Summary
A fundamental question in developmental biology is whether there are mechanisms to detect stem cells with mutations that, although not adversely affecting viability, would compromise their ability to contribute to further development. Here, we show that cell competition is a mechanism regulating the fitness of embryonic stem cells (ESCs). We find that ESCs displaying defective bone morphogenetic protein signaling or defective autophagy or that are tetraploid are eliminated at the onset of differentiation by wild-type cells. This elimination occurs in an apoptosis-dependent manner and is mediated by secreted factors. Furthermore, during this process, we find that establishment of differential c-Myc levels is critical and that c-Myc overexpression is sufficient to induce competitive behavior in ESCs. Cell competition is, therefore, a process that allows recognition and elimination of defective cells during the early stages of development and is likely to play important roles in tissue homeostasis and stem cell maintenance.
Graphical Abstract
Highlights
-
•
Defective ESCs are eliminated at the onset of differentiation by wild-type cells
-
•
Elimination of unfit cells is apoptosis dependent and mediated by secreted factors
-
•
Higher c-Myc levels are established in wild-type ESCs cocultured with unfit cells
-
•
c-Myc overexpression induces a competitive advantage in ESCs
Sancho et al. show that embryonic stem cells undergo cell competition to recognize and eliminate defective neighbors at the onset of differentiation. Cell competition, driven by differential c-Myc expression, provides a mechanism to monitor the fitness of pluripotent stem cells during early mammalian embryogenesis.
Introduction
Embryonic stem cells (ESCs) are the pluripotent counterparts of the preimplantation epiblast and are an invaluable model for understanding the first steps of mammalian development. These cells show rapid self-renewal and retain the potential to contribute to all derivatives of the three germ layers: endoderm, mesoderm, and ectoderm. Over the past few years, much has been learned about the mechanisms controlling ESC pluripotency (Nichols and Smith, 2009), but little is known regarding the mechanisms that control cell survival at the pluripotent stage and during the first stages of embryonic differentiation. It has been particularly hard to uncover whether there is any surveillance mechanism that detects cells that carry mutations that, although they would not adversely affect viability, would compromise their ability to contribute to further development. In the mouse embryo, apoptosis peaks just prior to the onset of gastrulation (Coucouvanis and Martin, 1999; Manova et al., 1998; Spruce et al., 2010). In addition to this, coincident with the start of embryonic differentiation, the embryo becomes hypersensitive to DNA damage induced by low-dose irradiation (Heyer et al., 2000). This suggests that, during these stages, cellular fitness and viability are likely to be tightly monitored.
Cell competition is a type of cell-cell interaction first studied in Drosophila, where the coexistence of two cell populations with different metabolic properties or growth rates results in the growth of the stronger population at the expense of the weaker one. This process of recognition and elimination of vulnerable, mispatterned, or abnormal cells during tissue growth has been proposed to play important roles in tissue homeostasis, organ size control, stem cell maintenance, and the expansion of precancerous cell fields (de Beco et al., 2012; Levayer and Moreno, 2013; Wagstaff et al., 2013). Here, we find that ESCs that display defective bone morphogenetic protein (BMP) signaling or defective autophagy or are tetraploid are eliminated at the onset of differentiation in an apoptosis-dependent manner, only in the presence of wild-type cells. Furthermore, we show that c-Myc is a key mediator in this process. We argue that these observations are remarkably reminiscent of what has been described as cell competition in other systems. Our data therefore demonstrate an involvement of cell competition in regulating cellular fitness during the first steps of embryonic differentiation in mammals.
Results
Cells with Defective BMP Signaling Are Eliminated in the Presence of Wild-Type Cells
BMP signaling is required for maintaining self-renewal and pluripotency of ESCs (Ying et al., 2003) and of the mouse postimplantation epiblast (Di-Gregorio et al., 2007). In embryos carrying a null mutation for Bmpr1a, the main type I BMP receptor during early postimplantation development, the entire epiblast undergoes neural differentiation (Di-Gregorio et al., 2007). However, in mosaic Bmpr1a−/− embryos, mutant cells are capable of contributing to all germ layers, suggesting that they have no autonomous defect during the first steps of germ layer differentiation (Davis et al., 2004; Di-Gregorio et al., 2007). Careful inspection of Bmpr1a−/− mosaics generated with the Mox2-Cre deletor line revealed that a proportion of mutant cells were being eliminated by apoptosis at the epiblast stage of postimplantation development (Figure 1A). In the Drosophila wing, cells that carry a mutation in the Bmpr1a homolog Thick veins (Tkv) have a competitive disadvantage over wild-type cells and are eliminated by apoptosis (Burke and Basler, 1996). Given that ESCs have proven to be a remarkable model for the first steps of differentiation, we turned to this system to test if the elimination of Bmpr1a null cells was due to the presence of wild-type cells.
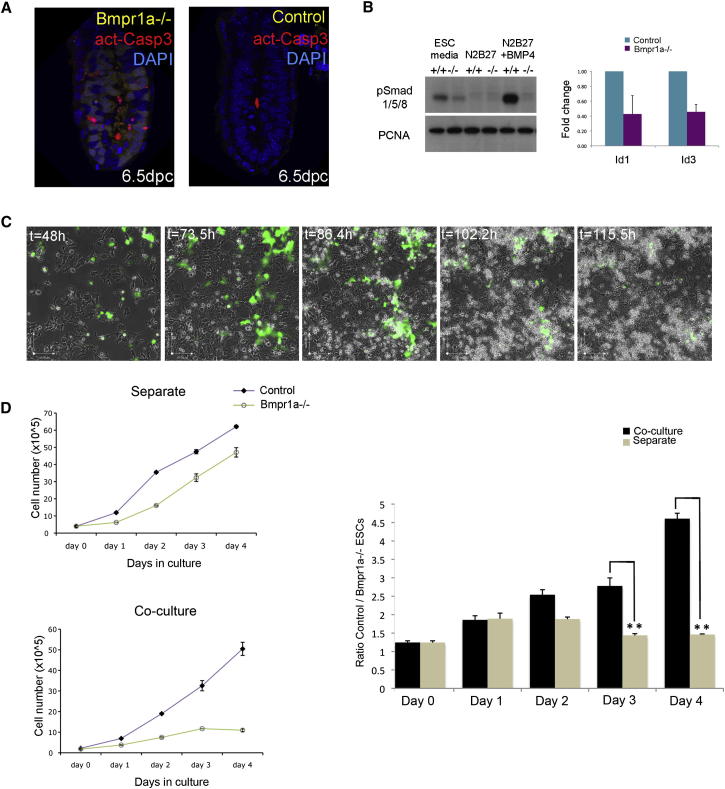
Figure 1.
Cells with Defective BMP Signaling Are Eliminated in the Presence of Wild-Type Cells
(A) High levels of apoptosis in Bmpr1a−/− cells in Bmpr1a−/fx;Mox2Cre+/− embryos (n = 5/7). act-Casp3, cleaved (activated) Caspase3.
(B) Phosphorylation of Smad1/5/8 in the described media (left) and Id gene activation in BMP4 + Lif (right) are decreased in Bmpr1a−/− ESCs. PCNA, proliferating cell nuclear antigen.
(C) Time-lapse imaging of cocultured control and Bmpr1a−/−-GFP ESCs.
(D) Growth curves (left) and ratio (right) of control to Bmpr1a−/− ESCs show that Bmpr1a−/−-GFP ESCs are outcompeted when cultured with control cells in N2B27. A minimum of three independent experiments were performed, and the average ± SEM was plotted. t, time. ∗∗p < 0.005, Student’s paired t test.
