Abstract
Concurrent spread of Tomato yellow leaf curl virus (TYLCV) with invasion of Bemisia tabaci Q rather than B in China suggests a more mutualistic relationship between TYLCV and Q than B. To assess this hypothesis, we quantified the impacts of TYLCV on the performance and competitiveness of B and Q in the laboratory. The results showed that relative to their non-infected counterparts feeding on cotton (a non-host for TYLCV), infected B exhibited significant reductions in life-history traits, whereas infected Q only showed marginal reductions. While Q performed better on TYLCV-infected tomato plants than on uninfected ones, the reverse was observed in B. Q displacement by B took one more generation on TYLCV-infected tomato plants than on healthy ones. These results demonstrate that TYLCV was indirectly mutualistic to Q but directly and indirectly parasitic to B.
With rapid economic development and increased international trading, bioinvasion has become an increasingly serious, worldwide problem. By bringing together previously disjunct taxa, bioinvasions have the potential to profoundly alter both natural and managed ecosystems1,2. Understanding the factors that influence the invasion ability of an exotic species is important for predicting future invasions and for managing current ones3. While a number of factors have been extensively investigated, how an exotic virus affects the performance of two closely-related invasive vectors of that virus has not been investigated. Here we chose the Begomovirus vector, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), to address this general question.
Bemisia tabaci, commonly known as the sweet potato whitefly, has been regarded as a species complex consisting of many putative species that are morphologically indistinguishable but differ in virus transmission, feeding behavior, insecticide resistance, host range, or the symbionts they harbor4,5,6,7,8,9. The two most invasive groups of B. tabaci are the Middle East – Asia Minor I (referred to B hereafter) and the Mediterranean (referred to Q hereafter) species. During the past two decades, B. tabaci B and Q have invaded nearly 60 countries and caused massive agricultural losses10,11.
In many regions of the world, invasion by B and/or Q has been followed by outbreaks of whitefly-transmitted viruses12,13,14,15. Tomato yellow leaf curl virus (TYLCV, genus Begomovirus, family Geminiviradae), which is transmitted by B. tabaci in a persistent and circulative manner, has devastated tomato plantings worldwide16. In China, TYLCV was first isolated from symptomatic tomato plants in 2006 in Shanghai17. Since then, it has spread to Zhejiang, Jiangsu, Hebei, Beijing, and Shandong, where it has caused great damage and economic loss5. TYLCV was not detected until Q became established, even though B is an important vector of TYLCV elsewhere and has been in China since the mid-1990's5,18.
Plant-mediated interactions between pathogenic pathogens and herbivorous arthropods are potentially important determinants of population dynamics of pathogens and arthropods in both managed and natural ecosystems19. Insects and the plant viruses they vector have complex interactions that can be direct, indirect, or both, and the ecological effects of the interactions are highly changeful depending on the species19,20. For example, viral infection of a host plant has been shown to exert positive, neutral, or negative effects on the performance of the vector19,20. Several studies indicated direct and/or indirect influence of begomoviruses on its vector B. tabaci, and the effects exerted on the whitefly vectors can be detrimental, neutral or beneficial, depending on the virus-whitefly-plant combinations21,22,23,24,25,26,27. Therefore, knowledge of the variational responses to virus-infection of host plants between B. tabaci B and Q is essential to understanding the epidemics of Begomovirus diseases in regions under invasion.
The concurrence of the spread of TYLCV with invasion of Q rather than B suggests a more mutualistic relationship between TYLCV and Q than B. We have recently demonstrated that Q is a better vector of TYLCV than B5 because Q acquired significantly more viral DNA per unit of time than the B, and reached the maximum viral load in a substantially shorter period of time. Although TYLCV was shown to be transmitted horizontally by both whiteflies, Q exhibited significantly higher viral transmission frequency than B5. In the present study, we conducted laboratory experiments to quantify how the two invasive species of B. tabaci, B and Q, are affected by TYLCV. The results indicate that TYLCV infection is more compatible with and beneficial to Q than B.
