Abstract
Nephrotoxicity severely limits the use of the anticancer drug cisplatin. Oxidative stress, inflammation, and endoplasmic reticulum (ER) stress contribute to cisplatin-induced nephrotoxicity. We developed novel orally active epoxyeicosatrienoic acid (EET) analogs and investigated their prophylactic effect in cisplatin-induced nephrotoxicity in rats. Cisplatin-induced nephrotoxicity was manifested by increases in blood urea nitrogen, plasma creatinine, urinary N-acetyl-β-(d)-glucosaminidase activity, kidney injury molecule 1, and histopathology. EET analogs (10 mg/kg/d) attenuated cisplatin-induced nephrotoxicity by reducing these renal injury markers by 40–80% along with a 50–70% reduction in renal tubular cast formation. This attenuated renal injury is associated with reduced oxidative stress, inflammation, and ER stress evident from reduction in related biomarkers and in the renal expression of genes involved in these pathways. Moreover, we demonstrated that the attenuated nephrotoxicity correlated with decreased apoptosis that is associated with 50–90% reduction in Bcl-2 protein family mediated proapoptotic signaling, reduced renal caspase-12 expression, and a 50% reduction in renal caspase-3 activity. We further demonstrated in vitro that the protective activity of EET analogs does not compromise the anticancer effects of cisplatin. Collectively, our data provide evidence that EET analogs attenuate cisplatin-induced nephrotoxicity by reducing oxidative stress, inflammation, ER stress, and apoptosis without affecting the chemotherapeutic effects of cisplatin.—Khan, Md. A. H., Liu, J., Kumar, G., Skapek, S. X., Falck, J. R., Imig, J. D. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity.
Keywords: chemotherapeutics, acute kidney injury, eicosanoids
Cisplatin, a platinum-based inorganic compound, is one of the most potent and widely used chemotherapy agents available to treat a variety of malignancies (1–3). Although cisplatin is widely used as an important chemotherapy drug in the clinic, it has potentially lethal adverse side effects. The most common adverse effect is severe nephrotoxicity, which occurs in 25–40% of cisplatin-treated patients and limits the safe and effective use of this chemotherapeutic agent (4–7). The pathology of cisplatin-induced nephrotoxicity involves enhanced oxidative stress, inflammation, increased endoplasmic reticulum (ER) stress, and renal cell apoptosis (5–10).
A number of studies have demonstrated that epoxyeicosatrienoic acids (EETs) display anti-inflammatory, antioxidative, and antiapoptotic activities that provide strong organ-protective potential (11, 12). Inhibition of soluble epoxide hydrolase (sEH; Ephx2), which hydrolyzes EETs to their less biologically active dihydroxyeicosatrienoic acid metabolite, increases EET bioavailability and provides kidney protection in a number of preclinical models of human diseases. Pharmacological inhibition of sEH in hypertensive Goto-Kakizaki rats and disruption of Ephx2 in Ephx2-knockout (KO) mice attenuated progression of renal damage associated with hypertension and diabetes (13, 14). These studies further demonstrated that the protective effect of sEH inhibition and Ephx2 KO in the kidney was strongly related to antioxidative and anti-inflammatory effects of EETs (13, 14). Apart from antioxidative and anti-inflammatory activities, EETs also protected cells from apoptosis. Overexpression of the EET-producing enzyme CYP epoxygenase inhibited caspase-3 activity and attenuated the down-regulation of antiapoptotic Bcl-2 and Bcl-xL expression during inflammation (15, 16). It was further reported that 11,12-EET inhibited anticancer drug arsenic trioxide-induced apoptosis, and this antiapoptotic effect of EETs was related to its strong antioxidant activity (17). Thus, strong evidence indicates that EETs provide organ protection through their anti-inflammatory, antiapoptotic, and antioxidative properties.
With such promising biological actions of EETs, interest in developing EET-based therapeutic strategies has grown enormously, and that has inspired the development of sEH inhibitors. Since sEH metabolizes EETs to their less active diols, sEH inhibition, in principle, indirectly increases endogenous EET levels. However, the major drawbacks of sEH inhibitors are that they result in a generalized increase in EETs and that their effectiveness depends entirely on CYP epoxygenase-mediated EET generation (18). This limitation is important because many renal and cardiovascular diseases are associated with impaired epoxygenase generation of EETs (12, 18, 19). It is likely that if CYP epoxygenase-mediated EET generation is impaired, then sEH inhibition will have a negligible effect on EET levels in these pathological conditions. It is further known that endogenously produced EETs are chemically and metabolically labile. As such, attempts have been made to develop EET analogs that possess EET-mimetic activity along with several key features important for stability and bioavailability (20, 21). Several recently developed EET analogs have demonstrated a number of biological actions, including organ protection (22, 23).
In the present study, we have investigated the kidney-protective effect of newly developed orally active EET analogs on cisplatin-induced nephrotoxicity. We demonstrated that these novel EET analogs offer marked kidney protection during cisplatin administration. This study further delineated the kidney protective action of EET analogs that were related to its antioxidative, anti-inflammatory, anti-ER, and antiapoptotic properties. Finally, we found that, while protecting the kidney from the deleterious nephrotoxic effect of cisplatin, the EET analogs did not compromise the anticancer cytotoxic activity of cisplatin.
MATERIALS AND METHODS
Materials
All chemicals and assay kits were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise mentioned. The EET analogs were designed and synthesized in the laboratory of J.R.F. (Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX, USA).
Animal experiments
Experiments were approved and carried out according to guidelines of the Institutional Animal Care and Use Committee, Medical College of Wisconsin. Male Wistar-Kyoto (WKY) rats weighing 180–200 g (Charles River, Wilmington, MA, USA) were used. Animals were kept in a temperature-controlled environment with a 12-h light-dark cycle and were allowed free access to food and water at all times. An acclimatization period of 6 d was allowed for the rats before experimentation. The rats were assigned into 4 groups (n=5–7/group). Group 1 rats received drinking water ad libitum and on d 7 were administered dimethylsulfoxide (DMSO; 300–500 μl i.p.), which is used to prepare cisplatin solution. Group 2, 3, and 4 rats were pretreated with vehicle [0.05% ethanol and 0.1% polyethylene glycol 400 (PEG-400) v/v], EET analog A (EET-A), or EET-B (10 mg/kg/d p.o.), respectively, in drinking water for 7 d. On d 7, these rats were administered a single dose of cisplatin (7 mg/kg i.p.) followed by another 5 d of treatment with vehicle or EET analogs. The experimental protocol is designed based on our observations in a pilot study, which revealed that a single cisplatin injection (5 mg/kg i.p.) in WKY rats increased plasma creatinine level by d 3, and this level reached a peak by d 5. We have also observed that the plasma creatinine remained elevated until d 15, with a level averaged at 2.3 ± 0.2 mg/dl.
