Abstract
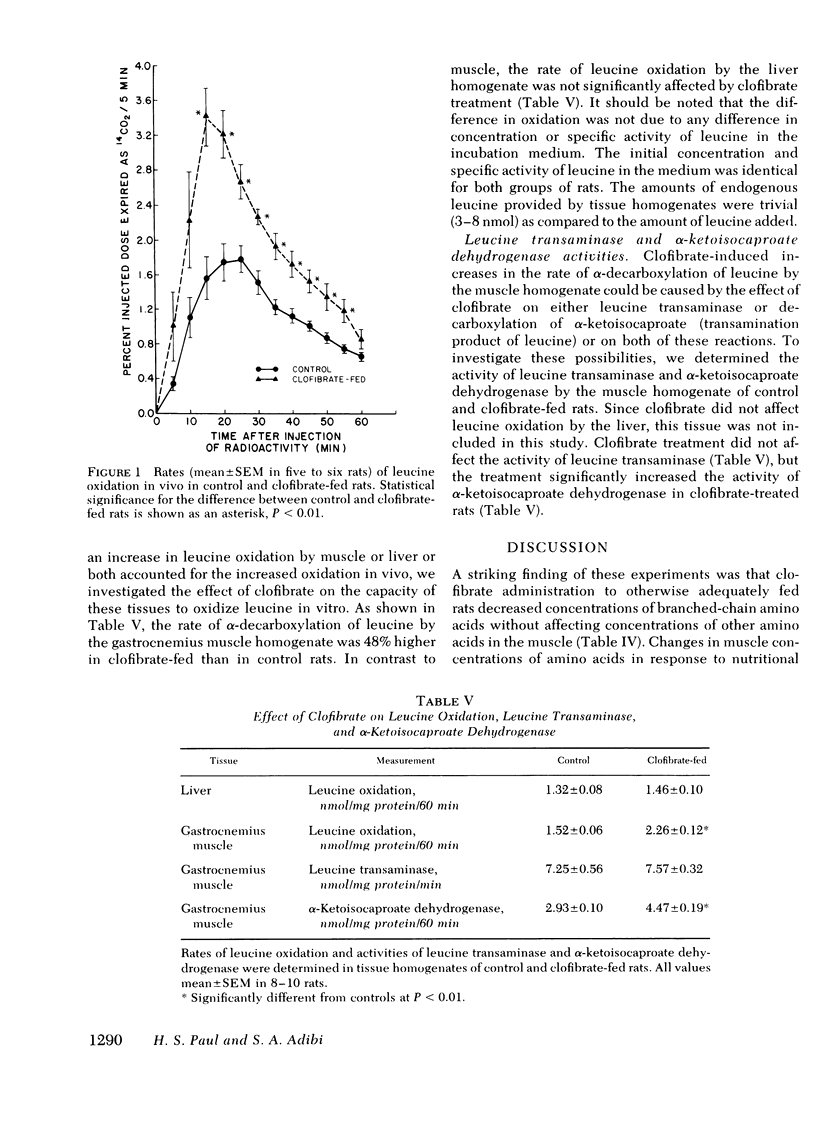
Treatment of hyperlipidemia with clofibrate may result in development of a muscular syndrome. Our previous investigation (1979. J. Clin. Invest.64: 405.) showed that chronic administration of clofibrate to rats causes myotonia and decreases glucose and fatty acid oxidation and total protein of skeletal muscle. In the present experiments we have investigated amino acid and protein metabolism in these rats. Clofibrate administration decreased the concentration of all three branched-chain amino acids without affecting those of others in muscle. Studies to examine the mechanism of decreases in muscle concentrations of branched-chain amino acids showed the following: (a) Plasma concentration of leucine was decreased, whereas there was no significant change in the concentration of isoleucine and valine. (b) Liver concentrations of all three branched-chain amino acids remained unaltered. (c) The uptake of cycloleucine (a nonmetabolizable analogue of leucine) by both muscle and liver was unaffected. (d) The percentage of a trace amount of injected [1-14C]leucine expired as 14CO2 in 1 h was significantly increased. (e) The capacity of muscle homogenate for α-decarboxylation of leucine was enhanced, whereas that of liver was unaffected. (f) The activity of leucine transaminase was unaffected, whereas that of α-ketoisocaproate dehydrogenase was increased in muscle.