Bmpr1a−/− ESCs can still transduce BMP signaling, but the level of activation of this pathway is reduced compared to that of control cells (Figure 1B). In spite of this, as we have previously reported (Di-Gregorio et al., 2007), Bmpr1a−/− ESCs have a similar pluripotent status to that of wild-type cells (Figure S1 available online). To analyze the behavior of Bmpr1a−/− ESCs in the presence of wild-type cells, we labeled the mutant cells with green fluorescent protein (GFP), and the two cell types were cocultured. N2B27 media was used for these experiments, as this allows ESCs cells to progress into a state that transcriptionally resembles the postimplantation epiblast (Figure S2A) (Brons et al., 2007; Tesar et al., 2007). Time-lapse analysis revealed that, over a 4-day period, the proportion of Bmpr1a−/−-GFP cells was dramatically reduced in these cultures (Figure 1C). To establish if this was due to the presence of wild-type cells, we cultured Bmpr1a−/−-GFP ESCs and control ESCs separately or together. Analysis of their growth curves and of the ratio of control ESCs to Bmpr1a−/− ESCs (obtained by flow cytometry) in each of these conditions revealed that, from day 3 in N2B27, the total number of Bmpr1a−/−-GFP ESCs decreased specifically in coculture (Figure 1D). This led to a significant increase in the proportion of control cells at days 3 and 4 of coculture, compared to separate populations (Figure 1D; Table S1). Calculation of the growth rate for each cell type in separate and coculture conditions indicated that accompanying the decrease in numbers of Bmpr1a−/−-GFP ESCs was a significant increase in the growth rate of control cells (Figure 2A; Table S1), suggesting that they undergo compensatory proliferation. When unlabeled Bmpr1a−/− ESCs were cultured with E14 (control) GFP-labeled cells to exclude a possible deleterious effect of GFP, we observed a similar phenomenon (Figures S2B and S2C; Table S1).
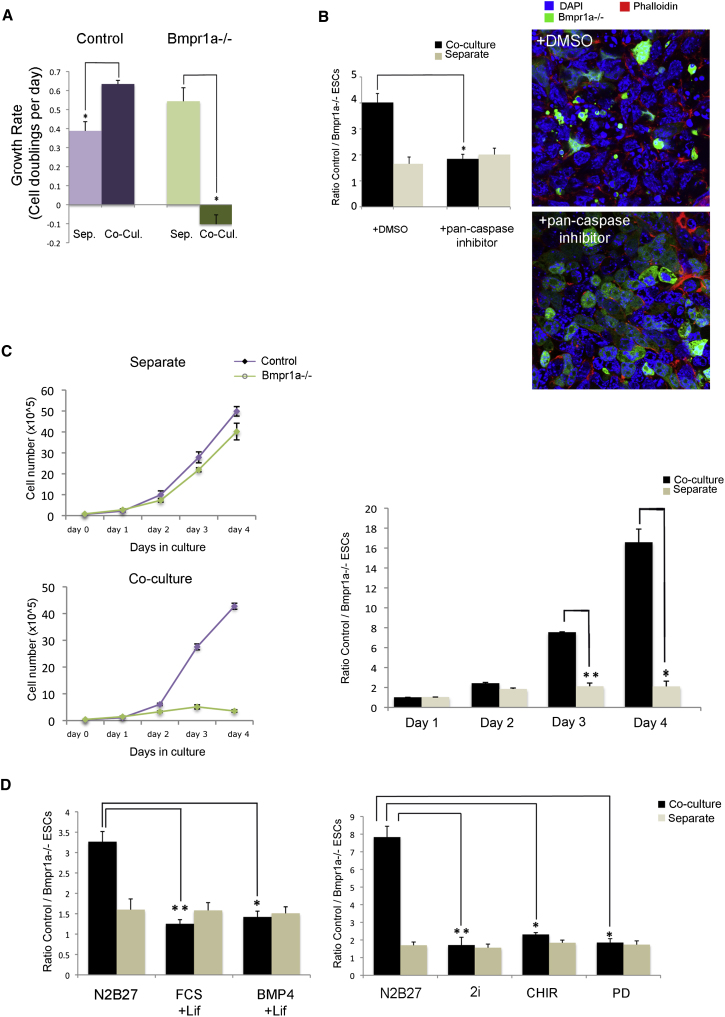
Figure 2.
ESCs with Defective BMP Signaling Are Eliminated by Apoptosis upon Exit of the Ground State of Pluripotency
(A) Growth rates (in cell doublings per day) of control and Bmpr1a−/− ESCs between days 3 and 4 of culture indicate that, while Bmpr1a−/− ESCs are eliminated, control cells undergo compensatory proliferation. Sep., separate culture; Co-Cul., coculture.
(B) Ratio obtained by flow cytometry and confocal images of Bmpr1a−/− and control cells indicates that addition between days 2 and 4 of coculture of the pancaspase inhibitor ZVAD-FMK blocks the elimination of Bmpr1a−/− ESCs. DMSO, dimethyl sulfoxide.
(C) Growth curves (left) and ratios (right) of control and Bmpr1a−/− ESCs showing that the elimination of mutant cells occurs under EpiSC culture conditions.
(D) Ratio of control to Bmpr1a−/− ESCs showing that the outcompetition of Bmpr1a−/−-GFP ESCs is prevented by FCS + Lif, BMP4 + Lif, 2i, CHIR99021, or PD0325901. A minimum of three independent experiments were performed, and the average ± SEM was plotted. ∗p < 0.05, and ∗∗p < 0.01; a one-way analysis of variance (ANOVA) followed by Tukey’s test.
See also Figures S2 and S3 and Table S1.
To address whether the decrease in the numbers of Bmpr1a−/− ESCs was due to apoptosis, we analyzed whether the addition of the pancaspase inhibitor ZVAD-FMK could prevent the elimination of Bmpr1a−/− ESCs in coculture. We observed that, at day 3, mixed colonies exhibited large amounts of cellular debris of Bmpr1a−/−-GFP ESCs that could be visualized as punctuate dots of GFP by confocal microscopy (Figures 2B and S2D). However, addition of ZVAD-FMK from the second day of culture abolished the elimination of Bmpr1a−/−-GFP cells and led to the disappearance of GFP-positive cellular debris in coculture (Figure 2B; Table S1).
To investigate the possibility that the elimination of Bmpr1a−/− ESCs was due to selection against a specific lineage, we analyzed the expression of lineage-specific markers in Bmpr1a−/−-GFP and control ESCs. We observed that Bmpr1a−/−-GFP ESCs in coculture showed an expression profile of mesoderm, endoderm, neural, and epidermal marker gene expression that was very similar to when they were cultured as a homogeneous population (Figure S2E). Together, these results indicate that Bmpr1a−/− ESCs are eliminated by apoptosis when in the presence of wild-type cells and that this elimination is not specific to any particular lineage.
Elimination of Bmpr1a−/− Cells Is Dependent on the Onset of Differentiation
Bmpr1a−/− embryos only display defects at postimplantation stages (Di-Gregorio et al., 2007), and in mosaics, a proportion of Bmpr1a−/− cells are eliminated by apoptosis at 6.5 days postcoitum (dpc) (Figure 1A). We therefore asked if the elimination of Bmpr1a−/− cells was dependent on exit of the ground state of pluripotency, which is thought to exist in preimplantation embryos and ESCs (Nichols and Smith, 2009). We first analyzed whether the elimination of Bmpr1a−/− cells occurred when these cells were differentiated to epiblast stem cells (EpiSCs). When this was done, we observed that the total numbers of Bmpr1a−/− ESCs decreased from the third day of coculture with control cells in EpiSC media (N2B27 containing Activin and fibroblast growth factor [FGF]) (Brons et al., 2007; Tesar et al., 2007) but not when they were cultured alone in these conditions (Figure 2C). We then analyzed what occurred when exit of pluripotency was suppressed by culturing the cells in serum plus Lif, BMP4 plus Lif, or in 2i (a MEK inhibitor and a GSK3β inhibitor). Interestingly, when we analyzed the ratios of control ESCs to Bmpr1a−/− ESCs in all of these conditions, we observed that they were similar for cells grown separately or in coculture (Figure 2D; Table S1). This indicates that, under conditions that maintain pluripotency, the elimination of Bmpr1a−/− ESCs is abolished and, therefore, that the outcompetition of these cells only occurs when differentiation has been initiated.