At 1 d before euthanasia, the urine of each rat was collected over a 24-h period. At 5 d after cisplatin or vehicle administration, rats were anesthetized for blood sample collection, followed by euthanasia and tissue collection. Urine and plasma samples were kept frozen at −80°C until analyzed. The kidneys were removed, washed with physiological saline, and stored at −80°C until used for real-time PCR analysis, malonaldehyde (MDA) and tumor necrosis factor α (TNF-α) measurement, and caspase-3 activity assay. A part of the kidney was preserved in 10% buffered formalin for histological examination.
Biochemical analysis
The levels of blood urea nitrogen (BUN; BioAssay Systems, Hayward, CA, USA) and serum creatinine (Cayman Chemical Co., Ann Arbor, MI, USA) were measured spectrophotometrically using commercial kits. Urinary content of creatinine and protein were measured using commercial kits (Cayman), and the activity of urinary N-acetyl-β-(d)-glucosaminidase (NAG) in the urine was measured by a kit from Diazyme Laboratories (Poway, CA, USA). Urinary content of kidney injury molecule 1 (KIM-1) was measured using ELISA (R&D Systems, Inc. Minneapolis, MN, USA). Kidney tissue MDA was measured in the kidney using a commercially available kit (Cayman). To determine the kidney tissue MDA level, the rat kidney was homogenized with buffer containing 1.5% potassium chloride to obtain a 1:10 (w/v) whole-kidney homogenate. MDA was measured spectrophotometrically after reaction with thiobarbituric acid. Urinary and plasma monocyte chemoattractant protein 1 (MCP-1; BD Bioscience, San Diego, CA, USA) and renal tissue TNF-α content (Thermo Scientific, Rockford, IL, USA) were measured using ELISA kits. Caspase-3 activity in the kidney homogenate was determined using a commercial fluorimetric assay kit. Kidney homogenate was prepared with a lysis buffer (50 mM HEPES, pH 7.4, with 5 mM CHAPS and 5 mM DTT), centrifuged at 10,000 g for 10 min, and then the resulting supernatant was used for the assay. The caspase-3 fluorimetric assay is based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-ValAsp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) by caspase-3, resulting in the release of the fluorescent AMC moiety. Caspase-3 activity is expressed as nanomoles of AMC per minute per microliter. Kidney tissue protein content was measured using a BCA protein assay kit (Thermo Scientific).
Terminal deoxynucleotidyltransferase-mediated triphosphate nick-end labeling (TUNEL) assay
The TUNEL technique was used to determine apoptosis. Deparaffinized and gradually hydrated 3-μm-thick sections of kidney tissues were used to assess apoptosis using TUNEL assay. The TUNEL assay was performed using the TUNEL Apoptosis Detection Kit (GenScript, Piscataway, NJ, USA).
Real-time PCR analyses
Real-time PCR analysis was carried out to assess the mRNA expression of oxidative [gp91phox, NOX1, and superoxide dismutase 1–3 (SOD1, SOD2, SOD3)], inflammatory [TNF-α, interleukin (IL)-6, and IL-1β], apoptotic [Bcl-2-associated X protein (Bax), Bcl-2 antagonist/killer protein (Bak), and Bcl-2], and ER stress [glucose regulatory protein 78 (GRP78)/BiP and caspase-12]. Total RNA was isolated from kidney homogenate using TRIzol LS reagents (Invitrogen Life Technologies, Carlsbad, CA, USA), according to the manufacturer's instructions. The isolated RNA was treated with RNase-free DNase (Invitrogen Life Technologies) to remove traces of genomic DNA contamination. The mRNA samples were quantified by spectrophotometry at 260 nm, and 1 μg of total RNA was reverse-transcribed to cDNA using iScript Select cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The target gene expression was quantified by iScript One-Step RT-PCR Kit with SYBR green using MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). Each amplified sample in all wells was analyzed for homogeneity using dissociation curve analysis using iQ5 Optical System 2.1 software (Bio-Rad). After denaturation at 95°C for 2 min, 40 cycles were performed at 95°C for 10 s and at 60°C for 30s. Each sample was run in triplicate, and the comparative threshold cycle (Ct) method was used to quantify fold increase (2−ΔΔCt) in the expression of the target genes compared to controls. In analyzing the relative expression of the target genes, the Ct values were normalized to a housekeeping gene (pgk1). Statistical analyses were carried out for ≥5–7 experimental samples in each experimental group.
Histopathology
After fixation of the kidneys with 10% buffered formalin, renal tissues were sectioned and stained with periodic acid-Schiff (PAS) reagents for histological examination. The numbers of tubules that contain proteinaceous casts were determined at ×200 view to assess tubular damage using NIS Elements AR 3.0 image analysis software (Nikon Instruments Inc., Melville, NY, USA). The percentage area positive for cast was calculated from the mean of 8 cortical and 5 medullary fields (×200) for each kidney sample. To minimize observer bias, the cast area calculation was performed by 2 observers in a blinded fashion without knowledge of the treatment group from which the tissues were originated.
Immunohistology
Formaldehyde (10%) fixed and paraffin-embedded kidney tissues were cut in 3-μm sections. The tissue slides were deparaffinized and incubated with rodent declocker solution (Biocare Medical, Concord, CA, USA) at 95°C for antigen retrieval. The primary antibodies (1:50 or 1:100 dilutions in 1% goat serum) used to detect GRP78/BiP, caspase-12, and C/EBP homologous protein (CHOP) were anti-GRP78 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-caspase-12 (Abcam, Cambridge, MA, USA) and anti-CHOP (Abcam), respectively. Biotinylated rabbit anti-goat (Abcam) secondary antibody was used for development with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC kits; Vector Laboratory, Burlingame, CA, USA). The slides were counterstained with hematoxylin and photographed.
Cytotoxicity of cisplatin in the presence and absence of EET analog
In this study, HEK293, U87MG, HeLa, and NCCIT cell lines were used to investigate the antitumor activity of cisplatin in the presence and absence of EET-A. All cell lines were maintained in DMEM or RPIM with 10% fetal bovine serum and penicillin/streptomycin purchased from Life Technologies (Grand Island, NY, USA). Cells were seeded in 96-well plates at 500–4000 cells/well depending on the cell type. After 24 h, the cells were treated with cisplatin, vehicle, or EET-A at various concentrations for 72 h. Cell viability was measured by alamar blue assay using resazurin according to the manufacturer's guidelines. Cell viability was measured by fluorescence/absorbance in a 96-well plate reader from BMG Labtech (Cary, NC, USA), and the IC50 was calculated by GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA).