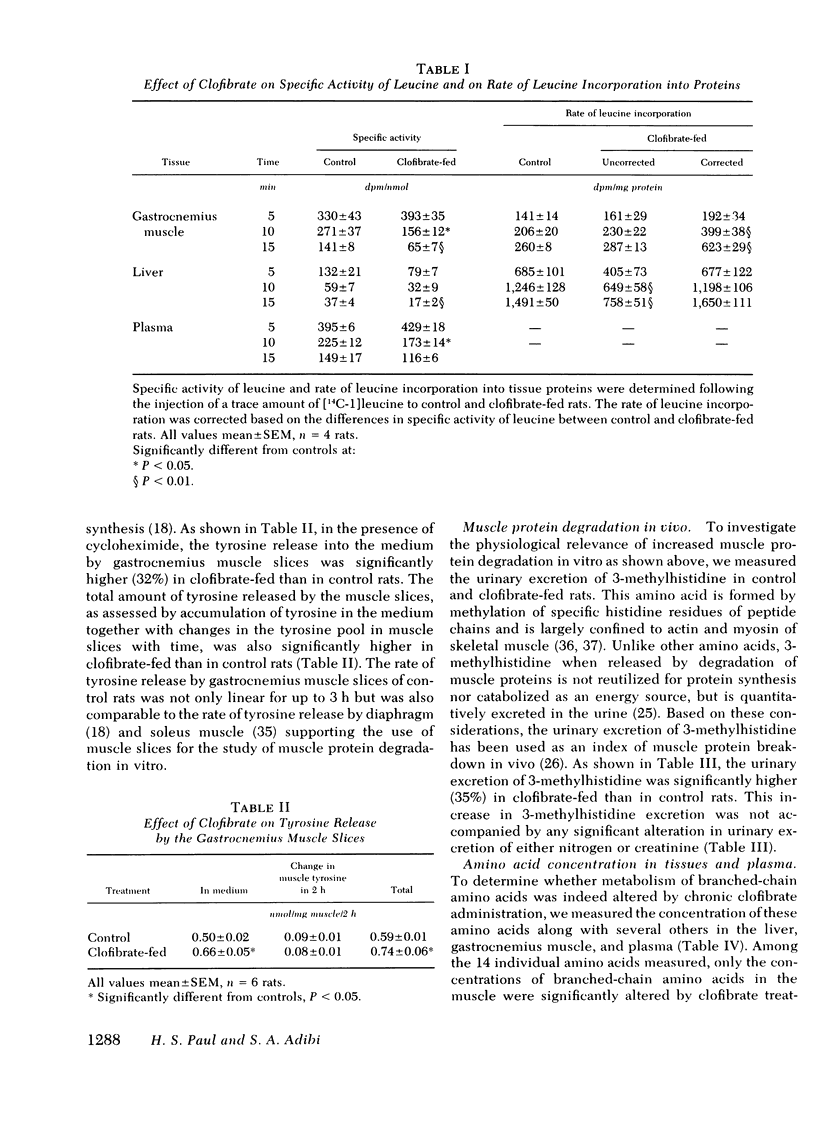
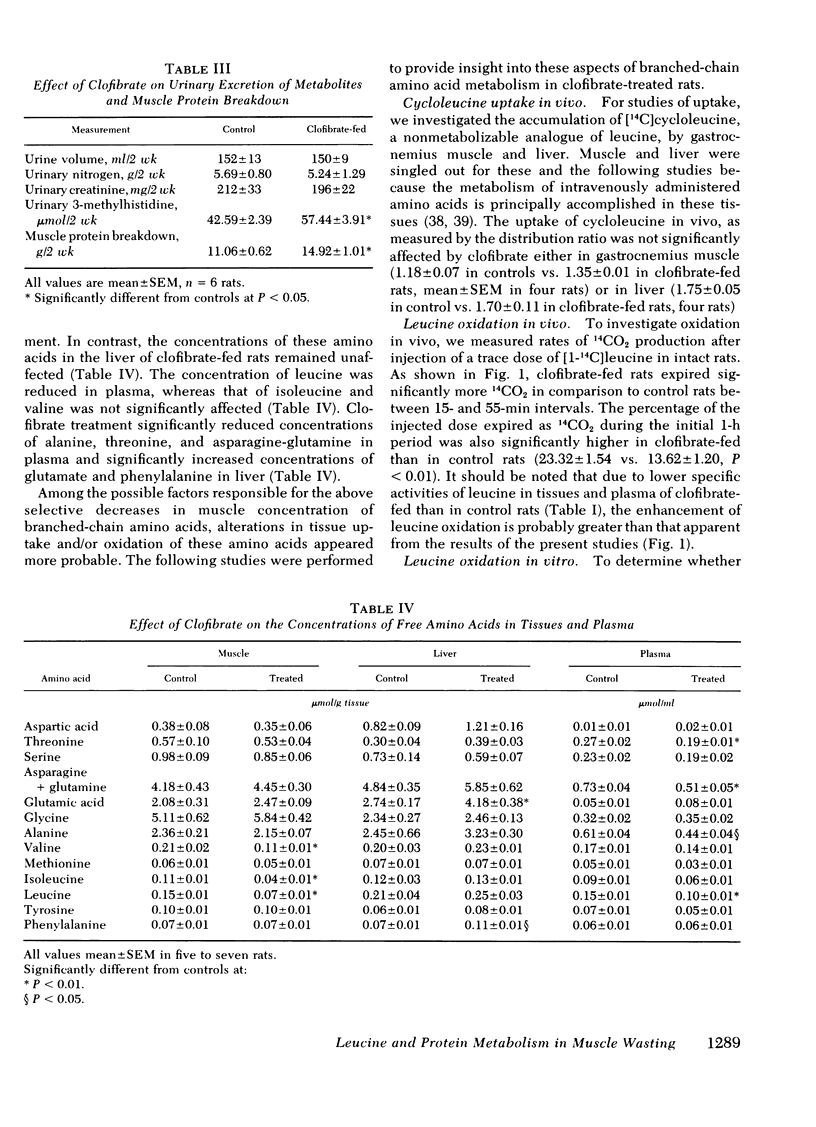
Studies of protein synthesis, carried out as incorporation of leucine into protein and corrected for differences in specific activity, showed no alteration in liver but enhanced synthesis in muscle of clofibrate-fed rats. Clofibrate stimulated muscle protein degradation, which was demonstrated by increased tyrosine release from gastrocnemius muscle slices and by increased urinary excretion of 3-methylhistidine.