Results
Direct impacts of TYLCV on B and Q whiteflies
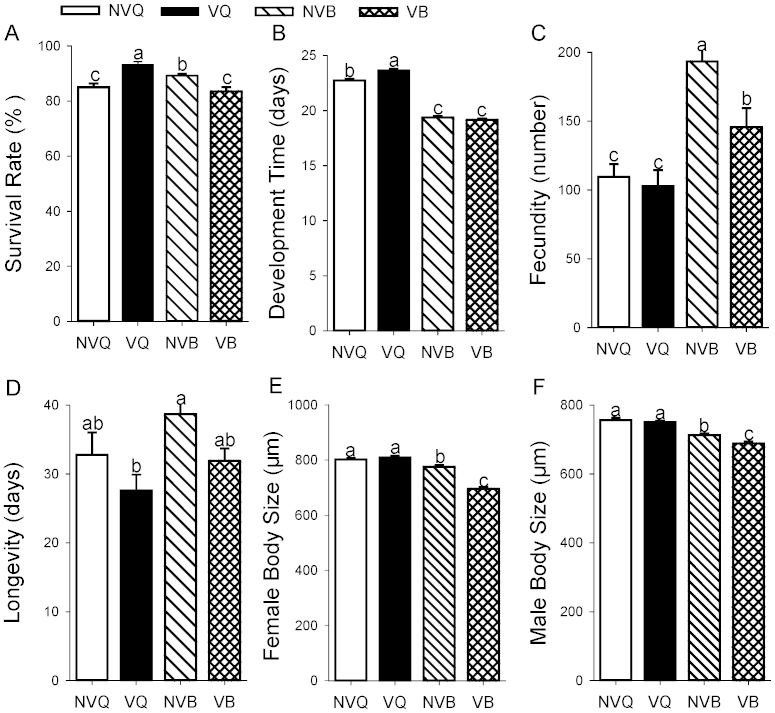
We first compared the life-history traits of the infected and non-infected whiteflies of each species on cotton, a non-host for TYLCV. Egg to adult survival was significantly affected by whitefly species (F1, 36 = 5.6, P = 0.023) and virus infection (F1, 36 = 27.0, P < 0.0001), but the species × infection status interaction was not significant (F1, 36 = 2.0, P = 0.16). Infected Q had significantly higher survival than non-infected Q (Figure 1A). In contrast, infected B had significantly lower survival than non-infected B (Figure 1A).
Figure 1. Life history traits as affected by whitefly species and whitefly virus status on cotton.
NVQ: non-infected Q; VQ: infected Q; NVB: non-infected B; VB: infected B. (A) Survival. (B) Development time. (C) Fecundity. (D) Longevity. (E) Female body size. (F) Male body size. Values are means ± SE. Within each panel, different letters indicate significant differences between treatments (P < 0.05).
Egg to adult development time was significantly affected by whitefly species (F1, 1536 = 1118.3, P < 0.0001), virus infection (F1, 1536 = 8.3, P < 0.01), and the interaction between the two factors (F1, 1536 = 22.7, P < 0.0001). Infected and non-infected Q had a longer development time than their B counterparts (Figure 1B). Infected Q had a significantly longer development time than non-infected Q, whereas development time did not differ between infected and non-infected B (Figure 1B).
Fecundity was significantly affected by whitefly species (F1, 90 = 27.8, P < 0.0001) and virus infection (F1, 90 = 5.1, P = 0.026), but the interaction between these factors was not significant (F1, 90 = 2.9, P = 0.090). Both non-infected and infected B laid markedly more eggs than their Q counterparts (Figure 1C). Non-infected B laid markedly more eggs than infected B, whereas the number of eggs laid per female did not differ between infected and non-infected Q (Figure 1C).
Female longevity was affected by virus infection (F1, 90 = 5.4, P = 0.023), but not by whitefly species (F1, 90 = 1.7, P = 0.193) and the interaction between the two factors (F1, 90 = 0.1, P = 0.789). Overall longevity was shorter in infected than non-infected whiteflies (Figure 1D). Longevity was similar in non-infected B and Q and in infected B and Q.
Female body size was significantly affected by whitefly species (F1, 160 = 132.7, P < 0.0001), virus infection (F1, 160 = 35.7, P < 0.0001), and the interaction between the two factors (F1, 160 = 50.8, P < 0.0001). Male body size was significantly affected by whitefly species (F1, 160 = 93.3, P < 0.0001) and virus infection (F1, 160 = 7.3, P < 0.01), but not by the interaction between the two factors (F1, 160 = 2.8, P = 0.096). Both females and males were significantly larger in Q than B. Female or male body size did not differ between infected and non-infected Q, whereas infected B were smaller than non-infected B in both females and males (Figure 1E, 1F).
