Abstract
Background and Purpose
The perihematomal hyperintensity is commonly interpreted to represent cerebral edema following ICH, but the accuracy of this interpretation is unknown. We therefore investigated the relationship between changes in PHH and changes in hemispheric brain volume as a measure of edema during the first week after ICH.
Methods
Fifteen individuals aged 66±13 with baseline hematoma size of 13.1 mL (range 3–43) were prospectively studied with sequential MRI 1.0±0.5, 2.6±0.9, and 6.5±1.0 days after spontaneous supratentorial ICH. Changes in hemispheric brain volume were assessed on MPRAGE using the Brain-Boundary Shift Integral. Hematoma and PHH volumes were measured on T2-weighted images.
Results
Brain volume increased a small but statistically significant amount (6.3±8.0 mL, 0.6±0.7%) between the first and second scans relative to 10 normal controls (−0.9±4.1 mL, p=0.02) and returned toward baseline at the third scan (1.5±9.5 mL vs. controls 0.9±4.0 mL, p=0.85). There were no significant differences in the volume changes between the two hemispheres at scan 2 or scan 3. At both scan 2 (p=0.04) and scan 3 (p=0.004), the change in PHH was significantly greater than and poorly correlated with the change in ipsilateral hemispheric volume. There were no significant correlations between change in NIHSS and change in PHH, ipsilateral, or total brain volume at scan 2 or scan 3 (all p> 0.05).
Conclusions
In patients with small-to-moderate-sized hematomas, change in PHH was a poor measure of brain edema in the first week following ICH. A small degree of bihemispheric brain swelling occurred but was of little clinical significance.
INTRODUCTION
There is considerable controversy regarding the clinical importance of perihematomal edema following acute intracerebral hemorrhage (ICH).(1–9) While experimental models of ICH have shown early blood-brain barrier breakdown leading to diffuse cerebral edema,(10–12) clinical data on the development and severity of edema are inconsistent,(2–5) likely reflecting different definitions, methodologies, and time periods studied. The largest study to date (1) suggested that perihematomal edema as defined by CT is unlikely to be clinically relevant, but addressed only the initial 72 hours and utilized an insensitive technique.
As opposed to experimental studies in which changes in brain water content are measured directly, most clinical studies have relied on measuring the extent of the CT perihematomal lucency or its MRI counterpart, the perihematomal hyperintensity (PHH). This approach is both technically and mechanistically problematic, however. On CT, the borders of the high and low density regions become increasingly indistinct over time after ICH, which may limit the accuracy of measurements and contribute to inconsistent results. In addition, interpretation of changes in the perihematomal lucency or PHH as indicating increased brain water content or brain swelling is complicated because of the phenomenon of clot retraction, in which the clot progressively shrinks and extrudes serum around it.(13, 14) Nevertheless, the PHH has been interpreted to represent both cytotoxic and vasogenic edema.(15)
In our previous study that circumvented the problem of the perihematomal signal abnormality and used change in midline shift to assess change in ipsilateral hemispheric volume, we did not find any evidence for brain edema within the first week following ICH; however, sensitivity was limited to detecting only changes in shift of 2 mm or greater.(5) We have previously demonstrated that sequential MRI scanning using the Brain-Boundary Shift Integral (BBSI) (16) is more sensitive for detecting brain volume changes than is measurement of midline shift.(17) In the absence of hematoma extension, accurate measurement of brain volume changes permits determination of the degree and timing of early edema after ICH.
Given the inconsistent data on the extent and significance of ICH-associated edema and unclear pathophysiology of the perihematomal signal change, we used sequential MRI scanning and the BBSI in the present study to measure change in brain volume and investigate the relationship between change in brain volume and change in PHH during the first week after ICH. The null hypotheses were that there would be no increase in brain volume in subjects with ICH compared to normal control subjects and that there would be no correlation between brain volume change and change in PHH, i.e., that the PHH is not due to an increase in brain water producing an increase in brain volume.
