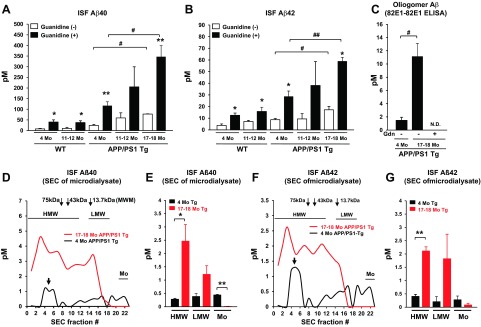
Levels of brain interstitial Aβ oligomers increase with age in Alzheimer APP/PS1 transgenic mice. A–C) Brain ISF samples were collected by using a large-pore-size membrane (1000-kDa MWCO) from the hippocampus of WT and APP/PS1 Tg mice at different ages (4, 11–12, and 17–18 mo). ISF samples were incubated with or without guanidine HCl (0.5 M final concentration), and levels of Aβ were quantified by ELISA [BNT77-BA27 ELISA for Aβ40 (A), BNT77-BC05 ELISA for Aβ42 (B), and 82E1-82E1 ELISA for Aβ oligomers (C)]; n=3–5. N.D., not detectable. *P < 0.05, **P < 0.01 vs. (−) guanidine treatment; #P < 0.05, ##P < 0.01. D–G) Brain ISF samples from young (4 mo) and old (17–18 mo) APP/PS1 Tg mice were separated by SEC, followed by ELISA quantification of Aβ levels in each fraction [BNT77-BA27 ELISA for Aβ40 (D, E) and BNT77-BC05 ELISA for Aβ42 (F, G)]. D, F) Representative graph of ISF Aβ40 (D) and Aβ42 (F) levels in SEC-separated samples. E, G) Mean Aβ40 (E) and Aβ42 (G) levels of HMW (fractions 1–11), LMW (fractions 14–19), and monomer (Mo; fractions 20–22) fractions; n=3–4. Gdn, guanidine HCl. *P < 0.05, **P < 0.01.
To clarify the molecular size of ISF Aβ oligomers and age-dependent changes in these levels, ISF samples from young and old APP/PS1 Tg mice were separated by SEC, followed by Aβ quantification in each fraction after guanidine HCl treatment (Fig. 3D–G). We detected HMW and LMW Aβ oligomers, in addition to monomer Aβ, in the brain ISF of young APP/PS1 Tg mice (Fig. 3D, F, black traces). Levels of HMW Aβ (fractions 1–11) significantly increased with age, while levels of monomer Aβ (fractions 20–22) decreased (Fig. 3D, F, red traces; E, G). Of note, a significant peak of HMW Aβ was detected in SEC fractions. 4–6 (100–160 kDa), even in young APP/PS1 Tg mice (Fig. 3D, F), which may suggest either oligomeric species of Aβ or association with a carrier protein in these fractions.
ISF HMW Aβ shows slower clearance rate from brain
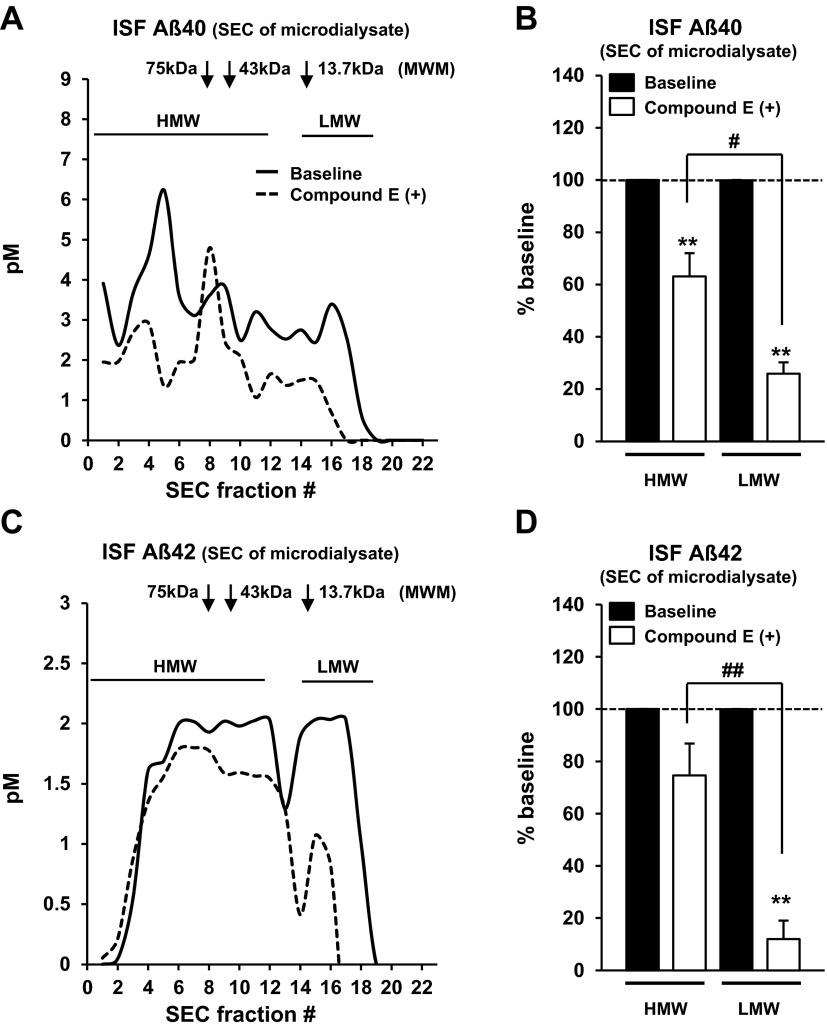
