Abstract
Eukaryotic cells maintain strict control over protein secretion, in part by using the pH gradient maintained within their secretory pathway. How eukaryotic proteins evolved from prokaryotic orthologs to exploit the pH gradient for biological functions remains a fundamental question in cell biology. Our laboratory previously demonstrated that protein domains located within precursor proteins, propeptides, encode histidine-driven pH sensors to regulate organelle-specific activation of the eukaryotic proteases furin and proprotein convertase-1/3. Similar findings have been reported in other unrelated protease families. By analyzing >10,000 unique proteases within evolutionarily unrelated families, we show that eukaryotic propeptides are enriched in histidines compared with prokaryotic orthologs. On this basis, we hypothesize that eukaryotic proteins evolved to enrich histidines within their propeptides to exploit the tightly controlled pH gradient of the secretory pathway, thereby regulating activation within specific organelles. Enrichment of histidines in propeptides may therefore be used to predict the presence of pH sensors in other proteases or even protease substrates.—Elferich, J., Williamson, D. M., Krishnamoorthy, B., Shinde, U. Propeptides of eukaryotic proteases encode histidines to exploit organelle pH for regulation.
Keywords: secretory pathway, cathepsins
Eukaryotes are descendants of distinct prokaryotic cells that united symbiotically and evolved complex cellular compartments called organelles (1). Secretory and endocytotic organelles maintain a precise pH gradient from the endoplasmic reticulum (ER; pH ∼7.2) to secretory vesicles (pH ∼5.5) and provide unique environmental conditions essential for optimal protein structure and function (2). How eukaryotic proteins differ from prokaryotic orthologs to be able to exploit the pH gradient for biological function is a fundamental question in cell biology and represents a major challenge to our understanding of protein trafficking, protein evolution, and organelle complexity. Comparing secreted eukaryotic proteases with bacterial orthologs will help to decipher the theoretical underpinnings that enable proteins to exploit the pH gradient within the secretory pathway and provide general principles for the relationship between the structure, dynamics, and function of biomolecules.
Proteases hydrolyze peptide bonds and probably arose during evolution as simple catabolic enzymes to generate amino acid nutrients for primitive organisms (3). Their ubiquitous distribution and the presence of orthologs in prokaryotes, eukaryotes, and archeae make proteases ideal models for analyzing the selective pressures that drove adaptation of eukaryotic proteins to complex organelle trafficking. Because uncontrolled proteolysis can have catastrophic consequences, cells have evolved at least two distinct mechanisms to maintain tight spatiotemporal control of protease activity. The first involves coevolution of specific endogenous inhibitors, typically within compartments distinct from those containing active enzymes. The second mechanism involves proteases being synthesized as inactive precursors called zymogens, which become active by limited intra- or intermolecular proteolysis. In some cases, the two regulatory mechanisms are combined: N-terminal propeptides coevolved to chaperone folding of cognate catalytic domains and act as potent temporary inhibitors after cleavage from the catalytic domain (4).
Here we hypothesize that one of the ways proteases adapted to eukaryotic organelle systems was by encoding histidine-based pH sensors in their N-terminal propeptides. When encoded at suitable positions within domains, the unique pKa of histidine side chains (∼6.5) can alter electrostatic interactions and modify conformations through changes in pH within the secretory pathway. These changes can then either affect the inhibitory potential of propeptides or increase their susceptibility to proteolysis.