MATERIALS AND METHODS
ICH subjects
Patients were eligible for inclusion if they suffered a spontaneous, non-traumatic supratentorial ICH evident on CT scan, were at least 18 years of age, and could be studied within 48 hours of onset. (Onset was defined as the time the patient was last known to be normal if symptoms were first noticed on waking from sleep or if no accurate history could be obtained.) Patients were excluded if pregnant; if the hemorrhage was thought to be associated with a vascular malformation, aneurysm, or tumor; if there were plans for immediate surgery; if there were contraindications to MRI; if the patient had received osmotic agents (in order to permit study of the natural history of edema after ICH); or if large portions of the hematoma abutted a cerebrospinal fluid surface (because intensity changes in the hematoma over time could obscure intensity changes due to tissue shifts). Finally, per the university Human Research Protection Office, patients with “critical illness,” defined by a Glasgow Coma Scale score <5, signs of brainstem dysfunction, or vasopressor requirement, were excluded.
Demographic data and risk factors for ICH were recorded. All subjects were admitted to the Neurology Neurosurgery Intensive Care Unit (NNICU) and transferred to the Neurology or Neurosurgery floor after remaining stable for 1–3 days. No subject was intubated, had an intraventricular catheter placed, had intracranial pressure monitoring, or received osmotic agents, corticosteroids, or blood products between the time of admission and the end of the study period. NIH Stroke Scale (NIHSS) was performed on admission and daily throughout the study period, and the medical record and nurses' notes were reviewed daily to look for any indication of neurological deterioration.
Normal control subjects
Ten individuals ranging in age from 29 to 62 who were free from neurological disease served as normal control subjects.
Imaging
ICH subjects were prospectively studied with MRI scanning of the brain at three time points after ICH onset: within 48 hours, at 2–5 days, and at 5–8 days. All subjects were studied using a Siemens Magnetom Sonata 1.5-Tesla scanner. A midsagittal scout T1-weighted spin-echo pulse sequence was used to position the subject. A 3-D MPRAGE sequence (TR=11.4 ms, TE=4.4 ms, TI=300 ms, flip angle=8, acquisition time=7:32 min, 256×256×128, 1×1×1.25 mm pixels) was acquired in a sagittally-oriented slab to produce a high-resolution T1-weighted image. The first five subjects had a T2-weighted image acquired using a turbo spin-echo sequence (TR=4100 ms, TE=120 ms, flip angle=140, acquisition time=6:54 min, 1×1×1 mm pixels). Because of its superior ability to define volumes of PHH, in the last ten subjects, a FLAIR sequence (TR=9999 ms, TE=119 ms, TI=2309 ms, flip angle=180, acquisition time=7:38 min, 256×256×32, 1×1×2 mm pixels) was substituted and acquired in an axially-oriented slab using two interleaved acquisitions. Images were examined immediately after acquisition and repeated if there was significant movement artifact. A physician or NNICU-trained research nurse was present throughout all studies.
Normal control subjects underwent the same MPRAGE sequence at intervals of 2.1±0.1 and 6.2±0.4 days.
Written informed consent was obtained from all participants or their legally authorized representative. The study was approved by the university Human Research Protection Office.
Image Analysis
All MR images for each subject were aligned to the baseline T1-weighted image (MPRAGE) from that subject using automated image registration.(18, 19) Each T1-weighted image was segmented to brain and normalized using the mean brain pixel intensity. The BBSI (16) was used to measure changes in brain volume. This technique measures the change in pixel intensity within a thin region of interest comprising the brain/cerebrospinal fluid boundaries of the entire brain. The total change in intensity within this region of interest is divided by the difference between gray matter and cerebrospinal fluid intensities to convert the measurement to change in brain volume. This measurement of change in brain volume was also calculated for the ipsilateral and contralateral hemispheres separately after the brain was divided by manually tracing the midline on all transverse image slices. The hematoma was defined on T2-weighted images by selecting the upper intensity value that defined the hypointense rim and bulk on each slice throughout the lesion and then manually editing to include hyper- or isointense foci within the bulk.(20) Perihematomal hyperintensity was similarly measured on T2-weighted images by intensity thresholding the region of hyperintense signal outside the hematoma boundary and manually editing as necessary. The resulting regions of interest were summed to yield hematoma and PHH volumes. Total lesion volume was calculated as the sum of the hematoma and PHH volumes. BBSI analysis was performed by a single investigator who was blinded to clinical information. Measurements of hematoma and PHH volume were performed by a single investigator who was blinded to scan order and brain volume results. Intrarater reliability of hematoma and PHH volume measurements was determined in a subset of 12 scans evaluated on two separate occasions. Intraclass correlation coefficient was 0.94.