Indirect impacts of TYLCV on B and Q whiteflies
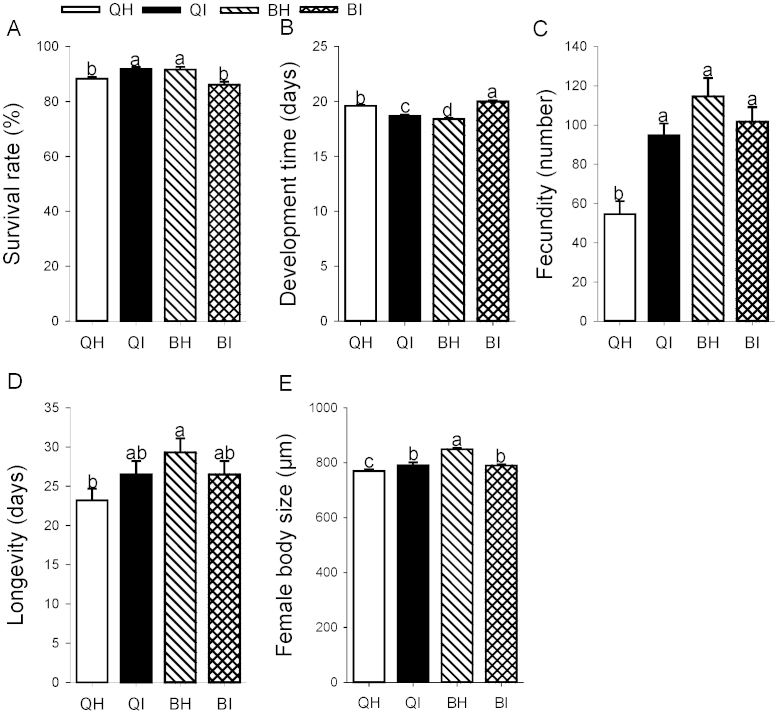
We also compared the life-history traits of non-infected whiteflies of each species fed on TYLCV-infected or non-infected tomato plants. Both whitefly species (F1, 36 = 0.3, P = 0. 570) and virus infection of the plant (F1, 36 = 1.0, P = 0.314) did not significantly affect egg to adult survival, although their interaction was significant (F1, 36 = 17.0, P < 0.0001). Survival of Q was significantly higher on TYLCV-infected tomato plants than on healthy ones, whereas survival of B was significantly lower on TYLCV-infected than on healthy tomato plants (Figure 2A).
Figure 2. Life history traits as affected by whitefly species and virus status of tomato plants.
QH: Q on healthy tomato; QI: Q on TYLCV-infected tomato; BH: B on healthy tomato; BI: B on TYLCV-infected tomato. (A) Survival rate. (B) Development time. (C) Fecundity. (D) Longevity. (E) Female body size. Values are means ± SE. Within each panel, different letters indicate significant differences between treatments (P < 0.05).
Although egg to adult development time was not affected by whitefly species (F1, 1014 = 0.1, P = 0.808), it was significantly affected by virus infection status of the plant (F1, 1014 = 17.8, P < 0.0001) and the interaction between them (F1, 1014 = 193.3, P < 0.0001). Q's development time was significantly shorter on TYLCV-infected tomato plants than on healthy ones (Figure 2B), whereas B's development time was significantly longer on TYLCV-infected than healthy tomato plants (Figure 2B).
Female fecundity was not significantly affected by virus infection of the plant (F1, 67 = 3.4, P = 0.07), although whitefly species (F1, 67 = 20.5, P < 0.0001) and the interaction between the two factors did significantly affect fecundity (F1, 67 = 12.9, P = 0.001). Fecundity did not differ between B reared on healthy and TYLCV-infected tomato plants, but was significantly greater for Q developing on infected than healthy plants (Figure 2C).
Female longevity was not affected by whitefly species (F1, 89 = 3.5, P = 0.065), virus infection of the plant (F1, 89 = 0.0, P = 0.845), or the interaction between these factors (F1, 89 = 3.3, P = 0.072). For both B and Q, longevity was similar on TYLCV-infected and healthy tomato plants (Figure 2D).
Female body size was significantly affected by whitefly species (F1, 135 = 36.1, P < 0.0001), virus infection of the plant (F1, 135 = 8.6, P < 0.01), and the interaction between these factors (F1, 135 = 37.9, P < 0.0001). Q females were significantly larger on TYLCV-infected than healthy tomato plants. In contrast, B females were significantly smaller on TYLCV-infected than healthy tomato plants (Figure 2E).