pH SENSORS IN THE PROPEPTIDES OF SUBTILASES
Subtilases, a ubiquitous superfamily of serine proteases, represent an ideal group of homologs to analyze protein adaptation to eukaryotic organelles. Bacterial subtilases are mostly secreted and undergo pH-independent activation, whereas eukaryotic subtilases undergo pH-dependent activation, usually in specific organelles (4, 5). Bacterial subtilisin and mammalian proprotein convertase (PC) subfamilies constitute the most extensively studied subtilases (6, 7). Despite evolutionary divergence, proteins in these subfamilies display common folds with conserved catalytic triads and are almost always expressed as zymogens, with N-terminal and occasionally C-terminal propeptide extensions. Similar to bacterial subtilases, propeptides of PCs assist folding and require 2 ordered steps of proteolytic cleavage for activation (4). Our understanding of PC activation is based on studies of profurin, a constitutively expressed PC homolog. The first cleavage occurs at a consensus site RTKR107↓ after protein folding in the ER and results in a noncovalent propeptide-protease complex. Activation requires additional cleavage at the internal site 69HRGVTKR75↓ in the furin propeptide, which only occurs when furin traffics into the trans-Golgi network (TGN)/endosomal system (7). Other PCs are activated in a similar manner but within different organelles (8). Studies established that a conserved histidine residue (His69) in the propeptide of furin acts as a pH sensor (9), the pH of the TGN is sufficient to trigger the second, activating cleavage of furin (5), and propeptide domains in PCs contain sufficient information to mediate pH-dependent activation of cognate proteases and undergo conformational changes that correlate with their respective pH of activation. Prokaryotic propeptides are stable over the pH range. Amino acid composition analysis shows increased histidine content in propeptides of furin and PC1, compared with the average content in the UniProt database (UniProt Consortium; http://www.uniprot.org/), whereas the bacterial propeptides show no such bias (10). This finding is consistent with the hypothesis that encoding of histidines in the propeptides allows for sensing organellar pH to direct activation as proposed in this article.
Such a broad hypothesis is difficult to test experimentally, because it would require biochemical studies on a large number of proteins. However, amino acid composition can be easily calculated from large amounts of available sequence data, and one would expect a consistent bias for histidine content in propeptides of eukaryotic but not prokaryotic proteins.
To examine whether residue-specific biases exist within eukaryotic propeptides, we computed the abundance of individual residues in propeptides and catalytic domains in 6533 unique subtilases from the PFAM database (Wellcome Trust Sanger Institute; http://pfam.sanger.ac.uk/) entry PF00082 (11). We calculated for each sequence the difference in abundance of histidine content (Δ[His]) between the propeptide ([His]Pro) and the catalytic domain ([His]Cat). Positive Δ[His] values indicate abundance of histidines in propeptides, negative values signify abundance in proteases, and values near 0 imply equal distribution. Whereas Δ[His] values in individual proteins may be subject to random fluctuations, the absence of any functional requirements would result in a distribution centered around 0. If histidine residues in propeptides are required for the experimentally observed function of sensing organelle-specific pH, they would be selected during evolution, and one would expect statistical bias for positive Δ[His] only within eukaryotic subtilases and near 0 or negative Δ[His] for prokaryotes.
For initial assessments, we plotted Δ[His] on a phylogenetic tree generated by the PFAM database (Fig. 1A), which is consistent with homology groups defined earlier (12), with the largest clades representing subtilisin, kexin, proteinase K, and pyrolisin, as well as the later characterized sedolisin family (13). Although the subtilisin family is exclusive to prokaryotes, the remaining 4 families contain eukaryotic and prokaryotic proteins, suggesting that these families diverged before speciation. Notably, 3 of the 4 families display predominantly positive Δ[His] in eukaryotes but not in prokaryotes. Only sedolisins show positive Δ[His] values in both prokaryotes and eukaryotes.
Figure 1.
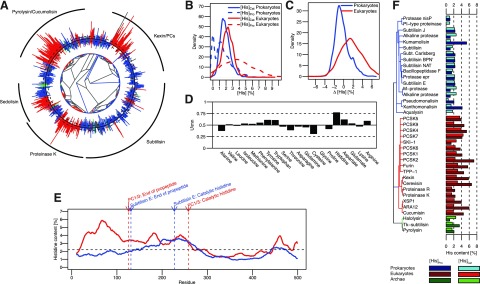
Histidines are enriched in propeptides of eukaryotic but not prokaryotic subtilases. A) Phylogenetic tree of subtilases from the PFAM database. Bars on the outside indicate the Δ[His] value of each sequence. Black circle represents 0%. Bars pointing outward and inward represent positive and negative Δ[His] values, respectively. Dashed circles outside and inside of the solid black circle represent Δ[His] values of ±1%. Eukaryotic, prokaryotic, and archean sequences are colored red, blue, and green, respectively. Black arcs on the outside mark the clades of major subtilase subfamilies. B) Kernel density estimation of the distribution of [His]Pro and [His]Cat in prokaryotes and eukaryotes. C) Kernel density estimation of the distribution of Δ[His] for prokaryotes and eukaryotes. D) Effect size (U/mn) of the Mann-Whitney test for differences between the distributions shown in panel C performed for all 20 natural amino acids. E) Sliding window analysis of average histidine content in eukaryotic and prokaryotic subtilases using a window of 20 residues. Black dashed line indicates average histidine content in the UniProt database. Arrows indicate relative position of annotations for the end of the propeptide domain and the catalytic histidine residue according to subtilisin E and PC1/3. F) Bar graph showing [His]Pro and [His]Cat values for selected subtilases. Blue, red, and green shades represent prokaryotic, eukaryotic, and archean sequences, respectively. Light shades indicate [His]Cat; dark shades indicate[His]Pro.