We have previously established the accuracy of the BBSI method (17) by measuring change in brain volume in normal control subjects between a baseline image and a copy of the image that had been resized by 0.5, 1.0, 1.5, and 2.0%. The precision of the method was established by measuring the change in volume for all repeated scans of normal control subjects. Based on those data, a sample size of 10 subjects would allow 95% power to detect a 0.6% change in brain volume (2-tailed t-test).
All continuous data are presented as mean ± standard deviation or median (range). The Shapiro-Wilk test was used to test data for normality of distribution. For data that significantly deviated from normality, non-parametric tests were used. Parametric tests were used in all other cases. Statistical analyses were performed with SPSS 17.0 for Windows (SPSS, Inc, Chicago, IL).
RESULTS
Of 221 consecutive patients with acute ICH screened, 26 were enrolled. Reasons for non-enrollment include the following: beyond time window (76), critical illness and/or large portion of hematoma abutting a cerebrospinal fluid surface (34), suspected hemorrhage related to tumor, arteriovenous malformation, or trauma (22), brainstem hemorrhage (18), declined participation (16), treatment with mannitol (10), MRI contraindication (8), surgery (6), and not consentable and no legally authorized representative available (5). Eleven of the 26 enrolled were excluded from this report because MRI data were not analyzable due to excessive movement (n=6), inability to complete the first MRI within 48 hours of onset (n=3), or hematoma evacuation after the first MRI (n=1) or because the hematoma was associated with warfarin use (n=1).
The remaining 15 subjects were studied with sequential MRI scans 1.0±0.5, 2.6±0.9, and 6.5±1.0 days after ICH. Age ranged from 36 to 86 years. Seven (47%) were female. Fourteen (93%) had hypertension. The single non-hypertensive subject had a second lobar hemorrhage two months after the index hemorrhage, meeting Boston criteria(21) for probable cerebral amyloid angiopathy. Hematomas were putamenal in nine, thalamic in two, and lobar in four. Four subjects (27%) had intraventricular hemorrhage (IVH score(22) 1 in two and 3 in two), and two (13%) had subarachnoid extension of blood. None had hydrocephalus on the baseline scan; one (subject 4) developed a progressive increase in ventricular size (8% increase at scan 2; 50% increase at scan 3). Median hematoma size on the baseline scan was 13.1 mL (range 3–43) and decreased across the study period. No subject showed an increase in hematoma size. Volumes of PHH and total lesion volume progressively increased. (Table 1.) Despite the 6.5 mL increase in total lesion volume, there were no changes in other markers of tissue shift (midline shift, uncal herniation, brainstem compression, or cisternal effacement).
Table 1.
Lesion volumes (mL) over first week after intracerebral hemorrhage*
| Scan 1 | Scan 2 | Scan 3 | |
|---|---|---|---|
| Hematoma volume | 13.1 (3–43) | 11.3 (2–42)† | 11.1 (2–39)‡ |
| PHH volume | 23.2 (11–48) | 28.0 (14–59)‡ | 30.8 (13–62)‡ |
| Total lesion volume | 37.0 (14–84) | 43.0 (16–89)† | 43.5 (15–102)† |
Values are median (range)
Significance level relative to scan 1:
p<0.005,
p<0.001
Scan 1 performed at 1.0±0.5, Scan 2 at 2.6±0.9, and Scan 3 at 6.5±1.0 days after hemorrhage.