Secreted Factors Regulate the Elimination of Bmpr1a−/− Cells
Given that initiation of differentiation is dependent on a wide array of growth factors, we first tested whether the elimination of Bmpr1a−/− cells requires cell contact. To test this, we cultured Bmpr1a−/− and control ESCs on Corning Transwell polycarbonate membrane cell culture inserts. In this system, cells of one genotype are cultured on the base of the well, and those of another genotype are cultured on a filter in an insert that lies 1.2 mm over the base of the well. We found that there was no significant difference in the numbers of control or Bmpr1a−/− ESCs when these were grown for 4 days with cells of the same genotype overlaying them or in the numbers of control cells when they were grown with overlaying Bmpr1a−/− ESCs (Figure 3A). In contrast, when Bmpr1a−/− ESCs were grown with overlaying control cells, their numbers were significantly lower than those of the other combinations tested (Figure 3A), indicating that secreted factors in the shared media must mediate their elimination. Interestingly, a similar finding has been made for cell competition in Drosophila (Senoo-Matsuda and Johnston, 2007).
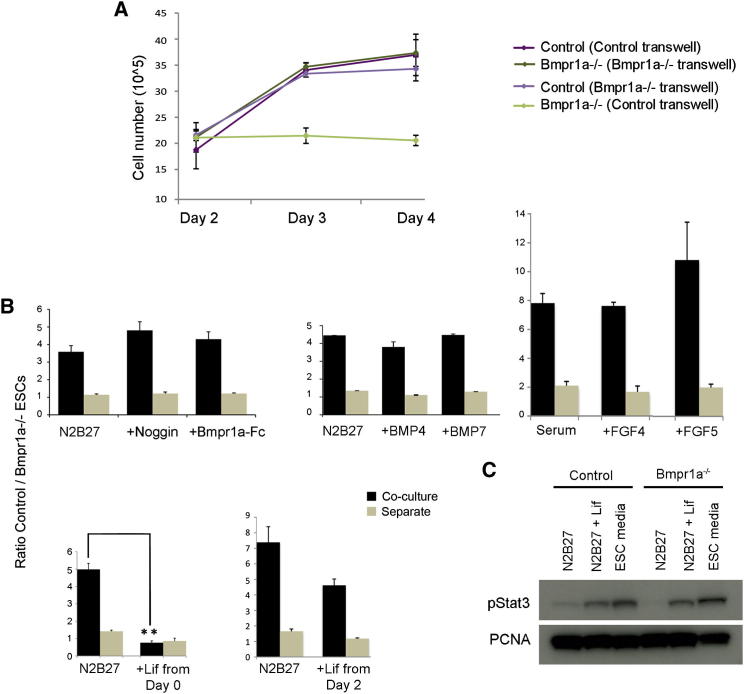
Figure 3.
Elimination of Bmpr1a−/− ESCs Depends on Secreted Factors
(A) Assays using Corning Transwell inserts indicate that the growth of Bmpr1a−/− ESCs is inhibited when these cells are grown with overlaying control cells in N2B27. Cell numbers refer to the cells growing underneath the insert; the genotype of the cells growing on the insert is indicated in brackets.
(B) Plot of the ratio of control to Bmpr1a−/− ESCs cultured as separate populations or cocultured in the presence of Noggin (p = 0.15), Bmpr1a-Fc (p = 0.48), BMP4 (p = 0.97), BMP7 (p = 0.51), FGF4 (p = 0.33), FGF5 (p = 0.93), and Lif from day 0 (p = 0.0001) or from day 2 (p = 0.06). The plot shows that only when Lif is added from day 0 of coculture is the outcompetition of Bmpr1a−/− ESCs significantly blocked.
(C) Western blot showing that Lif is less efficient in triggering Stat3 phosphorylation once differentiation is initiated. A minimum of three independent experiments were performed, and the average ± SEM was plotted. pStat3, phosphorylated Stat3. ∗∗p < 0.005, Student’s paired t test.
We next asked if the elimination of Bmpr1a−/− ESCs was due to defective BMP signaling. For this, we transfected Bmpr1a−/− ESCs with a CAG-Bmpr1a expression cassette (Bmpr1aGOF) and analyzed their behavior in coculture assays. We found that restoring BMP signaling in Bmpr1a−/− ESCs rescued their elimination in mixed cultures but also led them to induce elimination of nontransfected Bmpr1a−/− ESCs (Figures S3A–S3C; Table S1). These results indicate that the outcompetition of Bmpr1a−/− ESCs in coculture is due to the deficiency in transducing BMP signaling.
An attractive model to explain the elimination of Bmpr1a−/− ESCs is that defective BMP signaling made Bmpr1a−/− ESCs less capable of competing for limiting amounts of BMPs that act as a survival factor (Moreno and Basler, 2004; Moreno et al., 2002). To analyze whether limiting BMP availability would enhance the elimination of Bmpr1a−/− ESCs, we performed cocultures of Bmpr1a−/− and control ESCs in the presence of the BMP antagonist Noggin and of the fusion protein BMPR1A-FC, a soluble dominant-negative form of BMPR1A. To test whether providing an excess of BMPs would rescue the disadvantage of Bmpr1a−/− ESCs, we used either BMP4 or BMP7. We observed that, after 3 days of coculture in N2B27, Noggin decreased Smad1/5/8 phosphorylation in control and Bmpr1a−/− ESCs. In contrast to this, BMP4 increased Smad1/5/8 activation in control, but not Bmpr1a−/−, ESCs. This led to an overall increase in the difference of BMP signaling between control and Bmpr1a−/− ESCs (Figures S4A and S4B). In spite of this, BMP4, BMP7, Noggin, and BMPR1A-FC did not significantly alter the ratio of Bmpr1a−/− to control ESCs either separately or as cocultures (Figure 3B). These data suggest that, although defective BMP signaling leads to the elimination of mutant ESCs, cells are not competing for limiting amounts of BMPs, and the relative level of BMP signaling is not triggering their elimination.
Given these observations, we asked if other signaling pathways could be mediating the cell nonautonomous elimination of Bmpr1a−/− ESCs. Given that inhibition of FGF signaling and BMP plus Lif are two conditions that block the elimination of Bmpr1a−/− ESCs (Figure 2D), we asked whether excess FGF4, FGF5, or Lif affected this process. Interestingly, we observed that while FGF4 and FGF5 had little effect on the elimination of Bmpr1a−/− ESCs, Lif completely abolished their competitive disadvantage in coculture (Figure 3B; Table S1). This suggests that the signaling events downstream of Lif are a key element to the mechanism by which cells sense the defective status of Bmpr1a−/− ESCs. To address if the activity of Lif was specific to the ground state of pluripotency, we tested the effect of Lif when added from day 2 of coculture in N2B27. We observed that once differentiation had been initiated, Lif only partially rescued the elimination of Bmpr1a−/− ESCs (Figure 3B). Interestingly, analysis of the expression of components of the Lif pathway in ESCs after a 3 day culture in N2B27 indicated that the levels of gp130 and Stat3 were reduced (Figure S4C). Furthermore, we observed that Lif was also less efficient at triggering Stat3 phosphorylation when Bmpr1a−/− ESCs were cultured in N2B27 (Figure 3C). This indicates that, once ESCs have exited the ground state of pluripotency, Lif is less efficient at signaling and suggests that, although events downstream of Lif may be mediating the elimination of Bmpr1a−/− ESCs, Lif itself is not directly involved in cell competition.