The distributions of [His]Pro and [His]Cat (Fig. 1B) establish that catalytic domains in subtilases display a distribution centered on 2%, with eukaryotes having slightly higher [His]Cat values than prokaryotes, as expected from the average histidine content in the UniProt database (2.3%). Whereas the distribution of [His]Pro within propeptide domains in prokaryotes shifts toward lower values, with several lacking histidines, the [His]Pro distribution in eukaryotic propeptides is shifted to higher values, much greater than those in catalytic domains. This bias is clearly shown by the Δ[His] distributions (Fig. 1C, median values of −0.56 and 1.5% in prokaryotes and eukaryotes, respectively. To quantify the significance of the difference in distribution between species, we used a nonparametric Mann-Whitney test (Table 1) across all 20 amino acids. The test resulted in values of P < 0.05 for several amino acids, indicating statistically significant differences in amino acid change Δ[AA]) distributions between eukaryotes and prokaryotes. Because large sample sizes can result in statistically significant P values even for tiny differences, a more meaningful, sample size-independent measure of the difference in distribution can be obtained using effect sizes (U/mn; ref. 14). These values vary between 0.0 and 1.0 and estimate the probability that a random sample of Δ[AA] in eukaryotes is larger than a random sample of Δ[AA] in prokaryotes. Equal distribution of Δ[AA] in both species would result in an effect size of 0.5. As seen in Fig. 1D, histidine shows the highest deviation from 0.5, suggesting that this bias does not occur by pure chance. Only cysteine deviates substantially (>0.15 U) from 0.5, which is probably due to a higher frequency of disulfide bonds in eukaryotes than in prokaryotes. The fact that deviation from 0.5 in the effect size for histidine is considerably greater than that observed for cysteine suggests a biological significance for a histidine bias.
Table 1.
Results of Mann-Whitney tests to evaluate differences in distribution of Δ[AA] between eukaryotes and prokaryotes
| Residue | Eukaryotes | Prokaryotes | U | P | U/mn |
|---|---|---|---|---|---|
| A | −2.01 | −0.29 | 3,484,667 | 6.3 × 10−56 | 0.38 |
| V | −0.03 | −0.13 | 4,692,108 | 1.4 × 10−1 | 0.51 |
| L | 1.27 | 1.53 | 4,494,450 | 1.8 × 10−1 | 0.49 |
| I | −0.29 | −0.61 | 4,845,335 | 2.4 × 10−4 | 0.53 |
| M | −0.32 | −0.44 | 4,781,449 | 5.7 × 10−3 | 0.52 |
| F | 0.53 | 0.06 | 5,019,341 | 7.3 × 10−10 | 0.55 |
| Y | −0.27 | −1.00 | 5,564,109 | 3.6 × 10−44 | 0.61 |
| W | −0.46 | −0.92 | 5,529,692 | 3.2 × 10−41 | 0.60 |
| S | −0.47 | −0.18 | 4,329,195 | 2.2 × 10−4 | 0.47 |
| T | −1.36 | −0.07 | 3,616,488 | 9.2 × 10−44 | 0.39 |
| N | −1.63 | −1.86 | 4,339,854 | 3.9 × 10−4 | 0.47 |
| Q | 1.11 | 1.49 | 4,198,344 | 2.6 × 10−8 | 0.46 |
| C | −1.25 | −0.28 | 2,881,834 | 7.5 × 10−132 | 0.31 |
| G | −5.2 | −5.3 | 4,576,112 | 8.7 × 10−1 | 0.50 |
| P | −0.67 | 0.04 | 3,852,582 | 8.5 × 10−26 | 0.42 |
| D | −0.25 | −1.52 | 5,644,738 | 1.9 × 10−51 | 0.62 |
| E | 2.85 | 2.51 | 4,864,907 | 7.7 × 10−5 | 0.53 |
| H | 1.53 | −0.56 | 7,048,731 | 1.6 × 10−270 | 0.77 |
| K | 1.45 | 1.58 | 4,356,812 | 9.6 × 10−4 | 0.47 |
| R | 1.86 | 0.95 | 5,376,156 | 2.2 × 10−29 | 0.59 |
For each amino acid, the following numbers are reported: median of Δ[AA] for eukaryotes and prokaryotes, test statistic of the Mann-Whitney test, the resulting P value, and the effect size U/mn. Sample sizes were 2156 and 4256 for eukaryotes and prokaryotes, respectively.