Statistical analysis
Results are reported as means ± sem. Statistical significance between 2 measurements was determined by the 2-tailed unpaired Student's t test, and significance among groups by repeated-measures 1-way analysis of variance followed by Tukey's post hoc test, using GraphPad Prism 4.0 software. Probability values of P < 0.05 were considered significant where the critical value of P was 2-sided.
RESULTS
Structure of EET analogs
Structures of the novel EET analogs used in the present study are shown in Supplemental Fig. S1.
EET analog treatment attenuates renal injury in cisplatin-treated rats
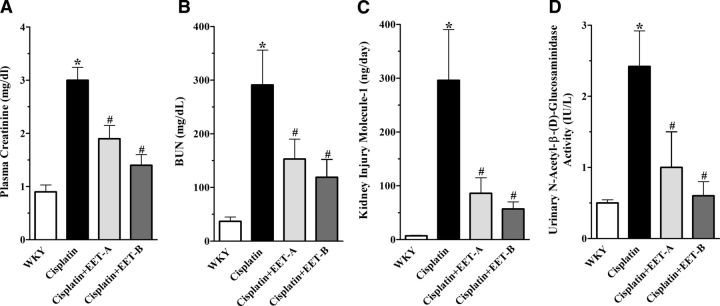
Cisplatin administration caused 3- and 9-fold increase in plasma creatinine and BUN levels, respectively. Treatment with EET-A and EET-B led to 40–60% reduction in serum creatinine and BUN levels (Fig. 1A, B). Cisplatin administration also caused 5- and 10-fold increase in urinary excretion of NAG and KIM-1, respectively. In cisplatin-treated rats, EET analogs reduced NAG and KIM-1 levels by 60–80% (Fig. 1C, D). Additional studies were conducted to determine the effect of EET analogs on plasma creatinine and BUN in control rats. No differences were found in BUN (37±8 vs. 35±9 mg/dl) and plasma creatinine (0.53±0.03 vs. 0.61±0.01 mg/dl) levels between rats treated with vehicle or EET analogs for 2 wk.
Figure 1.
Plasma creatinine (A), BUN (B), KIM-1(C), and urinary NAG (D) in cisplatin-treated rats pretreated with either EET analogs (EET-A and EET-B) or vehicle. Data are expressed as means ± sem, n = 5–7. *P < 0.05 vs. normal WKY rat; #P < 0.05 vs. vehicle-treated rat administered cisplatin.
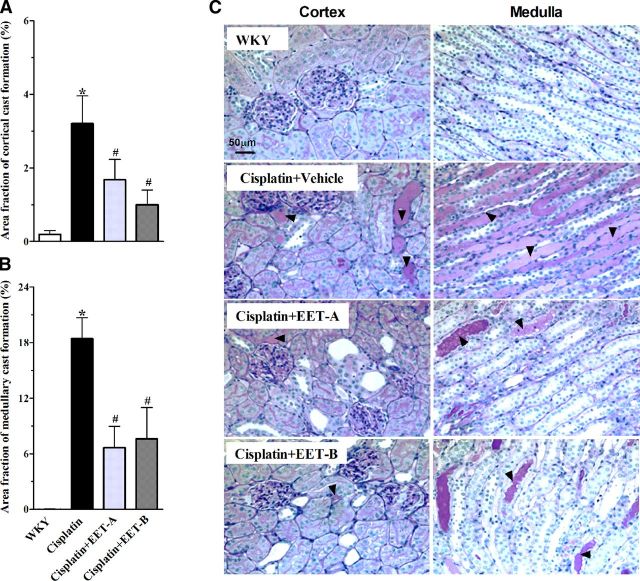
We further demonstrated that cisplatin administration caused marked proteinuria compared to vehicle administration (vehicle, 25.7±1; cisplatin, 53±5.1 mg/d), and EET analog reduced this proteinuria (EET-A, 33±8; EET-B, 32±3 mg/d; P<0.05). Cisplatin-induced kidney damage was also assessed using histological examination of the kidney. Cisplatin administration resulted in tubular injury, manifested by vacuolation and desquamation of the renal epithelial cells, along with intratubular proteinaceous cast formation involving 3 and 18% of the kidney cortical and medullary regions, respectively (Fig. 2). EET analog treatment decreased the tubular cast area by 50–70% in the renal cortex and medulla, as compared to vehicle (Fig. 2A, B).
Figure 2.
Calculated tubular cast area fraction (%) in the renal cortical (A) and medullary (B) sections of different experimental groups from representative photomicrographs of PAS staining (C; ×200 view) depicting tubular cast formation (arrowheads). Data are expressed as means ± sem, n = 5–7. *P < 0.05 vs. normal WKY rat; #P < 0.05 vs. vehicle-pretreated rat administered cisplatin.
EET analog treatment attenuates cisplatin-induced renal oxidative stress, inflammation, and ER stress
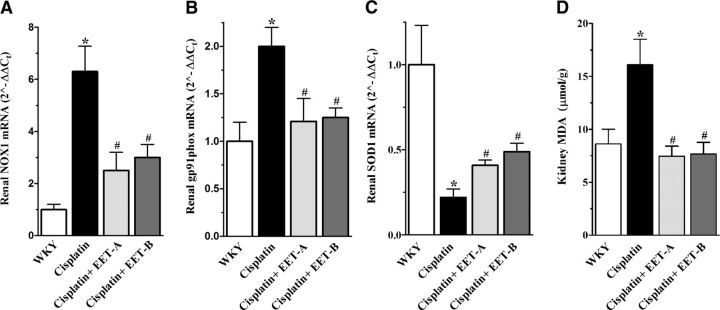
Real-time PCR analysis of the mRNA expressions of NADPH oxidase subunits NOX1 and gp91phox demonstrate increased expression of these oxidative marker genes following cisplatin administration. EET analog treatment caused 40–60% attenuation in the cisplatin-induced increase of renal NOX1 and gp91phox expression (Fig. 3A, B). It was also found that administration of cisplatin resulted in 50–80% reductions in the renal expression of SOD1 (Fig. 3C) and SOD3 (vehicle, 0.48±0.05; cisplatin, 1.0±0.1; P<0.05), while expression of SOD2 was unchanged (vehicle, 1.4±0.07; cisplatin, 1.0±0.6; P>0.05). In cisplatin-treated rats, EET analog treatment markedly increased the renal SOD1 expression compared to vehicle treatment (Fig. 3C), while the expression of SOD3 remained unaltered across the experimental groups (EET-A, 0.6±0.06; EET-B, 0.6±0.03; P>0.05). We further demonstrated that cisplatin administration elevated kidney MDA levels, an indicator of oxidative stress, and EET analog treatment reduced the MDA levels to values similar to WKY rats (Fig. 3D).
Figure 3.