Relative to sequential test-retest changes in 10 normal control subjects, brain volume increased a small but statistically significant amount of 0.6 ± 0.7% between the first and second scans (patients: 6.3 ±8.0 mL, controls: −0.9±4.1 mL, independent samples t-test, p=0.02) and returned toward baseline at the third scan (patients: 1.5±9.5 mL, controls: 0.9±4.0 mL, independent samples t-test, p=0.85). (Table 2.) There were no significant differences in the volume change between the two hemispheres at scan 2 or scan 3 (paired samples t-tests, p>0.05) (Figure 1).
Table 2.
Baseline volumes of hematoma (ICH) and perihematomal hyperintensity (PHH) and change in hemispheric volume in 15 individuals with intracerebral hemorrhage
| ID | Baseline ICH volume (mL) | Baseline PHH volume (mL) | Scan 2-1 change in hemispheric volume (mL) | Scan 3-1 change in hemispheric volume (mL) |
|---|---|---|---|---|
| 1 | 15.5 | 27.2 | 6.4 | −2.3 |
| 2 | 30.6 | 19.6 | 13.5 | −4.4 |
| 3 | 3.4 | 11.3 | −6.9 | −8.3 |
| 4 | 43.2 | 41.0 | 6.2 | −19.5 |
| 5 | 15.5 | 26.6 | 21.7 | 4.5 |
| 6 | 13.1 | 25.2 | 4.3 | 11.5 |
| 7 | 13.4 | 23.6 | −0.1 | 1.3 |
| 8 | 7.1 | 12.0 | 2.6 | −1.9 |
| 9 | 9.0 | 23.2 | 1.5 | −3.2 |
| 10 | 7.9 | 19.6 | 4.8 | 12.3 |
| 11 | 33.2 | 42.0 | −2.7 | −3.4 |
| 12 | 7.6 | 19.9 | 4.7 | 19.3 |
| 13 | 5.6 | 10.8 | 19.6 | 14.7 |
| 14 | 24.0 | 48.3 | 15.2 | 2.5 |
| 15 | 6.1 | 14.5 | 3.8 | −0.7 |
Scan 1 performed at 1.0±0.5, Scan 2 at 2.6±0.9, and Scan 3 at 6.5±1.0 days after hemorrhage.
Figure 1.
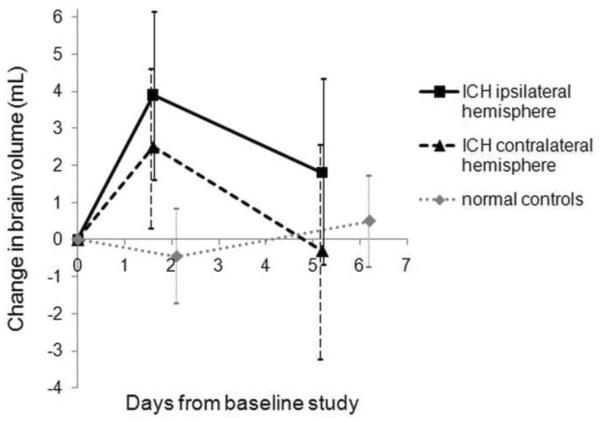
Mean change with 95% confidence intervals in brain volume (mL) in 15 ICH subjects (ipsilateral hemisphere, solid black line; contralateral hemisphere, dashed black line) and 10 normal control subjects (one hemisphere, dotted gray line). In ICH subjects, the baseline study was performed 1.0±0.5 days after hemorrhage. The peak change in brain volume represents a 0.7% increase in the ipsilateral hemisphere and 0.5% in the contralateral hemisphere in ICH subjects and a 0.1% decrease in control subjects. Hemispheric change for normal controls is bilateral change divided by 2 for the purposes of comparison to ICH subjects.
At both scan 2 (p=0.04, independent samples t-test) and scan 3 (p=0.004, Wilcoxon signed rank test), the change in PHH was significantly greater than and poorly correlated with the change in ipsilateral hemispheric volume. Between scan 1 and scan 2, an apparent weak correlation (r=0.52, p=0.045) was due to one outlier (Fig 2A). Removal of this outlier resulted in a non-significant correlation (r=0.10, p=0.87). There was no significant correlation between change in ipsilateral hemispheric volume and change in PHH between scan 1 and scan 3 (ρ=0.19, p =0.51).