Because errors in database annotation and differences in length between propeptides and catalytic domains may result in a false-positive bias, we developed a test that is independent of the start annotation in the PFAM database. We calculated histidine content in a 20-residue sliding window from the beginning of the sequence to the end of the catalytic domain for all sequences. After normalization to sequence length, we averaged the resulting histidine content profiles for eukaryotic and prokaryotic proteins. Eukaryotic proteins alone show an increase in histidine content in the first 100 residues corresponding to the propeptide (Fig. 1E), whereas both species have increased histidine content at positions 200−250, probably due to the active site histidine, along with a small increase at the C terminus due to a conserved histidine in the catalytic domain.
To decipher correlations that may exist between the histidine bias and experimental evidence of pH-dependent activation, we analyzed histidine contents in propeptides and catalytic domains of individual proteins (Fig. 1F). Most bacterial proteins display similar histidine content within propeptides and catalytic domains (∼2%), with only kumamolisin and xanthomonalisin displaying histidine enrichment (>4%) in cognate propeptides. Consistent with our hypothesis, both proteins undergo activation at acidic pH in vitro (15, 16), which is not surprising because their hosts display optimum growth under acidic conditions. Because intracellular pH within cells is maintained near neutral, pH sensing is an ideal mechanism for discerning intracellular and extracellular environments. Both proteins belong to the sedolisin family, which has evolved to function under acidic conditions (13), explaining the histidine bias in propeptides in eukaryotes as well as prokaryotes. Eukaryotic propeptides display histidine contents of >4% (except proteinase K and SKI-1). Expression of proteinase K in Escherichia coli produces active protease (17), and SKI-1 loses its propeptide in the ER (18), suggesting that activation occurs at neutral pH, relaxing the necessity for histidines.
pH SENSORS IN THE PROPEPTIDES OF CATHEPSINS
To investigate whether our hypothesis applies to other pH-activated, propeptide-dependent proteases, we analyzed histidine content in cathepsins, a large family of lysosomal cysteine peptidases (19), which, similar to subtilases, can activate at acidic pH. Because of these parallels, we hypothesized that eukaryotic cathepsins should show a similar bias for histidine in their propeptides.
We plotted the phylogenetic tree for cathepsin sequences (PFAM family PF00112) along with their Δ[His] values (Fig. 2A) in a manner identical to that for subtilases. The 2 major well-studied cysteine cathepsin subfamilies are the cathepsin L (CatL)-like and the cathepsin B (CatB)-like families, both of which activate at low pH (19–21). The CatL-like family includes human cathepsins L, V, H, K, and S, and the CatB-like family includes human cathepsin B. In addition, humans encode 5 more cathepsin genes, which we do not discuss here because of their inability to autoactivate (cathepsin C and O) or unusual long or short propeptides (cathepsin F and X, respectively; ref. 19). The CatL-like family shows positive Δ[His] values; the CatB-like family does not. Moreover, the distributions of [His]Pro and [His]Cat in the CatL-like family mimic eukaryotic subtilases (Fig. 2B), whereas the CatB-like family displays increased [His]Pro and [His]Cat values, leading to Δ[His] values near 0 (Fig. 2C). Prokaryotic cathepsins show distributions similar to those for prokaryotic subtilases. The small number of prokaryotic sequences precludes a statistical comparison between species with robustness similar to that of subtilases.