A–C) Renal mRNA expression of NOX1 (A), gp91Phox (B), and SOD1 (C) in cisplatin-treated rats treated with either EET analog or vehicle. D) Kidney MDA levels in cisplatin-treated rats pretreated with either EET analog or vehicle. Data are expressed as means ± sem, n = 5–7. *P < 0.05 vs. normal WKY rat; #P < 0.05 vs. vehicle-pretreated rat administered cisplatin.
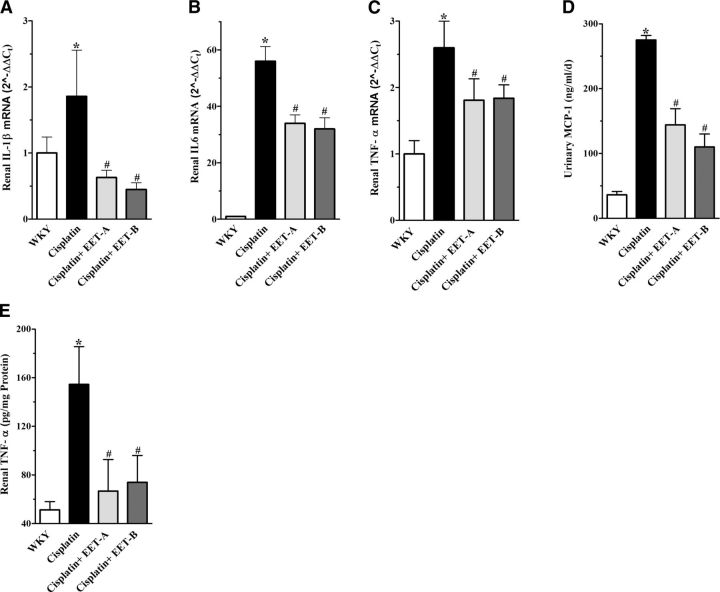
To investigate the effect of EET analog treatment on cisplatin-induced inflammation, we evaluated renal mRNA expression of IL-1β, IL-6, and TNF-α. We demonstrated a 2- to 50-fold increase in the renal expression of genes encoding these cytokines in cisplatin-treated rats. EET analogs reduced the renal expression of all inflammatory marker genes by 40–75% (Fig. 4A–C). We further studied urinary and plasma levels of MCP-1, as well as renal tissue levels of TNF-α. We found markedly elevated plasma MCP-1 levels in cisplatin-treated rats (156±39 ng/ml), compared to vehicle administration (1.4±1.0 ng/ml; P<0.05), and EET analog treatment attenuated the plasma MCP-1 levels in these cisplatin-treated rats (EET-A, 98±21; EET-B, 55±9 ng/ml; P<0.05). As in plasma, cisplatin-treated rats also had markedly elevated MCP-1 in the urine, and EET analog treatment attenuated the elevated urinary MCP-1 by 50–60% (Fig. 4D). We also demonstrated marked increase in the renal tissue levels of TNF-α in cisplatin-treated rats, and EET analog treatment attenuated the elevated TNF-α level by 65–70% (Fig. 4E).
Figure 4.
A–C) Renal mRNA expression of inflammatory markers IL-1β (A), IL-6 (B), and TNF-α (C) in cisplatin-treated rats pretreated with either EET analogs (EET-A and EET-B) or vehicle. D, E) Urinary MCP-1 (D) and renal tissue levels of TNF-α (E) in cisplatin-treated rats pretreated with either EET analogs or vehicle. Data are expressed as means ± sem, n = 5–7. *P < 0.05 vs. normal WKY rat; #P < 0.05 vs. vehicle-pretreated rat administered cisplatin.
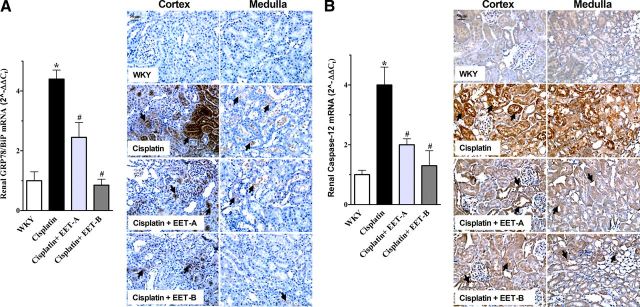
In the current study, we have further evaluated the renal expression of genes and proteins for ER stress markers GRP78/BiP, caspase-12, and CHOP. We found 4-fold increase in the renal expressions of GRP78/BiP and caspase-12 genes following cisplatin administration, while expression of CHOP was not affected (Fig. 5A, B and Supplemental Data). Interestingly, we demonstrated that EET analog treatment caused a 45–80% reduction in renal expression of GRP78/BiP and caspase-12 genes and proteins. Our results clearly demonstrate that EET analog treatment markedly attenuates the elevated renal oxidative stress, inflammation, and ER stress associated with cisplatin nephrotoxicity.
Figure 5.
A) Left panel: renal mRNA and protein expression of ER stress marker GRP78/BiP in cisplatin-treated rats pretreated with either EET analogs (EET-A and EET-B) or vehicle. Right panel: representative photomicrographs depicting immunopositive staining of GRP78/BiP in the kidney cortex and medulla (arrows). B) Left panel: renal mRNA and protein expression of ER stress marker caspase-12 in cisplatin-treated rats pretreated with either EET analogs or vehicle. Right panel: representative photomicrographs depicting immunopositive staining of caspase-12 in the kidney cortex and medulla (arrows). Data are expressed as means ± sem, n = 5–7. *P < 0.05 vs. normal WKY rat; #P < 0.05 vs. vehicle-pretreated rat administered cisplatin.
EET analog treatment attenuates cisplatin-induced renal apoptosis
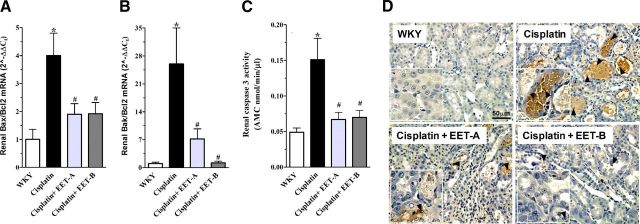
Cisplatin administration markedly reduced the renal expression of antiapoptotic Bcl-2 gene compared to vehicle administration (vehicle, 1.0±0.3; cisplatin, 0.1±0.05; P<0.05). Interestingly, EET analog treatment increased the expression of the antiapoptotic Bcl-2 gene in cisplatin-treated rats compared to vehicle treatment (EET-A, 0.48±0.15; EET-B, 2.0±0.4; P<0.05). Moreover, cisplatin administration resulted in 4- to 20-fold rise in the renal expression of proapoptotic Bax and Bak genes relative to antiapoptotic Bcl-2 gene (Bax/Bcl-2 and Bak/Bcl-2 ratios), and therefore indicated elevated apoptotic signaling in the cisplatin-treated rats. EET analog treatment caused a 50–90% reduction in Bax/Bcl-2 and Bak/Bcl-2 ratios, and therefore markedly attenuated the cisplatin-induced elevated apoptotic signaling (Fig. 6A, B). The cisplatin-induced elevation in apoptotic signaling was further characterized by significantly higher caspase-3 activity in cisplatin-treated rats. Treatment with EET analogs significantly attenuated cisplatin-induced caspase-3 activity to values similar to WKY (Fig. 6C). In addition, we demonstrated that the level of TUNEL-positive apoptotic cells was increased in the kidney of cisplatin-treated rats, and EET analog treatment markedly reduced apoptosis (Fig. 6D). These results clearly demonstrate that EET analog treatment markedly attenuates cisplatin-induced apoptotic signaling.