Figure 2.
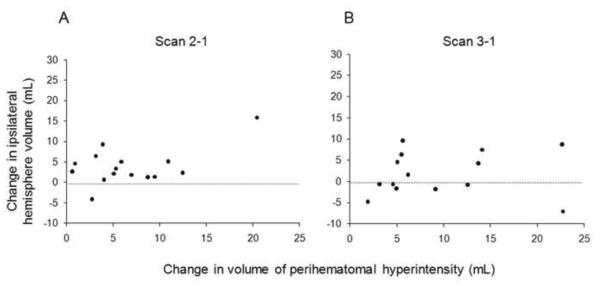
Scatter plot showing the correlation between change in ipsilateral hemisphere volume and change in perihematomal hyperintensity volume (a) between scan 1 and scan 2 (r=0.52, p=0.045) and (b) between scan 1 and scan 3 (ρ= 0.191, p =0.51) in 15 subjects with intracerebral hemorrhage.
Exploratory analyses (uncorrected for multiple comparisons) were performed to investigate further relationships between the hematoma and changes in hemispheric volume. No statistically significant relationships were found between change in ipsilateral hemispheric volume over each time interval and either 1) baseline volumes of the hematoma, PHH, and total lesion or 2) changes in volume of the hematoma, PHH, and total lesion. Change in hemispheric volume did not differ by hematoma location or presence of intraventricular hemorrhage. (All p>0.05). Baseline hematoma volume was significantly correlated with baseline PHH volume (r=0.78, p<0.001).
Median NIHSS was 8 (range 2–22) on admission and 9 (range 2–22) at the time of the baseline study. There were no significant correlations between change in NIHSS and change in ipsilateral or total brain volume between the first and second or the first and third study sessions (all p>0.05). In addition there were no significant correlations between change in NIHSS and change in PHH at scan 2 (r=0.20, p=0.48) or at scan 3 (ρ= −0.02, p=0.95)
DISCUSSION
Cerebral edema results when an increase in brain water content produces an increase in brain volume.(23) The poor correspondence between the increase in PHH and the lesser change in ipsilateral hemispheric volume indicates that change in the PHH is not a good measure of the degree of brain edema following ICH. While a small increase in brain water may contribute to the PHH, other processes that increase PHH without a matching increase in brain volume such as clot retraction or diffusion of serum from the initial clot must predominate. With these latter two processes, the increase in PHH is due to a redistribution of existing water from the initial hemorrhage, either as serum is extruded from the central clot (clot retraction) or diffuses along white matter tracts (serum diffusion). The volume of tissue with increased T2 signal increases, but the overall extracellular fluid volume contained within the tissue does not.
In the present study, we found a small but statistically significant 6 mL or 0.6% increase in hemispheric brain volume between post-hemorrhage day 1 and 3. Brain volume then returned towards baseline by the end of the first week. The magnitude of this change is consistent with other studies of the effect of osmotic factors on brain volume. We previously used this technique to detect a brain volume change of 0.6% in a clinical study of the acute effects of mannitol in patients with edema due to recent cerebral infarction.(17) Similar magnitude changes in brain volume of a 0.55% decrease during 16 hours of fluid restriction and a 0.72% increase during rehydration have been reported in normal subjects.(24)
Unexpectedly, the increase in brain volume occurred not only in the ipsilateral hemisphere, but the contralateral one as well. Similar to our results, an experimental model of ICH showed bilateral changes in brain water content. Brain water content increased after ICH, peaking between 1 and 4 days, and then gradually resolved.(25) The edema was most severe in perihematomal tissue, but was also present elsewhere in both the ipsilateral and contralateral hemispheres. Recently presented CT perfusion data from Xing et al(26) show increased blood-brain barrier permeability in 19 human subjects with ICH studied within 24 hours of onset, both focally around the hematoma as well as diffusely in both cerebral hemispheres. The mechanism for this bilateral blood-brain barrier permeability change is not known. Injury due to barotrauma from fluid percussion waves arising within the cranium due to the sudden expansion of the hematoma under arterial pressure has been suggested as a possible cause.(27)