Figure 2.
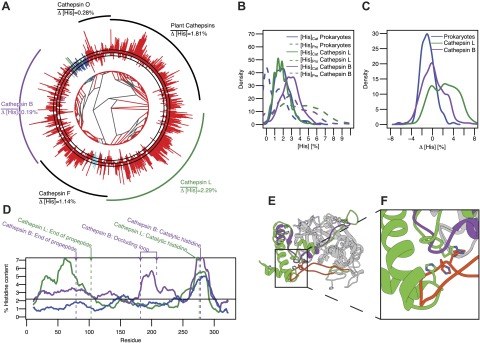
Histidine enrichment exists only in propeptide domains of the CatL family, whereas it is also present in the occluding loop of the CatB family. A) Phylogenetic tree of cathepsins from the PFAM database. Bars on the outside indicate the Δ[His] value of each sequence. Black circle represents 0%. Bars pointing outward and inward represent positive and negative Δ[His] values, respectively. Dashed circles outside and inside of the solid black circle represent Δ[His] values of ±1%. Eukaryotic, prokaryotic, archean, and viral sequences are colored red, blue, green, and cyan, respectively. Black arcs on the outside mark the clades of major cathepsin subfamilies, with the CatL family shown in green and the CatB family shown in purple. B) Kernel density estimation of the distribution of [His]Pro and [His]Cat in CatL and CatB families and in prokaryotes. C) Kernel density estimation of the distribution of Δ[His] in CatL and CatB families and in prokaryotes. D) Sliding window analysis of average histidine content in CatL and CatB families and in prokaryotes using a window of 20 residues. Black dashed line indicates average histidine content in the UniProt database. Arrows indicate relative position of annotations for the end of the propeptide domain and the catalytic histidine residue according to CatL and CatB, as well as the occluding loop in CatB. E) Structure superimposition of procathepsin L (Protein Data Bank: 1BY8) and procathepsin B (Protein Data Bank: 1MIR). The catalytic domains are shown in gray ribbon, whereas propeptides are shown in green and purple for CatL and CatB, respectively. The occluding loop of CatB is colored in orange and the corresponding loop in CatL is colored green. The side chains of histidine residues are depicted as stick representations. F) Close-up view of interactions between the occluding loop and the propeptide. Colors are as indicated above.
We next validated the increased histidine content in the CatL-like family propeptides and mapped the specific location of increased histidine content in the CatB-like sequences using sliding window analysis (Fig. 2D). Prokaryotic cathepsins showed low histidine content throughout the sequence, with one peak between residues 250 and 300, which is due to the catalytic histidine. Consistent with our hypothesis, an increase in histidine content exists within the first 100 residues of CatL-like sequences. Notably, CatB-like sequences show a moderate increase in histidine content within the first 100 residues compared with prokaryotes, along with a second peak corresponding to the occluding loop within the catalytic domain (Fig. 2D, E). A comparison of the crystal structures of CatL and CatB (Fig. 2E) shows that the catalytic domains of the 2 families are similar. However, compared with CatL, the CatB propeptide is truncated, whereas its occluding loop in the catalytic domain is longer and forms direct contacts with its propeptide by extending into the region occupied by the CatL propeptide in a complex with its cognate protease. Notably, histidines within the occluding loop of CatB occupy spatial locations similar to those for the histidine residues within the CatL propeptide (Fig. 2F). This finding suggests that the pH-sensing capability in CatB is encoded not only within the propeptide but also in the occluding loop within the catalytic domain, which is consistent with experimental data demonstrating that the occluding loop interacts with the propeptide in a pH-dependent manner and histidine to alanine substitutions within the occluding loop block activation (22). Moving pH sensitivity from propeptides onto catalytic domains provides evolutionary advantages to the CatB-like family by enabling members to switch between endo- and exopeptidases in a pH-dependent manner (23). In summary, histidine distribution in cathepsins is consistent with our hypothesis, although subtle variations can exist within individual propeptide-dependent protease families.