Figure 6.
A, B) Renal mRNA expression of proapoptotic Bak (A) and Bax (B) relative to the mRNA expression of antiapoptotic Bcl-2 in different experimental groups. C) Caspase-3 activity in different experimental groups. D) Representative photomicrographs show TUNEL-positive apoptotic cells in the outer strip of the outer medulla of kidney sections (brown stain; arrowheads). Insets: TUNEL-positive cells in the S3 segment (arrowheads) of the kidney proximal tubule present in the outer strip of the outer medullary region of the kidney. Data are expressed as means ± sem, n = 5–7. *P < 0.05 vs. normal WKY rat; #P < 0.05 vs. vehicle-pretreated rat administered cisplatin.
Effects of EET analogs on the cytotoxic activity of cisplatin
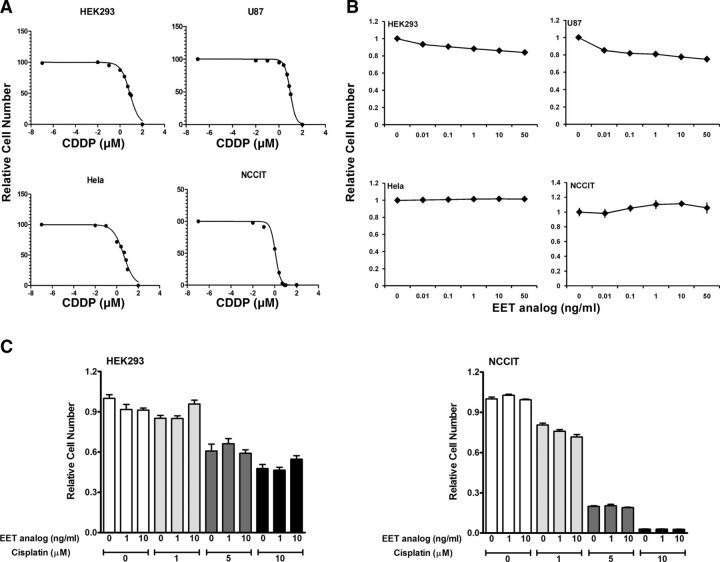
We demonstrate that in HEK293 cells derived from a normal human embryonic kidney, and in 3 different cancer cell lines, HeLa, NCCIT, and U87, cisplatin markedly inhibits cell growth, with IC50 ranging from 1.1 to 9.24 μM (Fig. 7A). In a similar approach with these cell lines, EET analog treatment had no observable effects on cell number (Fig. 7B). Moreover, we have evaluated the effect of EET analogs and cisplatin in HEK293 cells and found that concurrent application of EET analogs and cisplatin did not influence the cell growth. In a similar approach, we also found that concurrent application of EET analogs and cisplatin did not influence the cytotoxic activity of cisplatin in the NCCIT cancer cell line. During concurrent application of cisplatin and EET analogs on NCCIT cells, the IC50 for cisplatin was 2.60, 2.55, and 2.44 μM in the presence of EET analog at concentrations of 0, 1, and 10 ng/ml, respectively (Fig. 7C).
Figure 7.
A) Cytotoxic effect of cisplatin (CDDP) in normal kidney cell (HEK293) and cancer cells (HeLa, U87, NCCIT). B) Effect of EET analog on the cell growth of HEK293, HeLa, U87, and NCCIT. C) EET analog does not affect the cytotoxic effect of cisplatin in NCCIT cancer cells. Data are expressed as means ± sem, n = 5–7.
DISCUSSION
The defining limitation of cisplatin chemotherapy is the induction of tubulointestinal inflammation, renal oxidative stress, ER stress, and tubular cell apoptosis that lead to acute kidney injury (3, 5, 8, 10, 24). The main protective measure that is currently employed in clinical practice against cisplatin nephrotoxicity is hydration or diuretic therapy as an approach to decrease kidney cisplatin exposure (25, 26). Although considered useful, this approach is still inadequate, because 20–30% of the cancer patients receiving cisplatin develop nephrotoxicity (4, 6, 7, 10). Unfortunately, efficient pharmacotherapy for cisplatin-induced nephrotoxocity is not available. The current study investigated the kidney protective effect of EET analog treatment on cisplatin-induced nephrotoxicity.
Strong evidence indicates that EETs have the ability to protect organs by mechanisms involving anti-inflammatory, antiapoptotic and antioxidative activities. Indeed, it has been demonstrated that EETs provide organ protection in a number of preclinical models of human diseases, including diabetes, hypertension, and ischemic cardiac injury (27, 28). Consequently, in the present study we hypothesized that EET analogs, with their strong organ-protective potential, will attenuate cisplatin-induced nephrotoxicity. One approach to targeting EET for combating diseases is the development of EET analogs that are designed to resist metabolism and have improved bioavailability (29, 30). Several of these EET analogs have demonstrated organ protection in experimental disease models (27, 28). In line with these efforts, we have recently synthesized two novel orally active EET analogs and investigated their kidney protective potential in cisplatin-induced nephrotoxicity using a clinically relevant approach. In the current study, we clearly demonstrate that EET analog treatment provides marked kidney protection from cisplatin-induced nephrotoxicity, evidenced by a number of important and clinically relevant renal injury markers. Moreover, in the present study, we also demonstrate the plasma bioavailability of the EET analog in cisplatin-treated rats (Supplemental Data). These results provide strong support for the kidney-protective action of EET analogs in cisplatin-induced nephrotoxicity, and corroborate the clinical potential of the novel orally active EET analogs in preventing drug-induced nephrotoxicty.
In relation to our approach in the present study, it was recently reported that inhibition of the EET-degrading enzyme sEH reduced cisplatin-induced renal dysfunction in mice (31, 32). However, it is now known that current sEH inhibitors are limited in their effects, as they cause a generalized increase in EETs, and their biological effects depend on the generation of EETs by CYP epoxygenase enzymes. It is further known that in pathological conditions, endogenous EET generation can be low due to decreased levels or activities of CYP epoxygenase enzymes (33, 34). Therefore, it is highly plausible that exogenous administration of an EET analog will have better biological actions than sEH inhibition.