Neither increases in brain volume nor in PHH correlated with neurological worsening. This is consistent with a majority of previous reports showing no association between PHH and clinical status or outcome.(1, 2, 28, 29)
Generalizability of our results is limited by the small-to-moderate hematoma size (3–43 mL) and relatively good clinical grade of the individuals studied. These features were necessitated by the restrictions of our Human Research Protection office and by limitations of the BBSI technique when used in the setting of lesions that inherently produce MRI signal intensity changes over time. While these clinical characteristics permitted us to more accurately assess small changes in clinical grade or mass effect than we could have in subjects in coma with massive hematomas, we do not know whether PHH would be better correlated with brain volume in individuals with larger hematomas. We did not find that moderate hematomas behaved differently from smaller ones, but it is possible that we missed a “threshold effect,” in which a hematoma of sufficient size would produce a commensurate increase in hemispheric volume. Other pitfalls have been associated with the BBSI method,(30) but data from our normal control subjects show the error of the technique as we used it. The earliest baseline study was performed 8 hours after hemorrhage, so we cannot comment on brain volume before this time. Finally, since we excluded from the study individuals having ICH associated with aneurysm, vascular malformation, or warfarin use, we cannot extrapolate our results to these conditions.
CONCLUSIONS
In these patients with small-to-moderate sized hematomas, a small degree of bihemispheric brain swelling occurred over the first week after ICH, but it had no clinical consequence. Change in PHH was greater than and correlated poorly with changes in brain volume over this time period; thus change in PHH was a poor measure of brain edema. Due to the lack of correlation with brain swelling or with clinical course, our data do not support a strategy of treating isolated increases in perihematomal signal change within the first week after ICH with agents aimed at reducing brain swelling.
Acknowledgements
The authors would like to thank Angela Shackleford, R.N., and the NNICU nurses for their help in performing the MRI studies. This work was supported by NIH (NS35966 and NS044885) and the H. Houston Merritt Distinguished Professorship of Neurology at the University of North Carolina at Chapel Hill.
REFERENCES
- 1.Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Morgenstern LB, Chalmers J, Anderson CS. Significance of perihematomal edema in acute intracerebral hemorrhage: the INTERACT trial. Neurology. 2009;73:1963–1968. doi: 10.1212/WNL.0b013e3181c55ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inaji M, Tomita H, Tone O, Tamaki M, Suzuki R, Ohno K. Chronological changes of perihematomal edema of human intracerebral hematoma. Acta Neurochir.Suppl. 2003;86:445–448. doi: 10.1007/978-3-7091-0651-8_91. [DOI] [PubMed] [Google Scholar]
- 3.Mayer SA, Sacco RL, Shi T, Mohr JP. Neurologic deterioration in noncomatose patients with supratentorial intracerebral hemorrhage. Neurology. 1994;44:1379–1384. doi: 10.1212/wnl.44.8.1379. [DOI] [PubMed] [Google Scholar]
- 4.Gebel JM, Jr., Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–2641. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- 5.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- 6.Elijovich L, Patel PV, Hemphill JC., III Intracerebral hemorrhage. Semin.Neurol. 2008;28:657–667. doi: 10.1055/s-0028-1105974. [DOI] [PubMed] [Google Scholar]
- 7.Elliott J, Smith M. The acute management of intracerebral hemorrhage: a clinical review. Anesth.Analg. 2010;110:1419–1427. doi: 10.1213/ANE.0b013e3181d568c8. [DOI] [PubMed] [Google Scholar]
- 8.Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat.Rev.Neurol. 2010;6:593–601. doi: 10.1038/nrneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- 9.Sansing LH, Kaznatcheeva EA, Perkins CJ, Komaroff E, Gutman FB, Newman GC. Edema after intracerebral hemorrhage: correlations with coagulation parameters and treatment. J Neurosurg. 2003;98:985–992. doi: 10.3171/jns.2003.98.5.0985. [DOI] [PubMed] [Google Scholar]