CYTOSOLIC CASPASE FAMILY MEMBERS ENCODE NO pH SENSORS IN THEIR PROPEPTIDES
Our hypothesis assumes that eukaryotic proteases require histidines in their propeptides to sense the pH of the secretory pathway. Therefore, cytosolic proteases, such as the caspase family members, would be expected to show no histidine bias within their propeptides. Caspases are responsible for initiating apoptosis within eukaryotic cells (24) and are expressed as inactive zymogens that are activated by proteolytic processing, similar to subtilases and cathepsins.
We plotted the phylogenetic tree for caspase sequences (PFAM family PF00656) along with their Δ[His] values (Fig. 3A) in a manner identical to those for subtilases and cathepsins. The phylogenetic tree demonstrates that caspase homologs are found in metazoans, fungi, and plants. We excluded metacaspases (homologs in fungi and plants) from our analysis because their propeptides contain histidine residues that are involved in zinc binding (25). Metazoan caspases demonstrate increased [His]Cat values, whereas [His]Pro values are similar to those for prokaryotic caspases (Fig. 3B). Consistently, Δ[His] values were slightly smaller for eukaryotic proteins (Fig. 3C). The sliding window analysis of prokaryotic and eukaryotic caspases shows that there is no substantial histidine enrichment in the N-terminal residues (Fig. 3D). Overall, these results are consistent with the assumption that the functional requirement of histidines in propeptides is unique to proteases that need to sense pH to direct their activation.
Figure 3.
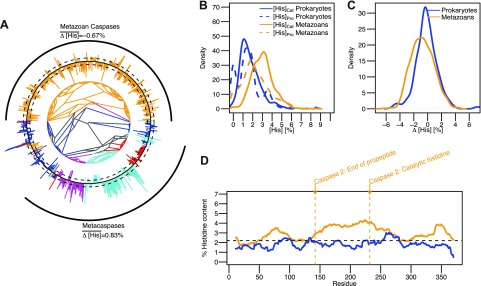
The cytosolic caspase family shows no histidine bias in propeptides. A) Phylogenetic tree of caspases from the PFAM database. Bars on the outside indicate the Δ[His] value of each sequence. Black circle represents 0%. Bars pointing outward and inward represent positive and negative Δ[His] values, respectively. Dashed circles outside and inside of the solid black circle represent Δ[His] values of ±1%. Prokaryotic, metazoan, plant, fungal, and other eukaryotic sequences are colored blue, yellow, cyan, purple, and red, respectively. Black arcs on the outside depict the metazoan caspase and metacaspase families. B) Kernel density estimation of the distribution of [His]Pro and [His]Cat in prokaryotes and metazoan is shown in blue and yellow, respectively. C) Kernel density estimation of the distribution of Δ[His] in metazoan and prokaryotic caspases is shown in yellow and blue, respectively. D) Sliding window analysis of average histidine content in metazoan and prokaryotic caspases using a window of 20 residues. Arrows indicate relative position of annotations for the end of the propeptide domain and the catalytic histidine residue according to caspase 2.
IMPLICATIONS FOR OTHER PROTEINS AND DISEASE
Because histidine enrichment correlates with pH-mediated activation in subtilases and cathepsins, we propose that it can be used to predict proteins that use a similar mechanism for activation. A list of all human proteins with annotated propeptides in the UniProt database, which have more histidines in their propeptides than expected, assuming a probability for histidine of 2.3% (Supplemental Table S1), includes 52 proteins that are either secreted or targeted to the secretory or endocytotic pathway. Although this bias can be random or caused by other factors, such as zinc binding sites, which could explain why metalloproteases such as ADAM and the matrix metalloprotease family members are frequent in the list, we propose that proteins with high histidine content in their propeptides use the pH of the secretory pathway to regulate their activation. This does not necessarily apply exclusively to proteases, because other proteins can also be inhibited by their propeptides. One example is bone morphogenic protein 4 (BMP4), which has a histidine content of 6.23% (Supplemental Table S1), suggesting a propeptide-mediated pH-sensing mechanism. Indeed, sequential processing of the propeptide of BMP4 by furin is pH dependent, and a histidine residue in the propeptide (His251) has been implicated as a pH sensor (26).