Impacts of TYLCV on B/Q competition
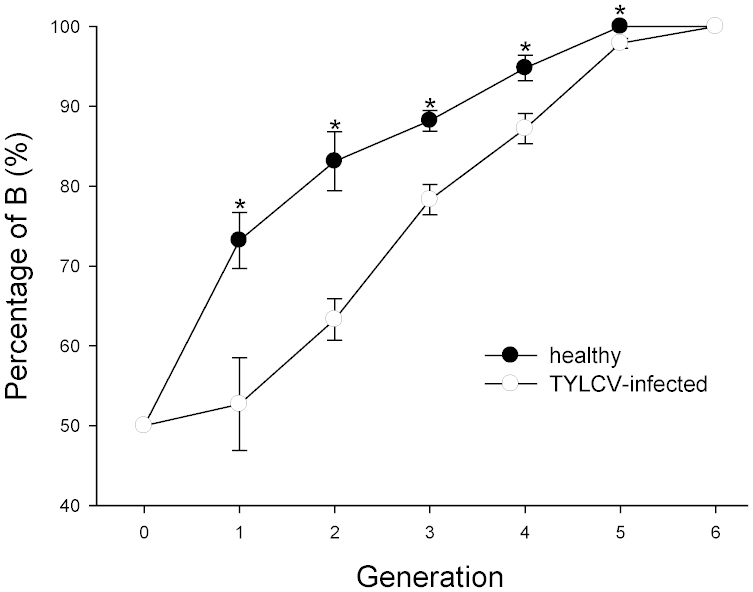
When B and Q were reared together, the percentage of B increased consistently over six generations on both healthy and TYLCV-infected tomato plants (Figure 3). Although B gradually displaced Q on both kinds of plants, TYLCV infection significantly slowed the rate of displacement (F1, 4 = 29.9, P = 0.005) (Figure 3). B completely displaced Q after five generations on healthy tomato and six generations on TYLCV-infected tomato. In addition, the percentage of B was higher on healthy than TYLCV-infected tomato from the 1st to the 5th generation (Figure 3).
Figure 3. Percentage of B on TYLCV-infected and healthy tomato plants.
TYLCV-infected and healthy tomato plants were infested with a mixture of B and Q at the start of the experiment (generation 0). The percentage of Q is equivalent to 100 minus the percentage of B. An asterisk indicates a significant difference between healthy and virus-infected tomato plants for that generation (P < 0.05).
Discussion
How an exotic virus affects the invasion and competition of two closely-related invasive vectors remains laregely unexplored. The triangular relationship among TYLCV and the B and Q species of B. tabaci provides an ideal system to address this question. The virus-vector relationship can range from parasitism to mutuallism, depending on whether impacts of a virus on its vector are deleterious, neutral, or beneficial. The concurrent outbreak and spread of TYLCV with invasion of Q rather than B in China suggests that the exotic TYLCV is mutualistic to Q but parasitic, neutral, or less mutualistic to B.
Several lines of evidence support this hypothesis. First and foremost, TYLCV infection of a host plant (tomato) indirectly improved the overall performance (higher survival, greater fecundity, shorter development time) of Q, whereas it directly and indirectly reduced the overall performance of B (Figure 1 and 2). Second, the global percentage of individuals carrying the TYLCV virus in fields was 5.8 times higher in Q than B (Pan et al. 2012)5. Third, data from Pan et al. (2012)5 demonstrate that Q is a better vector of TYLCV than B. Fourth, TYLCV infection of a host plant (tomato) delayed the displacement of Q by B by 1 generation in our laboratory competition experiments (Figure 3).
To further dissect this triangular relationship, we examined the direct and indirect impacts of TYLCV on life history traits of B and Q whiteflies. Previous studies indicated that the direct effects of a virus on its vectors can be deleterious, neutral, or beneficial depending on the specific combination of virus and vectors19,20,21,22,23,24,25,26,27. The data presented here show that TYLCV infection per se had limited impacts on the performance of Q on cotton (Figure 1), suggesting that TYLCV has largely neutral effects on Q. In contrast, TYLCV infection significantly decreased the survival, fecundity, longevity, and adult body size of B on cotton (Figure 1), indicating a parasitic relationship between TYLCV and B. Liu et al. (2010)22 and Li et al. (2011)21 showed that TYLCV did not affect the fecundity and longevity of Q individuals on cotton, which is consistent with our results. These results indicate that the direct negative effects of TYLCV on B are greater than on Q.