- 10.Wagner KR, Xi G, Hua Y, Kleinholz M, de Court, Myers RE, Broderick JP, Brott TG. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke. 1996;27:490–497. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- 11.Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272–278. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- 12.Keep RF, Xiang J, Ennis SR, Andjelkovic A, Hua Y, Xi G, Hoff JT. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir.Suppl. 2008;105:73–77. doi: 10.1007/978-3-211-09469-3_15. [DOI] [PubMed] [Google Scholar]
- 13.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 14.Kendall BE, Radue EW. Computed tomography in spontaneous intracerebral haematomas. Br.J Radiol. 1978;51:563–573. doi: 10.1259/0007-1285-51-608-563. [DOI] [PubMed] [Google Scholar]
- 15.Olivot JM, Mlynash M, Kleinman JT, Straka M, Venkatasubramanian C, Bammer R, Moseley ME, Albers GW, Wijman CA. MRI profile of the perihematomal region in acute intracerebral hemorrhage. Stroke. 2010;41:2681–2683. doi: 10.1161/STROKEAHA.110.590638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. J Magn Reson.Imaging. 1997;7:1069–1075. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- 17.Videen TO, Zazulia AR, Manno EM, Derdeyn CP, Adams RE, Diringer MN, Powers WJ. Mannitol bolus preferentially shrinks non-infarcted brain in patients with ischemic stroke. Neurology. 2001;57:2120–2122. doi: 10.1212/wnl.57.11.2120. [DOI] [PubMed] [Google Scholar]
- 18.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J.Comput.Assist.Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Freeborough PA, Woods RP, Fox NC. Accurate registration of serial 3D MR brain images and its application to visualizing change in neurodegenerative disorders. J Comput.Assist.Tomogr. 1996;20:1012–1022. doi: 10.1097/00004728-199611000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Alemany RM, Stenborg A, Sonninen P, Terent A, Raininko R. Detection and appearance of intraparenchymal haematomas of the brain at 1.5 T with spin-echo, FLAIR and GE sequences: poor relationship to the age of the haematoma. Neuroradiology. 2004;46:435–443. doi: 10.1007/s00234-004-1191-5. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 22.Ruscalleda J, Peiro A. Prognostic factors in intraparenchymatous hematoma with ventricular hemorrhage. Neuroradiology. 1968;28:34–37. doi: 10.1007/BF00341763. [DOI] [PubMed] [Google Scholar]
- 23.Fishman RA. Brain edema. N.Engl.J Med. 1975;293:706–711. doi: 10.1056/NEJM197510022931407. [DOI] [PubMed] [Google Scholar]
- 24.Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–550. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- 25.Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J.Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- 26.Xing L, Lee TY, Tamm A, Emery D, Jeerakathil T, Butcher K. Blood:brain barrier permeability is diffusely elevated in primary intracerebral hemorrhage. Stroke. 2010;41:e200–e253. abstract 40. [Google Scholar]
- 27.Powers WJ. Intracerebral hemorrhage and head trauma: common effects and common mechanisms of injury. Stroke. 2010;41:S107–S110. doi: 10.1161/STROKEAHA.110.595058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suga S, Sato S, Yunoki K, Mihara B. Sequential change of brain edema by semiquantitative measurement on MRI in patients with hypertensive intracerebral hemorrhage. Acta Neurochir.Suppl (Wien.) 1994;60:564–567. doi: 10.1007/978-3-7091-9334-1_156. [DOI] [PubMed] [Google Scholar]
- 29.Jauch E, Gebel J, Salisbury S, Broderick J, Brott T, Kothari R, Tomsick T, Pancioli A, Barsan W. Lack of association between early edema and outcome in spontaneous intracerebral hemorrhage. Stroke. 1999;30:249. [Google Scholar]
- 30.Preboske GM, Gunter JL, Ward CP, Jack CR., Jr. Common MRI acquisition non-idealities significantly impact the output of the boundary shift integral method of measuring brain atrophy on serial MRI. Neuroimage. 2006;30:1196–1202. doi: 10.1016/j.neuroimage.2005.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]