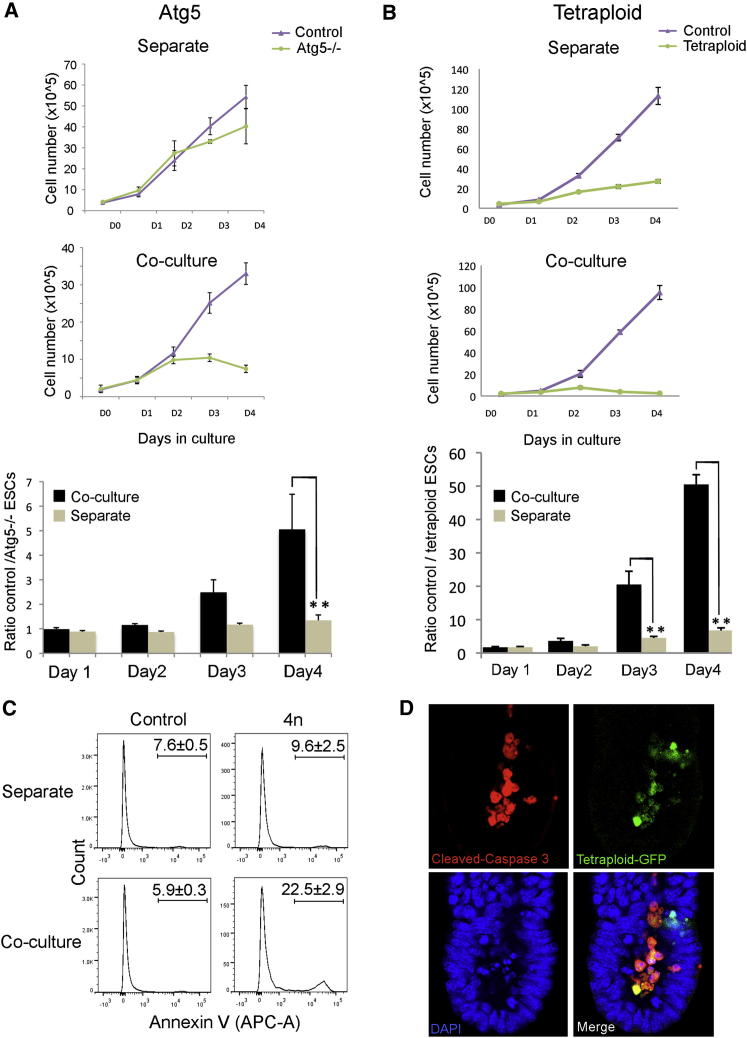
Autophagy-Deficient and Tetraploid ESCs Are Also Eliminated by Wild-Type Cells
Our finding that cells with defective BMP signaling are eliminated by wild-type cells prompted us to ask if this also occurred with cells carrying other defects. Autophagy is an intracellular degradation pathway that plays a pleiotropic role in the protection and maintenance of cellular life as well as in the response to starvation. Atg5 is a critical component of the autophagy pathway. Atg5-deficient ESCs are defective in ATP metabolism and, although they display apparently normal growth, upon differentiation are unable to engulf apoptotic cells (Qu et al., 2007). When control and Atg5−/− ESCs were cultured for 4 days in N2B27, we observed that, from the third day of culture, the numbers of Atg5−/− ESCs decreased specifically when cocultured with control cells (Figure 4A; Table S1). Interestingly, this competitive elimination was not observed when Atg5−/− ESCs were cocultured with Bmpr1a−/− ESCs (Figure S4D; Table S1), suggesting that ESCs must be capable of measuring their relative cellular fitness.
Figure 4.
Autophagy-Deficient and Tetraploid ESCs Are Eliminated by Cell Competition
(A) Growth curves and plots of the ratio of control to Atg5−/− ESCs in separate culture or coculture.
(B) Growth curves and plots of the ratio of control to tetraploid ESCs in separate culture or coculture.
Both (A) and (B) indicate that both autophagy deficiency and tetraploidy induce cell competition.
(C) Histograms for Annexin V levels in control and tetraploid ESCs in separate culture or coculture in EpiSC media.
(D) Immunostaining for cleaved-caspase 3 in chimeras generated with tetraploid-GFP ESCs (n = 10).
Both (C) and (D) indicate that the tetraploid ESCs are eliminated by apoptosis both in vitro and in vivo. A minimum of three independent experiments were performed, and the average ± SEM was plotted. ∗∗p < 0.005, Student’s paired t test.
Elimination of polyploid cells from a normal genetic environment has been shown to occur in vivo (Eakin et al., 2005). In tetraploid (or 4n) chimeras, 4n cells can be found in the epiblast at 6.5 dpc but are lost from this tissue thereafter. For this reason, we asked if tetraploid ESCs could also be subject to competition when cultured with diploid (or 2n) cells. Analysis of the growth rate of 4n ESCs indicated that they proliferated at a constant rate, but this proliferation was significantly lower than control cells (Figure 4B). In contrast to this, when 4n ESCs were cocultured with diploid ESCs, their numbers decreased from the second day of coculture (Figures 4B and 4C; Table S1). This decrease was found to be due to apoptosis, as tetraploid cells showed higher levels of annexin V staining than control cells and these levels were significantly higher in the cocultured condition compared to when these cells were cultured separately (Figure 4C). To test whether a similar phenomenon occurred in vivo, we generated chimeras using GFP-labeled tetraploid ESCs. We found that, at 6.5 dpc, the vast majority of tetraploid cells also showed cleaved-caspase 3 staining, indicating that, in the embryo, these cells were also eliminated by apoptosis (Figure 4D).
A possible explanation for the elimination of tetraploid or Atg5−/− ESCs is that they have defective BMP signaling. To address whether this could be the case, we analyzed the ability of both these lines to respond to BMP4 after a 3 day culture in N2B27, when the elimination of defective cells occurs. We observed that, after stimulation with BMP4, both tetraploid and Atg5−/− ESCs induced robust Smad1/5/8 phosphorylation, indicating that BMP signaling was normal in these cells (Figure S5A). We next analyzed if defective autophagy could be the cause for the elimination of Bmpr1a−/− or tetraploid cells. For this, we analyzed expression of LC3, a protein that switches from its LC3-I to its LC3-II isoform upon the induction of autophagy, and p62, a protein that is degraded during autophagy. We observed that, after a 3 day culture in N2B27, Bmpr1a−/− and tetraploid ESCs displayed a similar ratio of LC3-I to LC3-II expression and levels of p62 expression similar to that of control ESCs, indicating that they were not defective in autophagy (Figure S5B).