In the current study, we investigated the mechanism by which EET analogs provide kidney protection in cisplatin-induced nephrotoxicity. We demonstrate markedly increased expression of the genes that encode the major components of NADPH oxidase (NOX1 and gp91phox) in cisplatin-induced nephrotoxicty. Increased expression of these oxidative marker genes was accompanied by increased reactive oxygen species generation, evident from the elevated kidney MDA levels in the cisplatin-treated rats. We also found markedly reduced renal expression of antioxidant genes (SOD1 and SOD3) that would further contribute to the oxidative stress in cisplatin-treated rats. Similar observations have been reported in earlier studies where cisplatin-induced nephropathy was accompanied by increased MDA levels and elevated expression and activity of NADPH oxidase (35–37). Interestingly, our study demonstrated that EET analogs markedly reduced the renal oxidative stress by reducing the level of kidney MDA and renal expression of NOX1 and gp91phox genes, and also by attenuating the cisplatin-induced decrease in renal SOD1 expression. Indeed, in a recent study, it was reported that EETs up-regulate the expression and activity of SOD during toxic insult, and consequently enhance ROS scavenging and oxidative stress reduction (17). Another recent study demonstrated that renal NADPH oxidase and urinary MDA excretion were reduced in diabetic rats with a genetically disrupted sEH system and suggested the kidney-protective role of EETs in diabetic renal pathology in relation to their antioxidant effect (14).
Apart from oxidative stress, we further demonstrate that cisplatin-induced nephrotoxicity is accompanied by an elevated inflammatory response, as evident from increased renal MCP-1 and TNF-α levels. The elevated inflammatory response was also evident from the increased expression of renal TNF-α, IL-6, and IL-1β genes. Indeed, recent evidence suggests an important role for inflammatory mechanisms in the pathogenesis of cisplatin-induced nephrotoxicity (37–40). Cisplatin induces increased renal expression of a variety of inflammatory markers, such as TNF-α and IL-1β (38–40). In the present study, we demonstrated that EET analogs reduced plasma and urinary levels of MCP-1 and renal tissue levels of TNF-α and also attenuated the elevated renal expression of inflammatory marker genes in cisplatin-induced nephrotoxicity. Our results support earlier reports of anti-inflammatory activity of EETs that has also been implicated in organ protection in a number of pathologies characterized by kidney injury (13, 14, 41–43). Genetic disruption of sEH and the increase in EET levels reduces inflammation and provides kidney protection in streptozotocin-induced diabetes and in salt-sensitive hypertension (13, 14, 43). In these studies, our group demonstrated that genetic deletion or inhibition of sEH decreased renal NFκB activation and reduced renal MCP-1 levels in hypertension and diabetes (13, 14). We also demonstrated that genetic deletion of sEH decreased renal phospho-IKK levels in diabetes, supporting the notion that EETs could reduce renal injury via inhibition of phospho-IKK-derived NFκB activation (14). Moreover, overexpression of the EET-producing enzyme CYP2J2 has been shown to protect the kidney by reducing TGF-β in a chronic renal failure model of 5/6 nephrectomy (44). In the present study, we therefore clearly demonstrated that along with marked reduction in oxidative stress, an attenuation of the cisplatin-induced renal inflammatory response contributed to the EET analog-mediated kidney protection in cisplatin-induced nephrotoxicity.
Evidence shows that the ER is one of the subcellular targets of toxins and plays an important role in xenobiotic-induced nephrotoxicity (45). It was therefore of importance to investigate whether EET analog treatment could offset cisplatin-induced ER stress. Consequently, in the present study, we examined the renal expression of the genes and proteins of ER stress markers. We observed a marked up-regulation in the renal gene and protein expression of ER stress markers, and our findings are consistent with several earlier reports (45–48). Notably, we demonstrated that EET analog treatment attenuated the elevated renal expression of the proteins and the genes for the prominent ER stress marker GRP78/BiP and the ER stress-specific caspase, caspase-12 (10, 26, 46, 49). Indeed, the present study provided an interesting and novel finding regarding the biological action of EET analogs, and demonstrated an important aspect of the therapeutic potential of this in treating cisplatin-induced nephrotoxicity.
A significant feature of cisplatin and other drug-related nephrotoxicity is apoptosis (10) that is caused by elevated oxidative stress, inflammation, and ER stress (10, 26, 38, 39, 46–50). It is reported that during cisplatin-induced nephrotoxicity, the cellular stress caused by oxidative stress, inflammation, and ER stress leads to a reduction of antiapoptotic Bcl-2 and activation of the proapoptotic Bcl-2 family proteins, like Bax and Bak, in the kidney (10, 26, 51). This enhanced proapoptotic signaling leads to the activation of caspase-3, followed by apoptosis. Apoptosis of the renal cells is apparent from increased levels of TUNEL-positive cells localized mainly to the outer strip of the outer medulla and the S3 segment of the proximal tubule (5, 8, 10, 37). In the present study, we demonstrated that EET analog treatment protected the kidney from cisplatin-induced cell death by increasing antiapoptotic and decreasing proapoptotic signaling along with marked reductions in the activity of apoptosis executioner caspase-3 and apoptosis. We also demonstrated an EET analog-mediated attenuation in the renal expression of the gene for caspase-12 that plays an essential role in ER stress-mediated apoptosis. Indeed, it has been earlier reported that EETs attenuate several major apoptotic events, including elevated Bcl-2 protein-mediated proapoptotic signaling and caspase-3 activity (15–17). These observations supported our view on the ability of EET analogs to reduce renal cell death in cisplatin-induced nephrotoxicity through its attenuating effect on the Bcl-2 protein-mediated apoptotic signaling, reduction in the gene expression of ER stress-specific apoptosis mediator caspase-12, and a consequent reduction in the activity of apoptosis executioner caspase, caspase-3.
The present study clearly demonstrated that EET analog treatment provides strong protection from cisplatin-induced nephrotoxicity through multiple mechanisms, and indicates promising therapeutic potential. However, before considering the clinical use of any new cytoprotective agent against chemotherapy-related toxicity, protection from toxicity, as well as a lack of interference in the antitumor activity of the cytotoxic agents, must be demonstrated. To this end, in an in vitro approach, we investigated whether in vitro exposure of normal kidney cells (HEK293) or several human cancer cell lines (Hela, NCCIT, U87) to various concentrations of an EET analog influenced cell growth or the cytotoxic effect of cisplatin. We found that in the presence and absence of an EET analog, cisplatin was equally potent in exerting its cytotoxic effect in cancer cell lines. Comprehensive in vivo studies are required to demonstrate that EET analogs do not interfere with the chemotherapeutic activity of cisplatin. We have also investigated whether an EET analog influenced the growth of any of the cancer cell lines used in this study, and clearly demonstrated that an EET analog had no effect on the growth of any of the cancer cell lines.
