Abstract
OBJECTIVE:
Previous studies have suggested that marginal periodontitis is a risk factor for developing atherosclerosis. The objective of this study was to determine whether caries may also be associated with atherosclerosis.
METHODS:
The computed tomography data sets of 292 consecutive patients, 137 women and 155 men with a mean age of 54.1±17.3 years, were analyzed. Caries were quantified based on the number of decayed surfaces of all the teeth, and periodontitis was quantified on the basis of the horizontal bone loss in the jaw. The presence of chronic apical periodontitis (CAP) was assessed, and the aortic atherosclerotic burden was quantified using a calcium scoring method.
RESULTS:
The patients with <1 caries surfaces/tooth had a lower atherosclerotic burden (0.13±0.61 mL) than patients with ≥1 caries surfaces/tooth. The atherosclerotic burden was greater in patients with a higher number of lesions with pulpal involvement and more teeth with chronic apical periodontitis. In the logistical regression models, age (Wald 49.3), number of caries per tooth (Wald 26.4), periodontitis (Wald 8.6), and male gender (Wald 11) were found to be independent risk factors for atherosclerosis. In the linear regression analyses, age and the number of decayed surfaces per tooth were identified as influencing factors associated with a higher atherosclerotic burden, and the number of restorations per tooth was associated with a lower atherosclerotic burden.
CONCLUSION:
Dental caries, pulpal caries, and chronic apical periodontitis are associated positively, while restorations are associated inversely, with aortic atherosclerotic burden. Prospective studies are required to confirm these observations and answer the question of possible causality.
Keywords: Dental Caries, Atherosclerosis, Risk Factors, Dental Restoration, Computed Tomography, Cardiovascular Diseases
INTRODUCTION
Cardiovascular events attributed to atherosclerosis, such as heart attack and stroke, are the leading causes of death in the Western world (1). In addition to the classical risk factors, such as arterial hypertension, hypertriglyceridemia (2), diabetes mellitus, and smoking (3), periodontitis has been found to be a proatherogenic factor independent of the other risk factors (4,5). According to data from the National Health and Nutrition Examination Survey (NHANES III), periodontitis patients have a four-fold increased risk of suffering a heart attack (6).
Atherosclerosis and marginal periodontitis are chronic inflammatory diseases contributing to the biological plausibility of an association between them (7). The treatment of marginal periodontitis has been found to be significant for the primary prevention of cardiovascular events (8) because it can limit the extent of inflammation, reduce endothelial dysfunction, and normalize the intima-media thickness of the carotid arteries (9).
Streptococcus mutans, a significant contributor to caries, can be found in the atheromatous plaques of the vascular wall (10). It can be presumed that dental caries may also have a proatherogenic effect. Minimal carious lesions, caries with and without involvement of the pulpal cavity, and chronic apical periodontitis represent different stages of the same inflammatory process. The significance of this spectrum of inflammatory diseases with respect to their possible association with atherosclerosis is not clear, and previous studies have been unable to demonstrate a correlation between atherosclerosis and caries (11,12).
The objectives of this study were to test whether caries are associated with an increased aortic atherosclerotic burden, whether pulpal caries and chronic apical periodontitis (CAP) are associated with an increased aortic atherosclerotic burden, and whether restorative measures may be associated with a lower aortic atherosclerotic burden.
MATERIALS AND METHODS
Study design
This cross-sectional study used computed tomography (CT) data sets retrospectively and had no effect on the treatment of the patients involved. The study complied with the recommendations for conducting medical studies of the World Medical Association.
Patient population
The CT scans of 292 consecutive patients between August 2008 and June 2010 were analyzed. The data sets were from 137 women and 155 men with a mean age of 54.1±17.3 years. All the examinations included the viscerocranium, neck, and trunk. The indications for the examinations were tumor staging for 129 patients, staging of multiple myeloma for 52 patients, morphological evaluation of presumed arthritic degeneration for 68 patients, and trauma for 43 patients. The patients came from the Departments of Internal Medicine, Surgery, and Traumatology. None of the patients were referred from the School of Dentistry.
Equipment
A total of 249 examinations were conducted using a 16-row spiral CT scanner, and 43 examinations were conducted using a 64-row spiral CT scanner (GE LightSpeed and VCTXT; General Electric, Milwaukee, WI, USA). The secondary reconstructions of the images were created on a 3D image processing workstation (AW 4.4, General Electric). For the image analysis, a dedicated workstation with a high-resolution monitor (IBM 6659-HG2, IBM, Taoyuan, Taiwan) equipped with a 3D PACS (Picture Acquisition and Communication System) (Agfa IMPAX EE R20 VI, AGFA HealthCare, Mortsel, Belgium) was used.