Our hypothesis suggests a prominent role of the pH gradient in the secretory pathway in orchestrating proteolytic processing of secreted proteins. Any disturbances in this gradient could lead to disregulation of protease activity, which in PCs and cathepsins can have adverse effects and is associated with diseases such as cancer, atherosclerosis, and Dent's disease (8, 27). Because all these diseases are also associated with changes in cytosolic pH (28, 29), studies that address whether the secretory pH gradient is also affected are needed to address the question of whether pH disregulation plays a role in disturbing regulation of the secretory pathway.
After a review of sequences in 3 evolutionary unrelated protease families, we find a correlation of increased histidine content in propeptides with the requirement to sense pH. But does a correlation imply causality? Histidines play multiple unique roles in proteins because they can function as proton exchangers in enzyme catalysis, form complexes with soft metals, provide unique hydrogen bonding geometry, and alter protein structure and interactions in a pH-dependent manner. Because propeptides are not part of the active site that mediates proteolysis and because propeptides analyzed in this study do not bind metal ions, one can exclude the first two roles. It is also not likely that propeptides in eukaryotes have hydrogen bonding requirements that are different from those of their prokaryotic orthologs, thus endorsing their roles as pH sensors as the most likely explanation for the histidine bias observed.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Science Foundation (career award MCB0746589) and the American Heart Foundation (grant-in-aid to U.S.), the U.S. National Institutes of Health (training grant to D.M.W.), and the American Heart Association (predoctoral training grant 12PRE11470005 to J.E.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Δ[His]
- difference between [His]Pro and [His]Cat
- Δ[AA]
- amino acid change
- BMP4
- bone morphogenic protein 4
- CatB
- cathepsin B
- CatL
- cathepsin L
- ER
- endoplasmic reticulum
- [His]Cat
- histidine content of catalytic domain
- [His]Pro
- histidine content of propeptide
- PC
- proprotein convertase
- TGN
- trans-Golgi network
REFERENCES
- 1. Embley T. M., Martin W. (2006) Eukaryotic evolution, changes and challenges. Nature 440, 623–630 [DOI] [PubMed] [Google Scholar]
- 2. Casey J. R., Grinstein S., Orlowski J. (2010) Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 11, 50–61 [DOI] [PubMed] [Google Scholar]
- 3. Lopez-Otin C., Bond J. S. (2008) Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 283, 30433–30437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shinde U., Thomas G. (2011) Insights from bacterial subtilases into the mechanisms of intramolecular chaperone-mediated activation of furin. Methods Mol. Biol. 768, 59–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson E. D., VanSlyke J. K., Thulin C. D., Jean F., Thomas G. (1997) Activation of the furin endoprotease is a multiple-step process: requirements for acidification and internal propeptide cleavage. EMBO J. 16, 1508–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subbian E., Yabuta Y., Shinde U. P. (2005) Folding pathway mediated by an intramolecular chaperone: intrinsically unstructured propeptide modulates stochastic activation of subtilisin. J. Mol. Biol. 347, 367–383 [DOI] [PubMed] [Google Scholar]
- 7. Anderson E. D., Molloy S. S., Jean F., Fei H., Shimamura S., Thomas G. (2002) The ordered and compartment-specific autoproteolytic removal of the furin intramolecular chaperone is required for enzyme activation. J. Biol. Chem. 277, 12879–12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seidah N. G., Prat A. (2012) The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 11, 367–383 [DOI] [PubMed] [Google Scholar]