To further characterize the dynamic metabolism of ISF Aβ oligomers, we collected ISF samples before and after compound E (a potent γ-secretase inhibitor) perfusion, which can rapidly block Aβ production in surrounding brain tissue, from old (17–18 mo) APP/PS1 Tg mice (Fig. 4), then separated the samples by SEC, followed by Aβ quantification in each fraction by ELISA. Acute γ-secretase inhibition with compound E decreased the levels of ISF Aβ40 (Fig. 4A, B) and Aβ42 (Fig. 4C, D) and changed the distributions of ISF Aβ levels (Supplemental Fig. S5). SEC analysis revealed that the decrease in levels of LMW Aβ was more pronounced than that of HMW Aβ (Fig. 4B, D), indicating that HMW Aβ is eliminated more slowly than smaller MW Aβ species.
Figure 4.
ISF HMW Aβ shows slower clearance from brain. Effect of compound E (γ-secretase inhibitor, 200 nM) perfusion on levels of ISF Aβ40 (A, B) and Aβ42 (C, D) collected using large-pore-sized membrane probe (1000-kDa MWCO, flow rate 0.1 μl/min) from old APP/PS1 Tg mice (17–18 mo). After 8 h of baseline sample collection, compound E was added to the perfusion buffer, and samples were collected for another 8 h. ISF samples were separated by SEC, followed by ELISA quantification of Aβ levels in each fraction [BNT77-BA27 ELISA for Aβ40 (A, B) and BNT77-BC05 ELISA for Aβ42 (C, D)] after guanidine HCl treatment. A, C) Representative graph of ISF Aβ40 (A) and Aβ42 (C) levels in SEC-separated samples before (solid line) and after (dashed line) compound E treatment. B, D) Mean Aβ40 (B), and Aβ42 (D) levels of HMW (fractions 1–11) and LMW (fractions 14–19) fractions before (solid bar) and after (open bar) compound E treatment; n=3. MWM, molecular weight marker; Mo, monomer. **P < 0.01 vs. baseline; #P < 0.05, ##P < 0.01.
ISF apoE collected by large-pore-sized membrane microdialysis in freely moving animals
ApoE is secreted from astrocytes and microglia and participates in lipidated lipoprotein particles (19). It is becoming clear that apoE plays an important role in Aβ metabolism and its neurotoxicity in the brain (14, 20, 21, 31). Therefore, when analyzing the ISF Aβ metabolism, it is extremely important also to simultaneously evaluate ISF apoE and other possible carrier molecules.