A third possibility is that defective ploidy could be a defect common to the eliminated cells. This possibility can be discarded, as Bmpr1a−/− ESCs have a normal karyotype (data not shown) and the elimination of these cells can be rescued by reintroducing into them the Bmpr1a gene (Figures S3A–S3C). Together, these data point to a general mechanism that is monitoring ESC fitness upon the onset of differentiation in a non-cell-autonomous fashion rather than to a defect that is common among Bmpr1a−/−, Atg5−/−, and tetraploid ESC lines.
c-Myc Is a Key Mediator of the Elimination of Defective ESCs
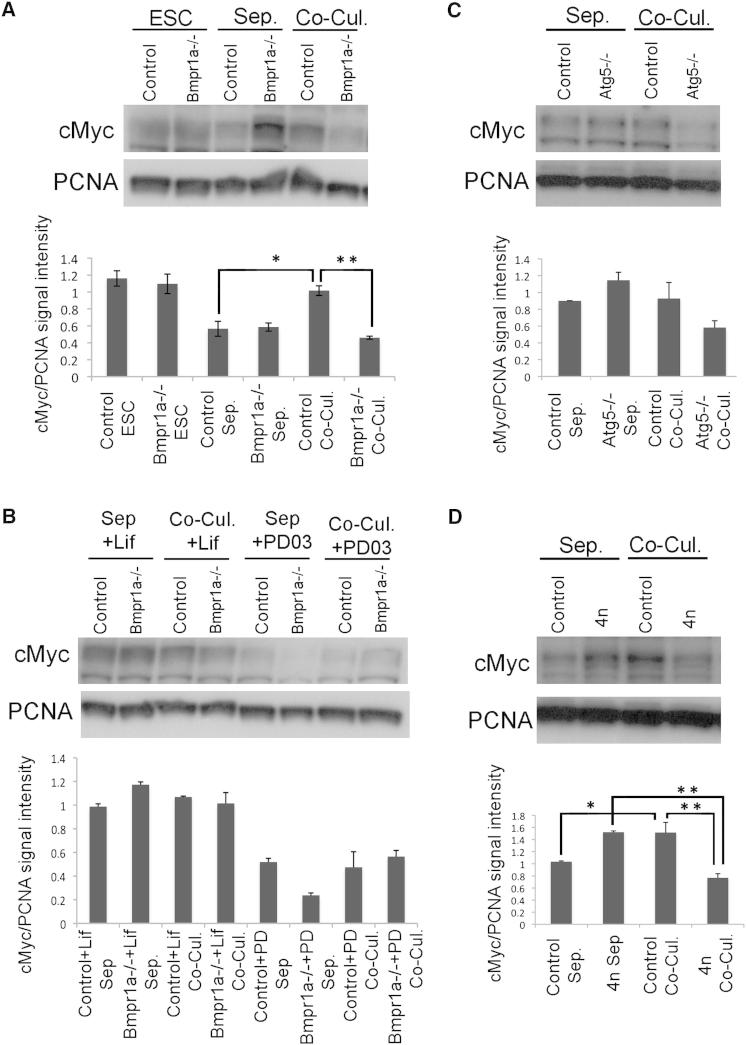
The MYC family of transcription factors has been implicated in a variety of biological processes, including apoptosis and cell cycle regulation (Dang, 2012). Furthermore, in Drosophila, d-Myc has been shown to be a key regulator of cell competition in the wing epithelium and in the developing ovary (de la Cova et al., 2004; Moreno and Basler, 2004; Rhiner et al., 2009). In ESCs, c-Myc acts downstream of Lif in the control of self-renewal (Cartwright et al., 2005; Singh and Dalton, 2009), and for these reasons, we addressed the possible involvement of c-Myc in the elimination of Bmpr1a−/− ESCs. Cells of different genotypes were sorted by fluorescence-activated cell sorting (FACS) on the basis of their GFP expression, and either messenger RNA (mRNA) or protein were isolated. Analysis of c-Myc mRNA levels revealed that these were similar in N2B27 for both Bmpr1a−/−-GFP and control ESCs, regardless of being in coculture or not (Figure S6A). In contrast to this, c-Myc protein levels were significantly increased specifically in wild-type ESCs only when cocultured with Bmpr1a−/−-GFP cells (Figure 5A). This suggests that differential c-Myc protein levels may be a key event in the elimination of Bmpr1a−/− ESCs. To test this, we first analyzed what occurred when these assays were performed in the presence of Lif or of MEK inhibition, two conditions that prevent the elimination of Bmpr1a−/− ESCs (Figure 2). We observed that both these treatments abolished the differential c-Myc protein levels found between control and Bmpr1a−/−-GFP ESCs (Figure 5B). Next, we analyzed what occurred in the cocultures of Atg5−/− or tetraploid-GFP ESCs with control cells and found that, when either of these cell types was cocultured with control cells, they downregulated c-Myc expression (Figures 5C and 5D).
Figure 5.
Establishment of Differential c-Myc Levels Is Critical for the Elimination of Defective ESCs
(A and B) In (A), western blot analysis and quantification of relative c-Myc/PCNA intensity indicate that c-Myc protein expression is significantly downregulated in Bmpr1a−/− ESCs at day 3 of coculture with control cells, but (B) shows that this difference is abolished by Lif and MEK inhibition using PD0325901.
(C and D) c-Myc levels are also lower in (C) Atg5−/− ESCs and in (D) tetraploid ESCs when cocultured with control ESCs. Cells in coculture were sorted by FACS based on GFP expression prior to analysis. A minimum of three independent experiments were performed, and the average ± SEM was plotted. ∗p < 0.05, and ∗∗p < 0.01; a one-way ANOVA was followed by Tukey’s test.
See also Figure S5.
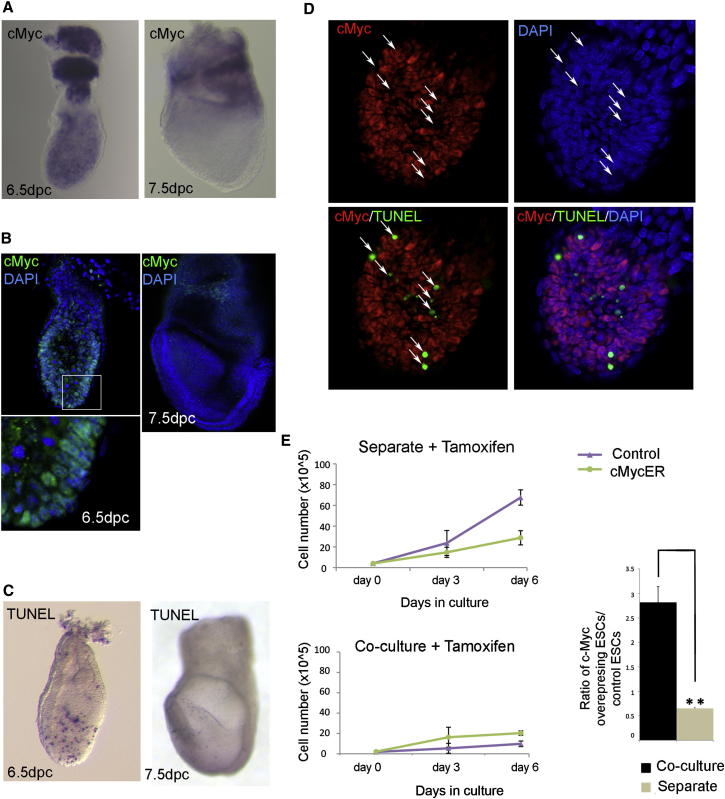
These observations prompted us to address if there was any correlation between c-Myc levels and cell death in vivo. Analysis of c-Myc mRNA and protein expression showed that it was heterogenous in embryos 6.5 dpc but was absent from the epiblast 7.5 dpc (Figures 6A and 6B). Interestingly, a similar pattern was observed for cell death, as high levels were observed at 6.5 dpc and dramatically reduced by 7.5 dpc (Figure 6C). Furthermore, costaining for cell death (using TUNEL) and c-Myc revealed that, in the epiblast 6.5 dpc, those cells that were dying preferentially showed low levels of c-Myc expression (Figure 6D). These results suggest that the establishment of differential levels of c-Myc is a common mechanism regulating the elimination of aberrant ESCs.