Other work has shown that the Tomato yellow leaf curl China virus (TYLCCNV) also reduces female longevity and fecundity of B and is thus directly parasitic to this species28. The direct negative impacts of TYLCCNV are due to TYLCCNV's suppression of the immune responses of B by down-regulation of genes in the Toll-like signaling and mitogen-activated protein kinase (MAPK) pathways28. Further research is needed to investigate whether the different direct effects of TYLCV on B and Q are related to dissimilar responses of their Toll-like signaling and MAPK pathway genes upon TYLCV infection.
The fact that Q whiteflies feeding on TYLCV-infected tomato plants had greater survival, fecundity, body size and longevity, and shorter egg to adult development time than those feeding on healthy plants (Figure 2) demonstrates the occurrence of an indirect mutualism between Q and TYLCV via their host plants. On the other hand, reduced performance of B on TYLCV-infected than on healthy tomato plants (Figure 2) indicates that TYLCV is indirectly parasitic to B. Apparently, TYLCV infection alters tomato plants and the resulting changes are beneficial to Q but detrimental to B.
Our results are consistent with findings by Rubinstein and Czosnek (1997)26, who reported that long-term association of TYLCV with females of the B species reduced lifespan and fertility compared to non-infected individuals. However, our results on the indirect effects of TYLCV on B and Q differ from other studies24,25. Matsuura and Hoshino (2009)25 found that symptomatic TYLCV infection of susceptible tomato plants has little effect on the reproduction of Q. Li et al. (2011)24 reported that TYLCV-infected tomato plants only marginally affected performance on the part of fecundity, survival rate, longevity, development time, and population increase of Q species. Jiu et al. (2007)21 and Liu et al. (2009)22 respectively showed that the performance with regard to fecundity, survival rate, longevity, development time of B feeding on TYLCV-infected tomato increased or was not significantly reduced. Differences in tomato cultivars, TYLCV isolates, and B and Q haplotypes could account for these discrepancies.
Vector-borne pathogens and parasites can induce changes in the phenotypes of their hosts that influence the frequency and nature of host–vector interactions, as documented by both empirical and theoretical studies29. In plant systems, such effects are largely restricted to vectors, because they are mobile and may exhibit preferences dependent upon plant host infection status30. Through these studies, we know that the transmission of persistently trans-mitted (PT) viruses would tend to enhance vector attraction to infected hosts, probably by improving host quality for vectors and by promoting long-term feeding29. In our latest study, we conducted two experiments testing the impact of TYLCV (one PT virus) infection of the host plant (tomato) and vector (B. tabaci B and Q) on whitefly feeding behavior using an Electrical Penetration Graph (EPG). When non-infected whiteflies fed on healthy and TYLCV-infected plants, respectively, B. tabaci Q engaged in more phloem salivation and phloem sap ingestion on both infected and healthy tomato plants than B. When non- infected and infected whiteflies, respectively, infested healthy plants, B. tabaci Q fed more readily than B on tomatoes, regardless of the infection status of whitefly31. The results of this feeding behavior study are consistent with the differential direct and indirect impacts of TYLCV on the performance of B. tabaci B and Q reported here.
Contrary to our expectations, the beneficial indirect effects of TYLCV on Q and the detrimental direct and indirect effects of TYLCV on B did not reverse the outcome of competition between B and Q, but rather only slowed displacement of Q by B in our laboratory experiments (Figure 3). One simple explanation is that the differential effects of TYLCV on B and Q were not great enough to override the asymmetric mating interference of Q by B32,33,34. This suggests that the triangular relationship between TYLCV, B and Q only contributes partially to the dominance of Q over B in the field in China. The competitive displacement between different species are mediated by many biotic and abiotic factors which are likely to vary from region to region35. Given that Q is usually more resistant to insecticides than is B36,37,38 and use of insecticides can shift the B/Q competition dynamics in favor of Q39, heavy insecticide use in China40,41 is probably a more important factor that helps Q to outcompete or at least coexist with B in China's open field systems.