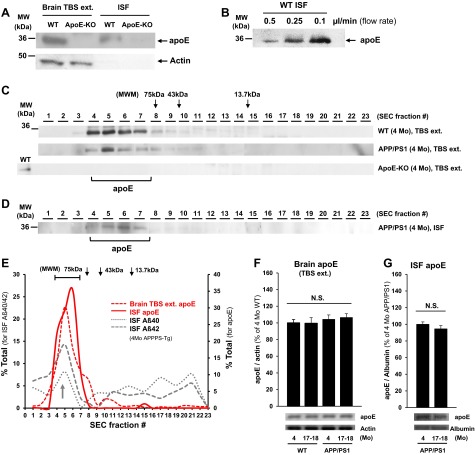
First, by immunoblot analysis, we confirmed that ISF apoE could be collected by 1000-kDa-MWCO membrane from WT mice (Fig. 5A, top panel). Notably, the absence of actin signals in the ISF samples demonstrates that this sampling system collects only extracellular molecules in the brain (Fig. 5A, bottom panel). Recovery efficiency of ISF apoE was dependent on flow rate of perfusion buffer (Fig. 5B) like other molecules (25). When brain TBS-soluble fractions were separated by SEC and analyzed by immunoblotting with an anti-apoE antibody, most apoE was detected in much larger fractions (fractions 4–7, 90–160 kDa) than its actual molecular weight (34 kDa), which likely reflects lipidation or polymer formation of apoE in the brain TBS-soluble fraction (Fig. 5C). To further characterize apoE in brain ISF under native conditions, SEC was also performed on ISF samples from APP/PS1 Tg mouse (4-mo) brain, followed by immunoblotting with an anti-apoE antibody. We found that most ISF apoE was detected in HMW fractions between fractions 4 and 7 (90–160 kDa), showing a similar distribution as that of apoE in the TBS-soluble fraction (Fig. 5D). Interestingly, these HMW fractions in which ISF apoE was detected (fractions 4–7) coincided with the significant peak of HMW Aβ detected in young APP/PS1 mice (Figs. 3D, F and 5E). The small amount of microdialysate precluded Co-IP experiments to determine whether there is a direct apoE-Aβ interaction in these fractions. Brain TBS-soluble and ISF apoE levels in young (4-mo) and old (17- to 18-mo) WT and APP/PS Tg mice were not significantly different (Fig. 5F, G).
Figure 5.
ISF apoE obtained by large-pore-sized membrane microdialysis in freely moving wild-type and APP/PS1 Tg mice. A) Brain lysate (TBS extracted) and ISF samples (1000-kDa MWCO, flow rate 0.1 μl/min) from WT (4 mo) and ApoE-KO (4 mo) mice were analyzed by immunoblot with anti-apoE and anti-actin antibodies; 20 μg of protein was loaded per well. B) Immunoblot analysis of ISF apoE levels at 3 different flow rates (0.5, 0.25, and 0.1 μl/min). ISF samples were collected from the same wild-type mouse (4 mo); 30 μg of samples was loaded per well. C, D) Immunoblotting (anti-apoE antibody) of SEC-separated fractions of brain TBS extract (C) and ISF sample (1000-kDa MWCO, flow rate 0.1 μl/min; D) from WT, APP/PS1 Tg, and apoE KO mice (4 mo). E) Distribution of ISF apoE, brain TBS-soluble ApoE, and ISF Aβ (Aβ40 and Aβ42) in young (4 mo) APP/PS1 Tg mice. Most ISF apoE was detected in SEC fractions 4–7 (90–160 kDa), in which peaks in ISF HMW Aβ levels (arrow) were also found. F, G) Brain TBS extract (F) and ISF (G) apoE levels in young (4 mo) and old (17–18 mo) WT and APP/PS1 Tg mice (n=3–6). MWM, molecular weight marker.
DISCUSSION
Growing evidence for the pathological role of Aβ oligomers in AD brain has drawn attention to the importance of understanding their dynamic metabolism in the central nervous system (8–13). In this study, we used in vivo microdialysis with a unique large-pore-sized membrane probe (1000-kDa MWCO) and push-pull perfusion system to monitor brain ISF Aβ oligomers in awake, freely moving mice with Alzheimer's disease (APP/PS1 Tg). We demonstrated that HMW and LMW Aβ oligomers are present in brain ISF collected with a large pore-sized membrane; levels of ISF Aβ oligomers increased with age; HMW Aβ oligomers are cleared from brain more slowly than LMW oligomers; and large-pore microdialysis also enables sampling of ISF apoE, which is a well-known Aβ-binding protein.