Figure 6.
c-Myc Is a Key Mediator of the Elimination of Defective ESCs
(A and B) WISH in (A) and immunostaining in (B) show that c-Myc is heterogeneously expressed in embryos at 6.5 dpc but that this expression is downregulated from the embryonic region by 7.5 dpc. The lower image is a magnification of the region highlighted by the square.
(C) TUNEL staining indicates that cell death peaks at 6.5 dpc in the embryo.
(D) Double staining for TUNEL and c-Myc shows that those cells that are dying (white arrows) show low levels of c-Myc expression.
(E) Growth curves and plot of the ratio of c-MycER and control ESCs when grown for 3 days in N2B27 and then treated with tamoxifen for 3 days in separate and coculture conditions, showing how c-Myc overexpression induces the elimination of control cells. A minimum of three independent experiments were performed, and the average ± SEM was plotted. ∗∗p < 0.005, Student’s paired t test.
To test this hypothesis further, we analyzed if c-Myc overexpression is sufficient to induce the elimination of wild-type cells. For this, we generated a tamoxifen-inducible c-Myc overexpression line and analyzed its behavior when cocultured with E14-GFP ESCs. Overexpression of c-Myc had been reported to maintain cells in the pluripotent state (Cartwright et al., 2005), and given that we have shown that cell competition only occurred once ESCs had initiated differentiation (Figure 2), we first allowed cells to differentiate and then stimulated c-Myc activation. Control cells carrying a tamoxifen-inducible c-Myc construct (E14-cMycER) and E14-GFP cells were cocultured or grown separately for 3 days without tamoxifen, and then tamoxifen was added for a further 3 days (Figure S6B). Analysis of the growth curves of E14-cMycER and E14-GFP cells in each condition indicated that, in the presence of tamoxifen, E14-cMycER cells grew at a similar rate in both the separate and coculture conditions (Figure 6E). In contrast to this, E14-GFP cells changed their behavior from an exponential growth rate under separate conditions to growth arrest when cocultured with c-Myc-overexpressing cells (Figure 6E; Table S1), indicating that they were being eliminated by E14-cMycER cells.
In separate cultures in the presence of tamoxifen, the growth of E14-cMycER cells was slower than that of E14-GFP ESCs. Given that we observed that the expression of c-MycER was heterogeneous in these cultures (data not shown), it is possible that, in E14-cMycER separate cultures, high-expressing c-Myc cells are eliminating low-expressing cells. Alternatively, it is possible that the overexpression of c-Myc is having toxic effects on these cells. We also observed that, when E14-cMycER cells were grown without tamoxifen, they were enriched in coculture compared to when grown separately (Figure S6C; Table S1), suggesting that the system may be leaky to some degree. Overall, our data suggest that the establishment of differential levels of c-Myc is a key event in the elimination of “unfit” embryonic stem cells.
Discussion
ESCs possess the unique ability to contribute to all the derivatives of the three germ layers as well as to self-renew in an indefinite fashion. In the embryo, their pluripotent counterparts will give rise to all the different tissues and cell types that compose the fetus. For this reason, the recognition and elimination of vulnerable, mispatterned, or abnormal cells is critical not only for the proper maintenance of the pluripotent stem cell pool but also for the correct development of the different organs of the newborn. In the mammalian embryo, the initiation of gastrulation marks the onset of embryonic differentiation and is associated with the start of rapid proliferation cycles (Mac Auley et al., 1993; Snow, 1977; Stuckey et al., 2011a). A potential cost of this very rapid proliferation rate is the likelihood of a high production of damaged cells. It is thus expected that, at this stage, mechanisms are set in place to monitor epiblast fitness.
Apoptosis in the early mouse embryo peaks just prior to the onset of gastrulation and has been associated to the process of cavitation (Coucouvanis and Martin, 1999; Manova et al., 1998; Spruce et al., 2010). Furthermore, coincident with the start of gastrulation, the embryo also becomes hypersensitive to DNA damage induced by low-dose irradiation and undergoes apoptosis (Heyer et al., 2000). Tetraploid cells are eliminated from the epiblast in 4n:2n chimeras at 6.5 dpc, when gastrulation is being initiated (Eakin et al., 2005), and here we show that this occurs by apoptosis. Similarly, we find that cells with defective BMP signaling or defective autophagy are also eliminated at the epiblast stage of embryonic development. This suggests the existence of a general mechanism monitoring stem cell fitness upon the onset of differentiation in a non-cell-autonomous fashion. Our studies indicate that the establishment of differential levels of c-Myc is crucial to the elimination of defective cells, leading to the death of those cells with lower c-Myc levels. This is a feature that occurs both during ESC differentiation and in the epiblast of the 6.5 dpc embryo. Interestingly, c-Myc expression is reduced when the ground state of pluripotency is captured using GSK3β and MEK inhibition (Ying et al., 2008). Given that blocking GSK3β activity increases c-Myc stability in ESCs (Cartwright et al., 2005), this suggests that the MEK inhibitor must decrease c-Myc levels in 2i. We find that MEK inhibition blocks the elimination of Bmpr1a−/− ESCs; therefore, one possible reason for why these cells are not eliminated in the ground state of pluripotency may be this effect of MEK inhibition on c-Myc levels. Alternatively, the mechanisms that govern cell death between the naive and primed states of pluripotency may differ.
Our findings are also of relevance to the tetraploid complementation experiments, where, to test the developmental potential of ESCs, they are injected into tetraploid embryos. In these chimeras, 4n cells can be found in the epiblast at 6.5 dpc (Eakin et al., 2005) but very rarely contribute to the embryonic tissue after 9.5 dpc. Our data indicate that this is because they are eliminated due to their competitive disadvantage. This observation, however, means that the conclusions from this type of complementation experiments should be treated with caution. If those ESCs that are used also have defects that affect their fitness, then it is possible that the tetraploid cells will persist for longer and, therefore, this will affect the interpretation of the experiment.