- 9. Feliciangeli S. F., Thomas L., Scott G. K., Subbian E., Hung C.-H., Molloy S. S., Jean F., Shinde U., Thomas G. (2006) Identification of a pH sensor in the furin propeptide that regulates enzyme activation. J. Biol. Chem. 281, 16108–16116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dillon S. L., Williamson D. M., Elferich J., Radler D., Joshi R., Thomas G., Shinde U. (2012) Propeptides are sufficient to regulate organelle-specific pH-dependent activation of furin and proprotein convertase 1/3. J. Mol. Biol. 423, 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Punta M., Coggill P. C., Eberhardt R. Y., Mistry J., Tate J., Boursnell C., Pang N., Forslund K., Ceric G., Clements J., Heger A., Holm L., Sonnhammer E. L., Eddy S. R., Bateman A., Finn R. D. (2012) The Pfam protein families database. Nucleic Acids Res. 40, D290–D301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siezen R. J., Leunissen J. A. (1997) Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 6, 501–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wlodawer A., Li M. G., Alla Oyama H., Dunn B. M., Oda K. (2003) Structural and enzymatic properties of the sedolisin family of serine-carboxyl peptidases. Acta Biochim. Pol. 50, 81–102 [PubMed] [Google Scholar]
- 14. Newcombe R. G. (2006) Confidence intervals for an effect size measure based on the Mann-Whitney statistic. Part 1: general issues and tail-area-based methods. Stat. Med. 25, 543–557 [DOI] [PubMed] [Google Scholar]
- 15. Oyama H., Hamada T., Ogasawara S., Uchida K., Murao S., Beyer B. B., Dunn B. M., Oda K. (2002) A CLN2-related and thermostable serine-carboxyl proteinase, kumamolysin: cloning, expression, and identification of catalytic serine residue. J. Biochem. 131, 757–765 [DOI] [PubMed] [Google Scholar]
- 16. Oda K., Sugitani M., Fukuhara K., Murao S. (1987) Purification and properties of a pepstatin-insensitive carboxyl proteinase from a gram-negative bacterium. Biochim. Biophys. Acta 923, 463–469 [DOI] [PubMed] [Google Scholar]
- 17. Gunkel F. A., Gassen H. G. (1989) Proteinase K from Tritirachium album Limber. Characterization of the chromosomal gene and expression of the cDNA in Escherichia coli. Eur. J. Biochem. 179, 185–194 [DOI] [PubMed] [Google Scholar]
- 18. Seidah N. G., Mowla S. J., Hamelin J., Mamarbachi A. M., Benjannet S., Touré B.B., Basak A., Munzer J. S., Marcinkiewicz J., Zhong M., Zhong M., Barale J. C., Lazure C., Murphy R. A., Chrétien M., Marcinkiewicz M. (1999) Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc. Natl. Acad. Sci. U. S. A. 96, 1321–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turk V., Stoka V., Vasiljeva O., Renko M., Sun T., Turk B., Turk D. (2012) Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim. Biophys. Acta 1824, 68–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turk B., Dolenc I., Turk V., Bieth J. G. (1993) Kinetics of the pH-induced inactivation of human cathepsin L. Biochemistry 32, 375–380 [DOI] [PubMed] [Google Scholar]
- 21. Nishimura Y., Kawabata T., Kato K. (1988) Identification of latent procathepsins B and L in microsomal lumen: characterization of enzymatic activation and proteolytic processing in vitro. Arch. Biochem. Biophys. 261, 64–71 [DOI] [PubMed] [Google Scholar]
- 22. Quraishi O., Nägler D. K., Fox T., Sivaraman J., Cygler M., Mort J. S., Storer A. C. (1999) The occluding loop in cathepsin B defines the pH dependence of inhibition by its propeptide. Biochemistry 38, 5017–5023 [DOI] [PubMed] [Google Scholar]
- 23. Illy C., Quraishi O., Wang J., Purisima E., Vernet T., Mort J. S. (1997) Role of the occluding loop in cathepsin B activity. J. Biol. Chem. 272, 1197–1202 [DOI] [PubMed] [Google Scholar]
- 24. Creagh E. M., Conroy H., Martin S. J. (2003) Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 193, 10–21 [DOI] [PubMed] [Google Scholar]
- 25. Tsiatsiani L., Van Breusegem F., Gallois P., Zavialov A., Lam E., Bozhkov P. V. (2011) Metacaspases. Cell Death Differ. 18, 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Degnin C., Jean F., Thomas G., Christian J. L. (2004) Cleavages within the prodomain direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Mol. Biol. Cell 15, 5012–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reiser J., Adair B., Reinheckel T. (2010) Specialized roles for cysteine cathepsins in health and disease. J. Clin. Invest. 120, 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Webb B. A., Chimenti M., Jacobson M. P., Barber D. L. (2011) Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677 [DOI] [PubMed] [Google Scholar]
- 29. Naghavi M., John R., Naguib S., Siadaty M. S., Grasu R., Kurian K. C., Van Winkle W. B., Soller B., Litovsky S., Madjid M., Willerson J. T., Casscells W. (2002) pH heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis 164, 27–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