Numerous studies have used conventional small-pore-sized (35- to 38-kDa-MWCO) membrane microdialysis to document the dynamic metabolism of ISF Aβ (15–18). A recent study reported that most of the ISF Aβ collected by using a 35-kDa-MWCO membrane probe was a 4-kDa monomer; no HWM/LMW Aβ oligomers were detected (16), which may have been due to the pore size of the microdialysis membrane. We analyzed the difference in the molecular size of Aβ species collected by 2 different pore-sized membranes (35- and 1000-kDa MWCO) in vitro. We found HWM and LMW Aβ species in the microdialysate collected by the 1000-kDa-MWCO membrane but only monomers and a small amount of LMW Aβ (presumably dimers according to MW markers) in that collected with the 35-kDa-MWCO membrane (Fig. 1E). More important, we also demonstrated the presence of HWM and LMW Aβ oligomers in brain ISF samples from APP/PS1 Tg mice by using a 1000-kDa-MWCO membrane probe (Fig. 3).
In this study, we confirmed the presence of Aβ oligomers in ISF samples in 3 different ways. First, we used the specificity of BNT77-BA27 (Aβ40) and BNT77-BC05 (Aβ42) ELISA to Aβ monomers (although note that these ELISAs are not completely specific to monomers) combined with guanidine HCl denaturation, which is known to dissociate oligomeric or protein-bound complex into Aβ monomers (32), to compare the levels of Aβ in the samples with and without guanidine HCl treatment (Fig. 3A, B). A significant increase in Aβ levels after guanidine treatment indicates the presence of Aβ oligomers or Aβ complex with other carrier proteins, which masked the epitope and inhibited antibody detection of Aβ. The rise in Aβ levels after guanidine HCl treatment was also observed in ISF samples from WT animals (Fig. 3A, B), indicating the presence of Aβ oligomers or an Aβ complex with other proteins in WT mouse brain, although there was no age-dependent increase in WT animals. Second, we used 82E1-82E1 oligomer Aβ ELISA, which is a single-antibody sandwich ELISA system to detect Aβ oligomers with multiple epitopes (and which cannot detect monomer Aβ; ref. 29). We found an age-dependent increase in ISF Aβ oligomers in APP/PS1 Tg mice (Fig. 3C) in association with an increase in Aβ oligomer levels in brain TBS-soluble fractions with age (Fig. 2C). Unfortunately, the small volume of ISF sample precluded the interpolated zero-flow method experiments (15) in this study; therefore, the absolute concentrations of ISF Aβ oligomer are still unclear. Third, to further characterize the presence of ISF Aβ oligomers and to identify their molecular size under native conditions, we separated brain ISF samples by SEC. SEC of the ISF samples clearly demonstrated the presence of HMW and LMW Aβ oligomers with levels that were elevated with age (Fig. 3D–G). The age-dependent decrease in ISF monomer Aβ observed in this study (Fig. 3D–G) is consistent with a previous report using a small-pore-sized membrane probe (16); taken together, however, the data suggest that ISF Aβ concentration increases with age in APP/PS1 mice. The extent to which this age-related change in Aβ oligomer in the ISF reflects altered deposition, equilibrium with amyloid deposits, or altered rates of generation or clearance remains uncertain. Positive correlation between the levels of ISF Aβ oligomers and brain FA fraction Aβ observed in this study (Supplemental Fig. S4) might indicate that these Aβ populations exist in an equilibrium state.
ISF HMW Aβ oligomers were more resistant to clearance from the brain than LMW Aβ oligomers (Fig. 4). The finding that HMW Aβ was more resistant to clearance from the brain might have clinical significance because it indicates that acute γ-secretase inhibition does not effectively reduce HMW Aβ oligomers with the same time course as monomers. In this current study, we could not determine the in vivo half-life of ISF Aβ oligomers due to the limited sample volume and sensitivity of detection system (ELISAs). It is also still uncertain which population of ISF Aβ oligomers, preexisting or newly generated oligomers, was affected by compound E treatment. Clearance of ISF monomer Aβ might affect the equilibrium state between various soluble Aβ populations, thereby establishing a new equilibrium state.