We conclude that (a) clofibrate treatment increased branched-chain amino acid oxidation by increasing the activity of branched-chain α-ketoacid dehydrogenase in the muscle, (b) increased oxidation results in selective decreases in the concentration of these amino acids in muscle, and (c) decreases in branched-chain amino acid concentration may be responsible for increased protein degradation in muscle.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abourizk N., Khalil B. A., Bahuth N., Afifi A. K. Clofibrate-induced muscular syndrome. Report of a case with clinical, electromyographic and pathologic observations. J Neurol Sci. 1979 Jun;42(1):1–9. doi: 10.1016/0022-510x(79)90149-7. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. Interrelationships between level of amino acids in plasma and tissues during starvation. Am J Physiol. 1971 Sep;221(3):829–838. doi: 10.1152/ajplegacy.1971.221.3.829. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Krzysik B. A., Morse E. L., Amin P. M., Allen E. R. Oxidative energy metabolism in the skeletal muscle: biochemical and ultrastructural evidence for adaptive changes. J Lab Clin Med. 1974 Apr;83(4):548–562. [PubMed] [Google Scholar]
- Adibi S. A. Metabolism of branched-chain amino acids in altered nutrition. Metabolism. 1976 Nov;25(11):1287–1302. doi: 10.1016/s0026-0495(76)80012-1. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Modesto T. A., Morse E. L., Amin P. M. Amino acid levels in plasma, liver, and skeletal muscle during protein deprivation. Am J Physiol. 1973 Aug;225(2):408–414. doi: 10.1152/ajplegacy.1973.225.2.408. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Peterson J. A., Krzysik B. A. Modulation of leucine transaminase activity by dietary means. Am J Physiol. 1975 Feb;228(2):432–435. doi: 10.1152/ajplegacy.1975.228.2.432. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Bryant S. H. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974 Jul;240(2):505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatoor A. M., Armstrong M. D. 3-methylhistidine, a component of actin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):168–174. doi: 10.1016/0006-291x(67)90229-x. [DOI] [PubMed] [Google Scholar]
- BIGGS H. G., COOPER J. M. Modified Folin methods for the measurement of urinary creatine and creatinine. Clin Chem. 1961 Dec;7:655–664. [PubMed] [Google Scholar]
- Bieber L. L., Choi Y. R. Isolation and identification of aliphatic short-chain acylcarnitines from beef heart: possible role for carnitine in branched-chain amino acid metabolism. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2795–2798. doi: 10.1073/pnas.74.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilmazes C., Uauy R., Haverberg L. N., Munro H. N., Young V. R. Musle protein breakdown rates in humans based on Ntau-methylhistidine (3-methylhistidine) content of mixed proteins in skeletal muscle and urinary output of Ntau-methylhistidine. Metabolism. 1978 May;27(5):525–530. doi: 10.1016/0026-0495(78)90018-5. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Friderici K. H., Buse J. F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972 Dec 25;247(24):8085–8096. [PubMed] [Google Scholar]
- Buse M. G., Herlong H. F., Weigand D. A. The effect of diabetes, insulin, and the redox potential on leucine metabolism by isolated rat hemidiaphragm. Endocrinology. 1976 May;98(5):1166–1175. doi: 10.1210/endo-98-5-1166. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Reid S. S. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975 Nov;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Yamada C., Swendseid M. E. Effect of L-leucine on amino acid levels in plasma and tissue of normal and diabetic rats. Am J Physiol. 1968 Dec;215(6):1324–1328. doi: 10.1152/ajplegacy.1968.215.6.1324. [DOI] [PubMed] [Google Scholar]
- Eaton R. P. Effect of clofibrate on arginine-induced insulin and glucagon secretion. Metabolism. 1973 Jun;22(6):763–767. doi: 10.1016/0026-0495(73)90045-0. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., KAPLAN E. Effects of glucose on fatty acid oxidation by diaphragms from normal and alloxan-diabetic fed and starved rats. Am J Physiol. 1960 Jan;198:39–44. doi: 10.1152/ajplegacy.1960.198.1.39. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- GUROFF G., UNDENFRIEND S. The uptake of tyrosine by isolated rat diaphragm. J Biol Chem. 1960 Dec;235:3518–3522. [PubMed] [Google Scholar]