In summary, the present study provides strong evidence of the kidney protective effect of EET analogs in cisplatin-induced nephrotoxicity. We have demonstrated that EET analogs provided kidney protection by the inhibition of multiple signaling pathways that are critically involved in the pathology of cisplatin-induced nephrotoxicity. This study highlighted several important biological actions of novel EET analogs in terms of their antioxidative, anti-inflammatory, anti-ER stress, and antiapoptotic activities. The results of the present study strengthen our view on the clinical translational implication of targeting EETs in ameliorating cisplatin-induced nephrotoxicity without compromising the chemotherapeutic activity of cisplatin.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
Acknowledgments
These studies were supported by U.S. National Institutes of Health (NIH) grants HL59699 and DK38226 to J.D.I.; Robert A. Welch Foundation grant GL625910 and NIH grants GM31278 and DK38226 to J.R.F.; and a postdoctoral fellowship from the Midwest Affiliate of the American Heart Association to Md.A.H.K.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AMC
- 7-amino-4-methylcoumarin
- Bak
- Bcl-2 antagonist/killer protein
- Bax
- Bcl-2-associated X protein
- BUN
- blood urea nitrogen
- CHOP
- C/EBP homologous protein
- DMSO
- dimethylsulfoxide
- EET
- epoxyeicosatrienoic acid
- EET-A/B
- epoxyeicosatrienoic acid analog A/B
- ER
- endoplasmic reticulum
- GRP-78
- glucose regulatory protein 78
- IL-1β/6
- interleukin-1β/6
- KIM-1
- kidney injury molecule 1
- KO
- knockout
- MCP-1
- monocyte chemoattractant protein 1
- MDA
- malonaldehyde
- NAG
- N-acetyl-β-(d)-glucosaminidase
- PAS
- periodic acid-Schiff
- PEG-400
- polyethylene glycol 400
- sEH
- soluble epoxide hydrolase
- SOD1–3
- superoxide dismutase 1–3
- TNF-α
- tumor necrosis factor α
- TUNEL
- terminal deoxynucleotidyltransferase-mediated triphosphate nick-end labeling
- WKY
- Wistar-Kyoto
REFERENCES
- 1.Bongers M. L., Coupé V. M., Jansma E. P., Smit E. F., Uyl-de Groot C. A. (2012) Cost effectiveness of treatment with new agents in advanced non-small-cell lung cancer: a systematic review. Pharmacoeconomics , 17–34 [DOI] [PubMed] [Google Scholar]
- 2.Levy A. R., Johnston K. M., Sambrook J., Donato B., Penrod J. R., Corral M., Chasen M. (2011) Indirect comparison of the efficacy of cetuximab and cisplatin in squamous cell carcinoma of the head and neck. Curr. Med. Res. Opin. , 2253–2259 [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Lippard S. J. (2005) Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. , 307–320 [DOI] [PubMed] [Google Scholar]
- 4.Kintzel P. E. (2001) Anticancer drug-induced kidney disorders. Drug Safety , 19–38 [DOI] [PubMed] [Google Scholar]
- 5.Miller R. P., Tadagavadi R. K., Ramesh G., Reeves W. B. (2010) Mechanisms of cisplatin nephrotoxicity. Toxins , 2490–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-González P. D., López-Hernández F. J., López-Novoa J. M., Morales A. I. (2011) An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit. Rev. Toxicol. , 803–821 [DOI] [PubMed] [Google Scholar]
- 7.Perazella M. A., Moeckel G. W. (2010) Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Semin. Nephrol. , 570–581 [DOI] [PubMed] [Google Scholar]
- 8.Lieberthal W., Triaca V., Levine J. (1996) Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am. J. Physiol. , F700–F708 [DOI] [PubMed] [Google Scholar]
- 9.Ma H., Jones K. R., Guo R., Xu P., Shen Y., Ren J. (2010) Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin. Exp. Pharmacol. Physiol. , 460–465 [DOI] [PubMed] [Google Scholar]
- 10.Pabla N., Dong Z. (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. , 994–1007 [DOI] [PubMed] [Google Scholar]
- 11.Imig J. D. (2012) Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. , 101–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imig J. D., Hammock B. D. (2009) Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. , 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olearczyk J. J., Quigley J. E., Mitchell B. C., Yamamoto T., Kim I. H., Newman J. W., Luria A., Hammock B. D., Imig J. D. (2009) Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin. Sci. (Lond.) , 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmarakby A. A., Faulkner J., Al-Shabrawey M., Wang M. H., Maddipati K. R., Imig J. D. (2011) Deletion of soluble epoxide hydrolase gene improves renal endothelial function and reduces renal inflammation and injury in streptozotocin-induced type 1 diabetes. Am. J. Physiol. , R1307–R1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B., Graham L., Dikalov S., Mason R. P., Falck J. R., Liao J. K., Zeldin D. C. (2001) Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol. Pharmacol. , 310–320 [DOI] [PubMed] [Google Scholar]
- 16.Zhao G., Wang J., Xu X., Jing Y., Tu L., Li X., Chen C., Cianflone K., Wang P., Dackor R. T., Zeldin D. C., Wang D. W. (2012) Epoxyeicosatrienoic acids protect rat hearts against tumor necrosis factor-α-induced injury. J. Lipid Res. 2012, , 456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., Chen C., Gong W., Li Y., Edin M. L., Zeldin D. C., Wang D. W. (2011) Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J. Pharmacol. Exp. Ther. , 451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imig J. D. (2010) Targeting epoxides for organ damage in hypertension. J. Cardiovasc. Pharmacol. , 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King L. M., Gainer J. V., David G. L., Dai D., Goldstein J. A., Brown N. J., Zeldin D. C. (2005) Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet. Genomics , 7–13 [DOI] [PubMed] [Google Scholar]
- 20.Morisseau C., Hammock B. D. (2005) Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu. Rev. Pharmacol. Toxicol. , 311–333 [DOI] [PubMed] [Google Scholar]
- 21.Falck J. R., Kodela R., Manne R., Atcha K. R., Puli N., Dubasi N., Manthati V. L., Capdevila J. H., Yi X. Y., Goldman D. H., Morisseau C., Hammock B. D., Campbell W. B. (2009) 14,15-epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J. Med. Chem. , 5069–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imig J. D., Elmarakby A., Nithipatikom K., Wei S., Capdevila J. H., Tuniki V. R., Sangras B., Anjaiah S., Manthati V. L., Sudarshan R. D., Falck J. R. (2010) Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive actions. Front. Physiol. , 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batchu S. N., Lee S. B., Qadhi R. S., Chaudhary K. R., El-Sikhry H., Kodela R., Falck J. R., Seubert J. M. (2011) Cardioprotective effect of a dual acting epoxyeicosatrienoic acid analogue towards ischaemia reperfusion injury. Br. J. Pharmacol. , 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagi R. (2009) Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron. Exp. Nephrol. , e1–e9 [DOI] [PubMed] [Google Scholar]