To the best of our knowledge, this is the first report that has demonstrated the detection and characterization of brain ISF apoE (Fig. 5), which is important since apoE plays an important role in Aβ metabolism, such as oligomerization (22) and clearance from the brain (20, 21). The use of the high-MWCO probe thereby allows detection of large complexes, potentially including even apoE complexed with lipid or other molecules, since its MW by SEC is substantially higher than the calculated MW of 34 kDa.
In summary, by using in vivo microdialysis with a large-pore-sized membrane probe, we characterized the dynamic metabolism of brain ISF Aβ oligomers in an awake, freely moving Alzheimer mouse model. Understanding the in vivo metabolism of Aβ oligomers in the brains of living animals will help to develop therapies aimed specifically at oligomeric forms of Aβ and provide insight into why Aβ-related pathological changes increase with increasing age in mice, and thus potentially in humans, as well. Furthermore, our unique microdialysis system could be a useful method for analyzing the pathogenesis of a variety of brain diseases.
Supplementary Material
Acknowledgments
This research is supported by U.S. National Institutes of Health grant P50AG05134 (B.T.H.). S.T. is supported by a fellowship from the Japan Society for the Promotion of Science (JSPS).
The authors also thank Dr. Alberto Serrano-Pozo for valuable discussions. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Aβ
- amyloid β
- aCSF
- artificial cerebrospinal fluid
- AD
- Alzheimer's disease
- apoE
- apolipoprotein E
- APP
- amyloid precursor protein
- FA
- formic acid
- HMW
- high molecular weight
- KO
- knockout
- LMW
- low molecular weight
- ISF
- interstitial fluid
- MWCO
- molecular weight cutoff
- SEC
- size-exclusion chromatography
- WT
- wild type
REFERENCES
- 1. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 2. Holtzman D. M., Morris J. C., Goate A. M. (2011) Alzheimer's disease: the challenge of the second century. Sci. Transl. Med. 3, 77sr71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sisodia S. S. (1999) Alzheimer's disease: perspectives for the new millennium. J. Clin. Invest. 104, 1169–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Strooper B. (2003) Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-secretase complex. Neuron 38, 9–12 [DOI] [PubMed] [Google Scholar]
- 5. Sinha S., Anderson J. P., Barbour R., Basi G. S., Caccavello R., Davis D., Doan M., Dovey H. F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S. M., Wang S., Walker D., Zhao J., McConlogue L., John V. (1999) Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402, 537–540 [DOI] [PubMed] [Google Scholar]
- 6. Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 [DOI] [PubMed] [Google Scholar]
- 7. Klein W. L. (2006) Synaptic targeting by A beta oligomers (ADDLS) as a basis for memory loss in early Alzheimer's disease. Alzheimers Dement. 2, 43–55 [DOI] [PubMed] [Google Scholar]
- 8. Walsh D. M., Selkoe D. J. (2007) A beta oligomers—a decade of discovery. J. Neurochem. 101, 1172–1184 [DOI] [PubMed] [Google Scholar]
- 9. Lesne S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 10. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 11. Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008) Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shankar G. M., Bloodgood B. L., Townsend M., Walsh D. M., Selkoe D. J., Sabatini B. L. (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li S., Hong S., Shepardson N. E., Walsh D. M., Shankar G. M., Selkoe D. (2009) Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koffie R. M., Meyer-Luehmann M., Hashimoto T., Adams K. W., Mielke M. L., Garcia-Alloza M., Micheva K. D., Smith S. J., Kim M. L., Lee V. M., Hyman B. T., Spires-Jones T. L. (2009) Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. U. S. A. 106, 4012–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cirrito J. R., May P. C., O'Dell M. A., Taylor J. W., Parsadanian M., Cramer J. W., Audia J. E., Nissen J. S., Bales K. R., Paul S. M., DeMattos R. B., Holtzman D. M. (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J. Neurosci. 