- Goldberg A. L., Odessey R. Oxidation of amino acids by diaphragms from fed and fasted rats. Am J Physiol. 1972 Dec;223(6):1384–1391. doi: 10.1152/ajplegacy.1972.223.6.1384. [DOI] [PubMed] [Google Scholar]
- Halliday D., McKeran R. O. Measurement of muscle protein synthetic rate from serial muscle biopsies and total body protein turnover in man by continuous intravenous infusion of L-(alpha-15N)lysine. Clin Sci Mol Med. 1975 Dec;49(6):581–590. doi: 10.1042/cs0490581. [DOI] [PubMed] [Google Scholar]
- Haverberg L. N., Omstedt P. T., Munro H. N., Young V. R. Ntau-methylhistidine content of mixed proteins in various rat tissues. Biochim Biophys Acta. 1975 Sep 9;405(1):67–71. doi: 10.1016/0005-2795(75)90315-3. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Johnson P., Harris C. I., Perry S. V. 3-methylhistidine in actin and other muscle proteins. Biochem J. 1967 Oct;105(1):361–370. doi: 10.1042/bj1050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzysik B. A., Adibi S. A. Comparison of metabolism of glycine injected intravenously in free and dipeptide forms. Metabolism. 1979 Dec;28(12):1211–1217. doi: 10.1016/0026-0495(79)90133-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li J. B., Goldberg A. L. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976 Aug;231(2):441–448. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- Meikle A. W., Klain G. J. Effect of fasting and fasting-refeeding on conversion of leucine into CO 2 and lipids in rats. Am J Physiol. 1972 May;222(5):1246–1250. doi: 10.1152/ajplegacy.1972.222.5.1246. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Nallathambi S. A., Goorin A. M., Adibi S. A. Hepatic and skeletal muscle transport of cycloleucine during starvation. Am J Physiol. 1972 Jul;223(1):13–19. doi: 10.1152/ajplegacy.1972.223.1.13. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- Ontell M., Paul H. S., Adibi S. A., Martin J. L. Involvement of transverse tubules in induced myotonia. J Neuropathol Exp Neurol. 1979 Nov;38(6):596–605. doi: 10.1097/00005072-197911000-00004. [DOI] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Assessment of effect of starvation, glucose, fatty acids and hormones on alpha-decarboxylation of leucine in skeletal muscle of rat. J Nutr. 1976 Aug;106(8):1079–1088. doi: 10.1093/jn/106.8.1079. [DOI] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Effect of carnitine on branched-chain amino acid oxidation by liver and skeletal muscle. Am J Physiol. 1978 May;234(5):E494–E499. doi: 10.1152/ajpendo.1978.234.5.E494. [DOI] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Leucine oxidation in diabetes and starvation: effects of ketone bodies on branched-chain amino acid oxidation in vitro. Metabolism. 1978 Feb;27(2):185–200. doi: 10.1016/0026-0495(78)90164-6. [DOI] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Paradoxical effects of clofibrate on liver and muscle metabolism in rats. Induction of myotonia and alteration of fatty acid and glucose oxidation. J Clin Invest. 1979 Aug;64(2):405–412. doi: 10.1172/JCI109476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotland D. L. An electron microscopic investigation of myotonic dystrophy. J Neuropathol Exp Neurol. 1970 Apr;29(2):241–253. [PubMed] [Google Scholar]
- Sekowski I., Samuel P. Clofibrate-induced acute muscular syndrome. Am J Cardiol. 1972 Oct;30(5):572–574. doi: 10.1016/0002-9149(72)90053-7. [DOI] [PubMed] [Google Scholar]
- Teräväinen H., Larsen A., Hillbom M. Clofibrate-induced myopathy in the rat. Acta Neuropathol. 1977 Aug 16;39(2):135–138. doi: 10.1007/BF00703319. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh V. W., Veerkamp J. H., Engelen P. J., Ghijsen W. J. Effect of L-carnitine on the oxidation of leucine and valine by rat skeletal muscle. Biochem Med. 1978 Aug;20(1):115–124. doi: 10.1016/0006-2944(78)90056-x. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Young V. R., Alexis S. D., Baliga B. S., Munro H. N., Muecke W. Metabolism of administered 3-methylhistidine. Lack of muscle transfer ribonucleic acid charging and quantitative excretion as 3-methylhistidine and its N-acetyl derivative. J Biol Chem. 1972 Jun 10;247(11):3592–3600. [PubMed] [Google Scholar]