Methods
Plant cultures, TYLCV agroinoculation, and whitefly laboratory populations
Tomato (Solanum lycopersicum Mill. cv. Zhongza 9) and cotton (Gossypium herbaceum L. cv. DP99BB) have been widely cultivated in China and thus were used as a host and a non-host plant of TYLCV, respectively, in our laboratory experiments. Zhongza 9 was breed by the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences. In 2000, it was acquired the National Science and Technology Progress Award. Zhongza 9 is suitable for both field and greenhouse conditions. In the late 1990s, it started as the main pink fruit tomato cultivars, and it is planted in every provinces of China, its cultivation area accounted for more than 40% of the area of the pink fruit tomato cultivation (Xiaoxuan Wang, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, personal communication). We cultivated healthy tomato and cotton in a potting mix in 1.5-L pots (one plant/pot) under natural light and controlled temperature (26 ± 2°C) in a glasshouse. When these plants grew to the 6–7 true-leaf stage, they were used in the experiments42.
TYLCV-infected tomato plants were obtained by agro-inoculation using a cloned TYLCV genome (GenBank accession number: AM282874) that was originally isolated from tomato plants in Shanghai, China17. Tomato plants were inoculated at the 3rd true-leaf stage. Tomato plants were assumed to be infected with TYLCV when they developed characteristic leaf-curl symptoms42.
The B laboratory population used in this study has been reared on cabbage (Brassica oleracea L. cv. Jingfeng 1) since its collection in Beijing, China, in 20045. The Q laboratory population has been reared on poinsettia (Euphorbia pulcherrima Wild. ex. Klotz.) since it was collected in Beijing, China, in 20095. Before specimens of B or Q were used in experiments, their source populations had been maintained on healthy and TYLCV-infected tomato plants, respectively, in separate whitefly-proof screen cages in a glasshouse under natural light and controlled temperature (26 ± 2°C) for six generations.
Life-history parameters of non-viruliferous and viruliferous whiteflies on cotton
The direct effects of TYLCV on the life history parameters or traits of B or Q whiteflies were examined by clip-caging B or Q whiteflies from non-infected or TYLCV-infected tomato plants on cotton, a non-host for TYLCV. Thus, there were four whitefly treatments (non-infected B, non-infected Q, infected B, and infected Q) for each parameter. To measure development time, survival, female and male body sizes, 10 replicates of 10 pairs each of newly emerged infected or non-infected B or Q adults were clip-caged to oviposit on cotton plants for 24 h (one clip-cage per cotton plant). The adults and the clip-cages were then removed. The eggs deposited on cotton leaves were counted and marked with a marker pen under a stereo microscope (Leica, M205C). From the 16th day onwards, adults emerged from each replicate plant were collected and recorded twice daily (at 9:00 and 15:00) until all the pupae had developed to adults. The length of each adult was measured with a stereo microscope (Leica, M205C) after the adult had been stored at −20°C for 15 min. The data recorded were used to calculate egg to adult development time, survival and adult body size.
To measure female longevity and fecundity, 30 replicates of 1 female each of newly emerged infected or non-infected B or Q adults were clip-caged to oviposit on cotton plants until their death. These virgin adults were newly emerged from the pupae that were individually excised from plant leaves and placed into individual test tubes (3 × 0.5 cm, 1 pupa per tube)43. Each cotton plant had 4 clip-cages containing the same type of whitefly. The 4 clip cages were attached to the third to sixth leaf of each plant from the top. The eggs laid by each female were counted once per week and the females were then clip-caged on a new cotton plant. Every female was checked daily until its death to calculate its longevity.
Life-history parameters of whiteflies on healthy and TYLCV-infected tomato
The indirect (through host plant) impacts of TYLCV on the life history parameters of B or Q whiteflies were examined by clip-caging newly emerged non-infected B or Q whiteflies from healthy tomato plants on healthy or TYLCV-infected tomato plants. The experiments for each parameter were set up and conducted in the similar manner as described in the previous section. The only difference was that the total number of eggs laid in the first 14 days by each female was considered as its fecundity.
Competition between B and Q on healthy and TYLCV-infected tomato plants
To measure the effect of TYLCV on the competition between B and Q, we quantified the proportional change in numbers of B and Q on the same healthy and TYLCV-infected tomato plants. In one treatment, healthy tomato plants were inoculated with a mixture of B and Q (20 pairs of newly-emerged B and 20 pairs of newly-emerged Q; the whiteflies were non-infected when added to the plants). There were three replicates, and each replicate included two healthy plants that were kept in the same whitefly-proof, ventilated cage (60 cm × 40 cm × 80 cm). The second treatment was identical to the first except that TYLCV-infected tomato plants were used.