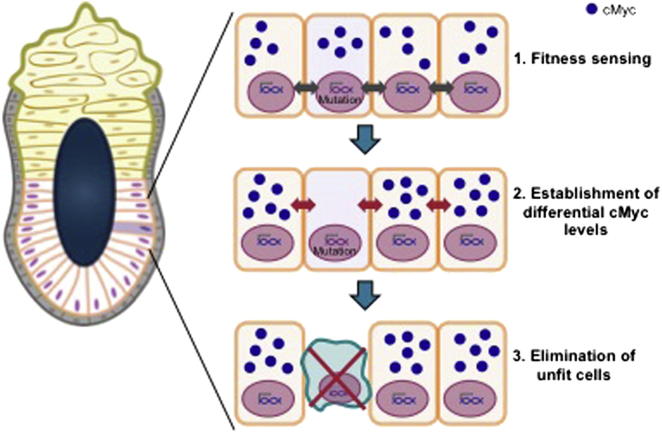
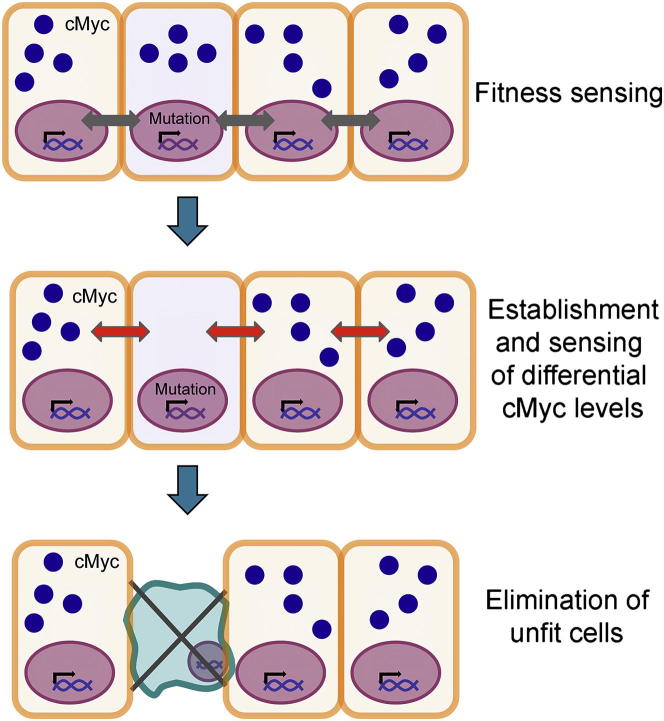
The mode of elimination of defective cells that we observe here shares several common features with what has been described as cell competition in Drosophila and other organisms (de Beco et al., 2012; Levayer and Moreno, 2013; Wagstaff et al., 2013); for this reason, we argue that cell competition may be monitoring cellular fitness in the early mammalian embryo. Together, our data suggest that the elimination of defective stem cells in the postimplantation epiblast follows sequential steps (Figure 7). First, a mutual sensing step between cells is likely to occur, allowing identification of those that present a lower “fitness” level. Our experiments indicate that this lower level of cellular “fitness” is a relative rather than an absolute measure, as defective cells are viable when cultured as a homogeneous population. Furthermore, our experiments show that this first step is likely to be mediated, at least in part, by secreted factors, but we cannot rule out the contribution of cell-cell contact in further downstream events. Our finding that mammalian cells are capable of measuring their relative signaling abilities, growth rates, or metabolic rates is of great importance for our understanding of how cells behave in a heterogeneous population, not just how they act as individual entities.
Figure 7.
Model for How Cell Competition Eliminates Defective Stem Cells during Early Mammalian Development
A first step during cell competition is the mutual sensing that allows identification of those stem cells that present a lower “fitness” level. In response to the mutual sensing, differential levels of c-Myc are established between “fit” and “unfit” cells. Next, a second “mutual sensing” event occurs that monitors c-Myc levels, leading to activation of the apoptotic pathway and elimination of the “weaker,” low c-Myc-expressing stem cells.
The second step in the process is that, in response to the mutual sensing between normal and defective ESCs, differential levels of c-Myc are established between “fit” and “unfit” cells. We propose that, once differential levels of c-Myc are established, a second “mutual sensing” event occurs, acting as a second checkpoint to establish the final outcome of the competition. Our data indicate that this second sensing event is also cell nonautonomous, as control cells are eliminated when cocultured with c-Myc-overexpressing cells. It is after this step that activation of the apoptotic pathway and elimination of the “weaker” cells occurs. We believe that this process of recognition and elimination of vulnerable, mispatterned, or abnormal cells during tissue growth is of broad interest, as it will be fascinating to unravel if it also plays an important role in tissue homeostasis, organ size control, and stem cell maintenance at later stages of development or in the adult.
Experimental Procedures
ESC Culture and Manipulation
E14 ESCs were a gift from Prof. A. Smith (Cambridge University). Bmpr1a−/− ESCs were described elsewhere (Di-Gregorio et al., 2007). Atg5−/− ESCs were a gift from Noboru Mizushima (Mizushima et al., 2001). Three tetraploid ESC lines were used, and these were derived during an electroporation experiment and determined to have 80 chromosomes by karyotyping. Cells were maintained following standard conditions and as described elsewhere (Cambray et al., 2012).
Cell Competition Assays
After dissociation with Trypsin-EDTA, cells were counted and 4.2 × 104 cells/cm2 plated into 0.1% gelatin-coated six-well plates. Cells were plated as each genotype separately or as a mixture of control and mutant ESCs at either a 50:50 or 40:60 ratio depending on the cell type. After plating, ESCs were incubated for 4 to 5 hr in ESC media without LIF and then washed and kept in N2B27 ESGRO Basal media (Milipore) for the specified times. Media were changed daily thereafter. We observed that competition also occurred when plating in Dulbecco’s modified Eagle’s medium plus serum; therefore, where indicated, this condition was also used. c-Myc induction was carried out with 4-hydroxytamoxifen (Sigma) at 10 nM. For analysis of the growth curves, 4.2 × 104 cells/cm2 were plated for separate cultures and cocultures, and then cells were counted daily. The cell count graphs represent the total cell number of each genotype counted. For cells in coculture, the total number of cells in the mix was counted, and the relative proportion of cells of each genotype was determined by flow cytometry. It is worth noting that, as control and mutant cells represent only a proportion of the total cell number in the cocultures, this number is lower than the totals for each genotype when grown separately.
The graph columns represent the ratio of control to mutant ESCs. For maximum accuracy, the precise proportion of cells of each genotype present in coculture was obtained by FACS analysis. For the separate cultures, cells were trypsinized, mixed in equal volumes, and analyzed by flow cytometry.
Growth Rate Calculations
The growth rate was calculated for each cell type in each condition for the different time points analyzed using the following equation: growth rate (in cell doublings per day) equals natural logarithm (final cell number/initial cell number) divided by the natural logarithm of two. A minimum of three independent experiments were performed, and the average ± SEM was plotted.
Caspase Inhibition
We plated 4.0 × 105 cells in a six-well plate and then cultured them from day 2 for 48 hr in the presence of the pan-caspase inhibitor Z-VAD-FMK (R&D Systems) at a final concentration of 100 nM. A minimum of three independent experiments were performed, and the average ± SEM is plotted.
Growth Factor/Inhibitor Treatments
Cells were cultured in the presence of 1,500 U/ml LIF (Chemicon), 10 ng/ml BMP4, 25 ng/ml BMP7, 50 ng/ml Noggin, 250 ng/ml BMPR1A-FC, 5 ng/ml FGF4, 10 ng/ml FGF5 (all from R&D Systems), 1 μM PD0325901 (Sigma), or 3 μM CHIR99021 (Millipore) in serum-free N2B27.
Mice and Embryos
Bmpr1a−/fx-; Mox2Cre+/− mice were generated as described elsewhere (Miura et al., 2006). Mice on a mixed CD1 and 129/Sv background were used for the cell death and expression analysis. All mice were maintained on a 10 hr-14 hr light-dark cycle. Noon of the day of finding a vaginal plug was designated 0.5 dpc. Embryo dissection and chimera generation were carried out as described elsewhere (Nagy et al., 2003). All mice were maintained and treated in accordance with the Home Office’s Animals (Scientific Procedures) Act 1986.
Immunostaining and Whole-Mount In Situ Hybridization
Immunostaining was carried out as described elsewhere (Nowotschin et al., 2013), with minor modifications. Briefly, embryos were fixed in 4% paraformaldehyde (PFA) + 0.1% Tween + 0.01% Triton for 20–30 min at room temperature (RT) and then washed three times in PBS + 0.1% Triton (PBT) for 5 min. Embryos were permeabilized with 0.4% Triton in PBS for 20 min and then washed three times for 10 min in PBT. Embryos were then blocked in 2% horse serum in PBT for 45 min at RT and then incubated with the antibody overnight at 4°C in (1:2) blocking solution in PBS. Antibodies used were 488-conjugated rabbit anti-GFP (1:300; Invitrogen), rabbit anti-cleaved caspase 3 (1:400; Cell Signaling), and rabbit anti-c-Myc (1:250; Abcam Y69 clone). Phalloidin-TRITC (Sigma) was used at 1:100. Whole-mount in situ hybridization (WISH) was carried out as described elsewhere (Stuckey et al., 2011b).