23, 8844–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hong S., Quintero-Monzon O., Ostaszewski B. L., Podlisny D. R., Cavanaugh W. T., Yang T., Holtzman D. M., Cirrito J. R., Selkoe D. J. (2011) Dynamic analysis of amyloid beta-protein in behaving mice reveals opposing changes in ISF versus parenchymal Aβ during age-related plaque formation. J. Neurosci. 31, 15861–15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bero A. W., Yan P., Roh J. H., Cirrito J. R., Stewart F. R., Raichle M. E., Lee J. M., Holtzman D. M. (2011) Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat. Neurosci. 14, 750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang J. E., Lim M. M., Bateman R. J., Lee J. J., Smyth L. P., Cirrito J. R., Fujiki N., Nishino S., Holtzman D. M. (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bu G. (2009) Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 10, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castellano J. M., Kim J., Stewart F. R., Jiang H., DeMattos R. B., Patterson B. W., Fagan A. M., Morris J. C., Mawuenyega K. G., Cruchaga C., Goate A. M., Bales K. R., Paul S. M., Bateman R. J., Holtzman D. M. (2011) Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med. 3, 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeMattos R. B., Cirrito J. R., Parsadanian M., May P. C., O'Dell M. A., Taylor J. W., Harmony J. A., Aronow B. J., Bales K. R., Paul S. M., Holtzman D. M. (2004) ApoE and clusterin cooperatively suppress Aβ levels and deposition: evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron 41, 193–202 [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto T., Serrano-Pozo A., Hori Y., Adams K. W., Takeda S., Banerji A. O., Mitani A., Joyner D., Thyssen D. H., Bacskai B. J., Frosch M. P., Spires-Jones T. L., Finn M. B., Holtzman D. M., Hyman B. T. (2012) Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid beta peptide. J. Neurosci. 32, 15181–15192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savonenko A., Xu G. M., Melnikova T., Morton J. L., Gonzales V., Wong M. P., Price D. L., Tang F., Markowska A. L., Borchelt D. R. (2005) Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol. Dis. 18, 602–617 [DOI] [PubMed] [Google Scholar]
- 24. Holtzman D. M., Bales K. R., Tenkova T., Fagan A. M., Parsadanian M., Sartorius L. J., Mackey B., Olney J., McKeel D., Wozniak D., Paul S. M. (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 97, 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takeda S., Sato N., Ikimura K., Nishino H., Rakugi H., Morishita R. (2011) Novel microdialysis method to assess neuropeptides and large molecules in free-moving mouse. Neuroscience 186, 110–119 [DOI] [PubMed] [Google Scholar]
- 26. Fukumoto H., Tokuda T., Kasai T., Ishigami N., Hidaka H., Kondo M., Allsop D., Nakagawa M. (2010) High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 24, 2716–2726 [DOI] [PubMed] [Google Scholar]
- 27. Cao C., Cirrito J. R., Lin X., Wang L., Verges D. K., Dickson A., Mamcarz M., Zhang C., Mori T., Arendash G. W., Holtzman D. M., Potter H. (2009) Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer's disease transgenic mice. J. Alzheimers Dis. 17, 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Townsend M., Shankar G. M., Mehta T., Walsh D. M., Selkoe D. J. (2006) Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J. Physiol. 572, 477–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xia W., Yang T., Shankar G., Smith I. M., Shen Y., Walsh D. M., Selkoe D. J. (2009) A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch. Neurol. 66, 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia-Alloza M., Robbins E. M., Zhang-Nunes S. X., Purcell S. M., Betensky R. A., Raju S., Prada C., Greenberg S. M., Bacskai B. J., Frosch M. P. (2006) Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 24, 516–524 [DOI] [PubMed] [Google Scholar]
- 31. Koffie R. M., Hashimoto T., Tai H. C., Kay K. R., Serrano-Pozo A., Joyner D., Hou S., Kopeikina K. J., Frosch M. P., Lee V. M., Holtzman D. M., Hyman B. T., Spires-Jones T. L. (2012) Apolipoprotein E4 effects in Alzheimer's disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain 135, 2155–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamada K., Yabuki C., Seubert P., Schenk D., Hori Y., Ohtsuki S., Terasaki T., Hashimoto T., Iwatsubo T. (2009) Aβ immunotherapy: intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high affinity to soluble Aβ. J. Neurosci. 29, 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.