Whiteflies were sampled and identified to species by PCR at two times for each of six successive generations. The first sample was taken 2 days after progeny began emerging in a generation, and the second was taken 3 days later. One-hundred whiteflies per replicate cage were randomly selected and identified to species (B or Q) for each sampling within each generation. To avoid overcrowding of the populations in each replicate cage, the following was done immediately after the first sampling of each generation: one plant (along with all whiteflies on it) of the two in each cage was removed and replaced with a new plant that was not infested with whiteflies but was infected or not infected by TYLCV as indicated by the treatment.
Statistical analysis
Two-way ANOVAs were used to compare the life history parameters of non-infected and infected B and Q on cotton and of B and Q on the healthy and TYLCV-infected tomato. A repeated measure ANOVA was used to compare the percentage of B on the healthy and TYLCV-infected tomato across generations. Means were compared with Tukey tests at P < 0.05. Proportional data were arcsine square root transformed before analyses. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Author Contributions
Y.J.Z., X.C.L., H.P.P. conceived and designed the experiments. H.P.P., B.M.L., X.B.S., L.T.G., W.X. performed the experiments. H.P.P., X.C.L., Y.J.Z., Y.C. analyzed the data. H.P.P., X.C.L., D.C., Y.J.Z., Y.C. wrote the paper. All authors read and approved the final manuscript.
Acknowledgments
This research was supported by the Agricultural Science and Technology Innovation Program (ASTIP), the National Science Fund for Distinguished Young Scholars (31025020), the 973 Program (2013CB127602), and the Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables. The granting agencies have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Lounibos L. P. Invasions by insect vectors of human disease. Ann. Rev. Entomol. 47, 233–266 (2002). [DOI] [PubMed] [Google Scholar]
- Juliano S. A. & Lounibos L. P. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 8, 558–574 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder D. W. et al. Niche partitioning and stochastic processes shape community structure following whitefly invasions. Basic Appl. Ecol. 12, 685–694 (2011). [Google Scholar]
- Brown J. K., Frohlich D. R. & Rosell R. C. The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 40, 511–534 (1995). [Google Scholar]
- Pan H. P. et al. Rapid spread of Tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS ONE 7, e34817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H. P. et al. Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci. PLoS ONE 7, e30760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perring T. M. The Bemisia tabaci species complex. Crop Prot. 20, 725–737 (2001). [Google Scholar]
- Liu B. M. et al. Difference in feeding behaviors of two invasive whiteflies on host plants with different suitability: implication for competitive displacement. Int. J. Biol. Sci. 8, 697–706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 109, 195–219 (2003). [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M. & Dinsdale A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011). [DOI] [PubMed] [Google Scholar]
- Dinsdale A., Cook L., Riginos C., Buckley Y. M. & De Barro P. J. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase I to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 103, 196–208 (2010). [Google Scholar]
- Varma A. & Malathi V. G. Emerging geminivirus problems: a serious threat to crop production. Ann. Appl. Biol. 142, 145–164 (2003). [Google Scholar]
- Seal S. E., VandenBosch F. & Jeger M. J. Factors influencing begomovirus evolution and their increasing global significance: implications for sustainable control. Crit. Rev. Plant Sci. 25, 23–46 (2006). [Google Scholar]
- Brown J. K. in Bionomics and Management of a Global Pest (eds Stansly P. A., & Naranjo S. E.) 31–67 (Springer Ltd., 2010). [Google Scholar]
- Navas-Castillo J., Fiallo-Olivé E. & Sánchez-Campos S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Physiol. 49, 219–248 (2011). [DOI] [PubMed] [Google Scholar]
- Brown J. K. & Czosnek H. Whitefly transmission of plant viruses. Adv. Bot. Res. 36, 65–76 (2002). [Google Scholar]
- Wu J. B., Dai F. M. & Zhou X. P. First report of Tomato yellow leaf curl virus in China. Ann. Appl. Biol. 155, 439–448 (2006). [DOI] [PubMed] [Google Scholar]
- Pan H. P. et al. Further spread of and domination by Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q on field crops in China. J. Econ. Entomol. 104, 978–985 (2011). [DOI] [PubMed] [Google Scholar]