TUNEL and c-MYC Staining on Embryos
TUNEL cell death staining was carried out according to manufacturer’s conditions (Promega DeadEnd Fluorometric TUNEL System), with the following modifications. Once dissected, embryos were fixed in 4% PFA + 0.1% Tween + 0.01% Triton for 20–30 min at RT, washed three times in PBT for 5 min, and permeabilized with 0.4% Triton in PBS for 20 min. Then, TUNEL staining was performed as indicated in the kit; once completed, embryos were stained for c-MYC using a tyramide signal amplification system (PerkinElmer TSA Cyanine Plus 3 Evaluation Kit) as described elsewhere (Clements et al., 2011).
Western Blot Analysis
For the analysis of cells in cocultures, the different genotypes were separated by FACS based on GFP expression. Cells were lysed in radio immunoprecipitation assay buffer containing Complete Mini Protease Inhibitors (Roche) and Phosphatase Inhibitors Set II (Calbiochem). Protein extracts (5–40 μg) were resolved by a 10% SDS-PAGE gel and subjected to western blot analysis. Primary antibodies used were rabbit anti-c-MYC (N-262 clone, 1:200), rabbit anti-phospho-Smad1/5/8 (1:1,000; Cell Signaling), rabbit anti-LC3 (1:300; Abgent), mouse anti-p62 (1:1,500; BD Bioscience), (rabbit anti-PCNA (1:5,000; Santa Cruz), and rabbit anti-tubulin (1:5,000; Cell Signaling).
For clonal assays, flow cytometry, annexin V staining, electroporation, RNA isolation, quantitative real-time PCR, and microarray analysis, see Supplemental Experimental Procedures.
Acknowledgments
We thank Mel Clements for help with the embryos, members of the Molecular Embryology group, Vasso Episkopou, Jesus Gil, and Helle Jorgenssen for critical discussions, and Marion Leleu for help with microarray data analysis. We also thank Elodie Ndjetehe for the blastocyst injections. We are grateful to Professor Mizushima for the gift of the Atg5−/− ESCs. We also thank the Fundação para a Ciência e Tecnologia (Portugal), Lister Institute of Preventive Medicine, Medical Research Council, and British Heart Foundation for funding.
Published: July 15, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes Supplemental Experimental Procedures, six figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2013.06.012.
Accession Numbers
The GEO accession number for our microarray data is GSE48092.
Supplemental Information
References
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Burke R., Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996;122:2261–2269. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- Cambray S., Arber C., Little G., Dougalis A.G., de Paola V., Ungless M.A., Li M., Rodríguez T.A. Activin induces cortical interneuron identity and differentiation in embryonic stem cell-derived telencephalic neural precursors. Nat. Commun. 2012;3:841. doi: 10.1038/ncomms1817. [DOI] [PubMed] [Google Scholar]
- Cartwright P., McLean C., Sheppard A., Rivett D., Jones K., Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- Clements M., Pernaute B., Vella F., Rodriguez T.A. Crosstalk between Nodal/activin and MAPK p38 signaling is essential for anterior-posterior axis specification. Curr. Biol. 2011;21:1289–1295. doi: 10.1016/j.cub.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucouvanis E., Martin G.R. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- Dang C.V. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Miura S., Hill C., Mishina Y., Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev. Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- de Beco S., Ziosi M., Johnston L.A. New frontiers in cell competition. Dev. Dyn. 2012;241:831–841. doi: 10.1002/dvdy.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C., Abril M., Bellosta P., Gallant P., Johnston L.A. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Di-Gregorio A., Sancho M., Stuckey D.W., Crompton L.A., Godwin J., Mishina Y., Rodriguez T.A. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development. 2007;134:3359–3369. doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- Eakin G.S., Hadjantonakis A.K., Papaioannou V.E., Behringer R.R. Developmental potential and behavior of tetraploid cells in the mouse embryo. Dev. Biol. 2005;288:150–159. doi: 10.1016/j.ydbio.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Heyer B.S., MacAuley A., Behrendtsen O., Werb Z. Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Genes Dev. 2000;14:2072–2084. [PMC free article] [PubMed] [Google Scholar]
- Levayer R., Moreno E. Mechanisms of cell competition: themes and variations. J. Cell Biol. 2013;200:689–698. doi: 10.1083/jcb.201301051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Auley A., Werb Z., Mirkes P.E. Characterization of the unusually rapid cell cycles during rat gastrulation. Development. 1993;117:873–883. doi: 10.1242/dev.117.3.873. [DOI] [PubMed] [Google Scholar]
- Manova K., Tomihara-Newberger C., Wang S., Godelman A., Kalantry S., Witty-Blease K., De Leon V., Chen W.S., Lacy E., Bachvarova R.F. Apoptosis in mouse embryos: elevated levels in pregastrulae and in the distal anterior region of gastrulae of normal and mutant mice. Dev. Dyn. 1998;213:293–308. doi: 10.1002/(SICI)1097-0177(199811)213:3<293::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Miura S., Davis S., Klingensmith J., Mishina Y. BMP signaling in the epiblast is required for proper recruitment of the prospective paraxial mesoderm and development of the somites. Development. 2006;133:3767–3775. doi: 10.1242/dev.02552. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Yamamoto A., Hatano M., Kobayashi Y., Kabeya Y., Suzuki K., Tokuhisa T., Ohsumi Y., Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Moreno E., Basler K., Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Nagy A., Gertsenstein M., Vintersten K., Behringer R. Third Edition. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2003. Manipulating the Mouse Embryo. [Google Scholar]
- Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Nowotschin S., Costello I., Piliszek A., Kwon G.S., Mao C.A., Klein W.H., Robertson E.J., Hadjantonakis A.K. The T-box transcription factor Eomesodermin is essential for AVE induction in the mouse embryo. Genes Dev. 2013;27:997–1002. doi: 10.1101/gad.215152.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Zou Z., Sun Q., Luby-Phelps K., Cheng P., Hogan R.N., Gilpin C., Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- Rhiner C., Díaz B., Portela M., Poyatos J.F., Fernández-Ruiz I., López-Gay J.M., Gerlitz O., Moreno E. Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development. 2009;136:995–1006. doi: 10.1242/dev.033340. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N., Johnston L.A. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc. Natl. Acad. Sci. USA. 2007;104:18543–18548. doi: 10.1073/pnas.0709021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.M., Dalton S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell. 2009;5:141–149. doi: 10.1016/j.stem.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow M.H.L. Gastrulation in the mouse: growth and regionalization of the epiblast. J. Embryol. Exp. Morphol. 1977;42:293–303. [Google Scholar]
- Spruce T., Pernaute B., Di-Gregorio A., Cobb B.S., Merkenschlager M., Manzanares M., Rodriguez T.A. An early developmental role for miRNAs in the maintenance of extraembryonic stem cells in the mouse embryo. Dev. Cell. 2010;19:207–219. doi: 10.1016/j.devcel.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Stuckey D.W., Clements M., Di-Gregorio A., Senner C.E., Le Tissier P., Srinivas S., Rodriguez T.A. Coordination of cell proliferation and anterior-posterior axis establishment in the mouse embryo. Development. 2011;138:1521–1530. doi: 10.1242/dev.063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey D.W., Di Gregorio A., Clements M., Rodriguez T.A. Correct patterning of the primitive streak requires the anterior visceral endoderm. PLoS ONE. 2011;6:e17620. doi: 10.1371/journal.pone.0017620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Wagstaff L., Kolahgar G., Piddini E. Competitive cell interactions in cancer: a cellular tug of war. Trends Cell Biol. 2013;23:160–167. doi: 10.1016/j.tcb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Nichols J., Chambers I., Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.