- 25.Benoehr P., Krueth P., Bokemeyer C., Grenz A., Osswald H., Hartmann J. T. (2005) Nephroprotection by theophylline in patients with cisplatin chemotherapy: a randomized, single-blinded, placebo-controlled trial. J. Am. Soc. Nephrol. , 452–458 [DOI] [PubMed] [Google Scholar]
- 26.Hanigan M. H., Deng M., Zhang L., Taylor P.T., Lapus M.G. (2005) Stress response inhibits the nephrotoxicity of cisplatin. Am. J. Physiol. Renal Physiol. , F125–F132 [DOI] [PubMed] [Google Scholar]
- 27.Seubert J. M., Zeldin D. C., Nithipatikom K., Gross G. J. (2007) Role of epoxyeicosatrienoic acids protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. , 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross G. J., Gauthier K. M., Moore J., Falck J. R., Hammock B. D., Campbell W. B., Nithipatikom K. (2008) Effects of the selective EET antagonist, 14,15- EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am. J. Physiol. Heart Circ. Physiol. , H2838–H2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imig J. D., Falck J. R. (2009) Compositions and methods for the treatment of renal and cardiovascular disease. U.S. Patent 7,550,617
- 30.Sodhi K., Inoue K., Gotlinger K. H., Canestraro M., Vanella L., Kim D. H., Manthati V. L., Koduru S. R., Falck J. R., Schwarzman M. L., Abraham N. G. (2009) Epoxyeicosatrienoic acid agonist rescues metabolic syndrome phenotype of HO-2-null mice. J. Pharmacol. Exp. Ther. , 906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrish A. R., Chen G., Burghardt R. C., Watanabe T., Morisseau C., Hammock B. D. (2009) Attenuation of cisplatin nephrotoxicity by inhibition of soluble epoxide hydrolase. Cell Biol. Toxicol. , 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Webb H. K., Fukushima H., Micheli J., Markova S., Olson J. L., Kroetz D. L. (2012) Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor κB signaling. J. Pharmacol. Exp. Ther. , 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaergel E., Muller D. N., Honeck H., Theuer J., Shagdarsuren E., Mullally A., Luft F. C., Schunck W. H. (2002) P450-dependent arachidonic acid metabolism and angiotensin II-induced renal damage. Hypertension , 273–279 [DOI] [PubMed] [Google Scholar]
- 34.Marino J. P. (2009) Soluble epoxide hydrolase, a target with multiple opportunities for cardiovascular drug discovery. Curr. Top. Med. Chem. , 452–463 [DOI] [PubMed] [Google Scholar]
- 35.Jia Z., Wang N., Aoyagi T., Wang H., Liu H., Yang T. (2011) Amelioration of cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int. , 77–88 [DOI] [PubMed] [Google Scholar]
- 36.El-Beshbishy H. A., Bahashwan S. A., Aly H. A., Fakher H. A. (2011) Abrogation of cisplatin-induced nephrotoxicity in mice by alpha lipoic acid through ameliorating oxidative stress and enhancing gene expression of antioxidant enzymes. Eur. J. Pharmacol. , 278–284 [DOI] [PubMed] [Google Scholar]
- 37.Mukhopadhyay P., Horváth B., Zsengellér Z., Zielonka J., Tanchian G., Holovac E., Kechrid M., Patel V., Stillman I. E., Parikh S. M., Joseph J., Kalyanaraman B., Pacher P. (2012) Cisplatin-induced nephropathy. Free Radic. Biol. Med. , 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramesh G., Reeves W. B. (2002) TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. , 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faubel S., Lewis E. C., Reznikov L., Ljubanovic D., Hoke T. S., Somerset H., Oh D. J., Lu L., Klein C. L., Dinarello C. A., Edelstein C. L. (2007) Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. , 8–15 [DOI] [PubMed] [Google Scholar]
- 40.Zhang B., Ramesh G., Norbury C. C., Reeves W. B. (2007) Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. , 37–44 [DOI] [PubMed] [Google Scholar]
- 41.Falck J. R., Reddy L. M., Reddy Y. K., Bondlela M., Krishna U. M., Ji Y., Sun J., Liao J. K. (2003) 11,12-epoxyeicosatrienoic acid (11,12-EET): structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg. Med. Chem. Lett. , 4011–4014 [DOI] [PubMed] [Google Scholar]
- 42.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science , 1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manhiani M., Quigley J. E., Knight S. F., Tasoobshirazi S., Moore T., Brands M.W., Hammock B. D., Imig J. D. (2009) Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am. J. Physiol. Renal Physiol. , F740–F748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao G., Tu L., Li X., Yang S., Chen C., Xu X., Wang P., Wang D. W. (2012) Delivery of AAV2-CYP2J2 protects remnant kidney in the 5/6-nephrectomized rat via inhibition of apoptosis and fibrosis. Hum. Gene Ther. , 688–699 [DOI] [PubMed] [Google Scholar]
- 45.Cribb A. E., Peyrou M., Muruganandan S., Schneider L. (2005) The endoplasmic reticulum in xenobiotic toxicity. Drug Metab. Rev. , 405–442 [DOI] [PubMed] [Google Scholar]
- 46.Mandic A., Hansson J., Linder S., Shoshan M. C. (2003) Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J. Biol. Chem. , 9100–9106 [DOI] [PubMed] [Google Scholar]
- 47.Peyrou M., Hanna P. E., Cribb A. E. (2007) Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol. Sci. , 346–353 [DOI] [PubMed] [Google Scholar]
- 48.Rabik C. A., Fishel M. L., Holleran J. L., Kasza K., Kelley M. R.,., Egorin M. J., Dolan M. E. (2008) Enhancement of cisplatin [cis-diamminedichloroplatinum (II)] cytotoxicity by O6-benzylguanine involves endoplasmic reticulum stress. J. Pharmacol. Exp. Ther. , 442–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breckenridge D. G., Germain M., Mathai J. P., Nguyen M., Shore G. C. (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene , 8608–8618 [DOI] [PubMed] [Google Scholar]
- 50.Park M. S., De Leon M., Devarajan P. (2002) Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J. Am. Soc. Nephrol. , 858–865 [DOI] [PubMed] [Google Scholar]
- 51.Wei Q., Dong G., Franklin J., Dong Z. (2007) The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. , 53–62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.