- Stout M. J., Thaler J. S. & Thomma B. P. H. J. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Ann. Rev. Entomol. 51, 663–689 (2006). [DOI] [PubMed] [Google Scholar]
- Belliure B., Janssen A., Maris P. C., Peters D. & Sabelis M. W. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70–79 (2005). [Google Scholar]
- Jiu M. et al. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2, e182 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhao H., Jiang K., Zhou X. P. & Liu S. S. Differential indirect effects of two plant viruses on an invasive and an indigenous whitefly vector: implications for competitive displacement. Ann. Appl. Biol. 155, 439–448 (2009). [Google Scholar]
- Liu J. et al. Viral infection of tobacco plants improves the performance of Bemisia tabaci but more so for an invasive than for an indigenous biotype of the whitefly. J. Zhejiang Univ.-Sci. B 11, 30–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Liu J. & Liu S. S. Tomato yellow leaf curl virus infection of tomato does not affect the performance of the Q and ZHJ2 biotypes of the viral vector Bemisia tabaci. Insect Sci. 18, 40–49 (2011). [Google Scholar]
- Matsuura S. & Hoshino S. Effect of tomato yellow leaf curl disease on reproduction of Bemisia tabaci Q biotype (Hemiptera: Aleyrodidae) on tomato plants. Appl. Entomol. Zool. 44, 143–148 (2009). [Google Scholar]
- Rubinstein G. & Czosnke H. Long-term association of tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: effect on the insect transmission capacity, longevity and fecundity. J. Gen. Virol. 78, 2683–2689 (1997). [DOI] [PubMed] [Google Scholar]
- McKenzie C. L. Effect of tomato mottle virus (ToMoV) on Bemisia tabaci biotype B (Homoptera: Aleyrodidae) oviposition and adult survivorship on healthy tomato. Fla. Entomol. 85, 367–368 (2002). [Google Scholar]
- Luan J. B. et al. Global analysis of the transcriptional response of whitefly to Tomato yellow leaf curl China virus reveals their relationship of coevolved adaptations. J. Virol. 85, 3330–3340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck K., Bosque-Pérez N., Eigenbrode S. D., De Moraes C. & Mescher M. Transmission mechanisms shape pathogen effects on host-vector interactions: evidence from plant viruses. Funct. Ecol. 26, 1162–1175 (2012). [Google Scholar]
- Ingwell L. L., Eigenbrode S. D. & Bosque-Perez N. A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2, 578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. M. et al. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and tomato yellow curl leaf virus. J.Virol. 10.1128/JVI.03571-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsueda H. & Tsuchida K. Reproductive differences between Q and B whiteflies, Bemisia tabaci, on three host plants and negative interactions in mixed cohorts. Entomol. Exp. Appl. 141, 197–207 (2011). [Google Scholar]
- Crowder D. W. et al. Mating behavior, life-history, and adaptation to insecticides determine species exclusion between whiteflies. J. Anim. Ecol. 79, 563–570 (2010). [DOI] [PubMed] [Google Scholar]
- Crowder D. W., Sitvarin M. I. & Carrière Y. Plasticity in mating behavior drives competitive displacement of whitefly biotypes. Anim. Behav. 79, 579–587 (2010). [Google Scholar]
- Reitz S. R. & Trumble J. T. Competitive displacement among insects and arachnids. Annu. Rev. Entomol. 47, 435–465 (2002). [DOI] [PubMed] [Google Scholar]
- Dennehy T. J. et al. Extraordinary resistance to insecticides reveals exotic Q biotype of Bemisia tabaci (Gennadius) in the New World. J. Econ. Entomol. 103, 2174–2186 (2010). [DOI] [PubMed] [Google Scholar]
- Horowitz A. R., Kontsedalov S., Khasdan V. & Ishaaya I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect. Biochem. Physiol. 58, 216–225 (2005). [DOI] [PubMed] [Google Scholar]
- Kontsedalov S. et al. Bemisia tabaci biotype dynamics and resistance to insecticides in Israel during the years 2008–2010. J. Integr. Agr. 11, 312–320 (2012). [Google Scholar]
- Sun D. B. et al. Competitive displacement between two invasive whiteflies: insecticide application and host plant effects. B. Entomol. Res. 103, 344–353 (2013). [DOI] [PubMed] [Google Scholar]
- Luo C. et al. Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 29, 429–434 (2010). [Google Scholar]
- Lu Y. H. et al. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328, 1151–1154 (2010). [DOI] [PubMed] [Google Scholar]
- Su Q. et al. Insect symbiont facilitates vector acquisition, retention, and transmission of plant virus. Sci. Rep. 3, 1367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J. B., Ruan Y. M., Zhang L. & Liu S. S. Pre-copulation intervals, copulation frequencies, and initial progeny sex ratios in two biotypes of whitefly, Bemisia tabaci. Entomol. Exp. Appl. 129, 316–324 (2008). [Google Scholar]