CT protocols and image processing
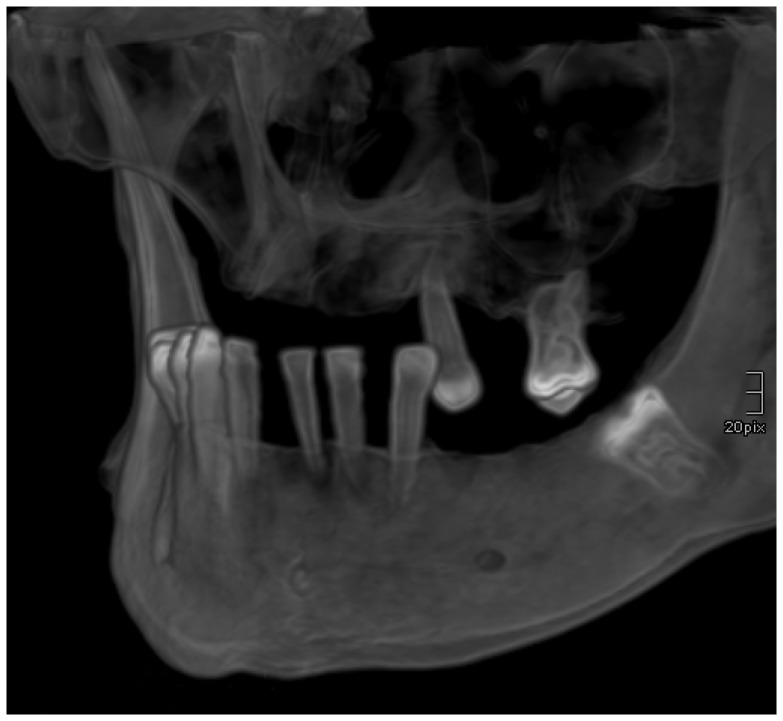
The CT protocols were low-dose protocols designed for bone imaging (N = 120) or high-dose protocols (N = 172) of the entire body designed for detection of the consequences of accidents. The primary scan field of view was between 40 and 47 cm in diameter for the trunk and between 19 and 27 cm in diameter for the viscerocranium. With an image matrix of 512×512 pixels, the primary data associated with a slice thickness of 0.6 mm reconstructed in a standard kernel were available for all the regions, and axial reconstructions were available in a display field of view with a diameter between 12 and 15 cm for the viscerocranium. The resolution was between 0.25 and 0.35 mm in the x and y axis and between 0.3 and 0.6 mm in the z axis. The coronal and sagittal reconstructions of the jaws were performed with a slice thickness and interval of 0.5 mm each, as well as with semitransparent and opaque volume rendering reformatting (Figure 1). Using what are known as curved, thick-slice maximum-intensity projections, zonographies of the jawbones were reconstructed. Each tooth was individually reformatted.
Figure 1.
A 3D volume rendering reconstruction of the jaw in a left anterior oblique projection. There is severe periodontitis causing bone loss and loss of teeth, but no caries is present.
Calculation of aortic calcification
The aortic calcification was quantified without knowledge of the jaw or dental status using a volumetric calcium scoring method (13). For this process, a defined segment of the abdominal aorta from the celiac trunk to the bifurcation was used. First, the segments located caudal to the trunk and cranial to the bifurcation were sent to the image processing workstation. In the next step, all the structures with a density less than 160 Hounsfield units were electronically excluded. Then, in a 3D image, the aorta was manually separated with a scissors tool from the spinal column, ribs, and all the calcified structures before introducing a lower density threshold of 600 Hounsfield units to exclude all the non-calcified structures in the data set. The volume of the remaining calcified plaques of the aortic wall was automatically measured, and the data were presented in mL. This method was developed to quantify the calcified plaques in human coronary arteries in CT scans of the thorax which were not specifically intended for the purpose of quantification of calcified plaques. It was shown that this method could replace the Agatston method (14) in this respect, while the Agatston method cannot be used to analyze volume data sets (13). The quantification of the atherosclerotic burden correlates well with the classic atherosclerosis scores, such as the Friesinger score (15), as was shown for the superficial femoral artery using a series of CT angiographies of the thigh (N = 60; Spearman's rho = 0.7580; CI 0.6195 to 0.8507; p<0.0001, unpublished data).
Evaluation of jawbone and dental status using CT images
The evaluation of dental status with respect to caries was based on 32 teeth (16). Impacted teeth (N = 10) were excluded from the analysis as they are not affected by caries and cannot have fillings. Based on the Decayed/Missing/Filled Surface Index (DMFS) (17), the number of decayed surfaces was categorized as 0, 1, 2, 3, or 4 on front teeth and as 0, 1, 2, 3, 4, or 5 on lateral teeth. The maximum possible value of all the surfaces of all the teeth that were decayed was thus 140. The individual value was then divided by the number of existing teeth. The number of decayed surfaces per tooth calculated by this method was analyzed as an independent parameter, not as part of an index. The caries that had verifiably reached the pulpal cavity were included as a separate parameter.
The DMFS M (missing) component was measured precisely and analyzed as a separate parameter. For the F (filling) component, the presence or absence of a filling was recorded. No point was given if a tooth had no restorations. If a restoration was present, 1 point was given, regardless of whether it was a filling or a crown or whether there were multiple fillings. This parameter was analyzed separately.
Apical radiolucency that was more than twice the width of the adjacent periodontal space on the crown side was considered to indicate chronic apical periodontitis (18). If a tooth with several roots had CAP at a minimum of one apex, it was considered to be affected by CAP.
Two parameters were used as surrogate parameters for periodontitis. To measure the horizontal bone loss across the width of the alveolar crest, the lowest bone height was determined (19) for each of the positions 16//17, 13//14, 11//21, 23//24, 26//27, 36//37, 33//34, 31//41, 43//44, and 46//47 and summed to obtain the total value. In a modification of a cone-beam volume tomography measurement technique (20), the distance between the lowest level of the alveolar crest and the nearest incisal point of the teeth was determined and totaled for each of the positions indicated. In the upper jaw, a perpendicular line was drawn from the selected incisal point to the palatal level. At the shortest projection of this perpendicular line onto the alveolar process of the maxilla, the segments between the incisal point, the lowest level of the alveolar crest, and the intersection with the palatal level were removed. In the lower jaw, the perpendicular line was drawn from the selected incisal point to a level determined by the inferior margin of the lower jaw. At the shortest projection of this perpendicular line onto the mandible, the segments between the incisal point, the lowest level of the alveolar crest, and the inferior margin of the mandible were removed.
Study procedure and other parameters measured
The jawbones were evaluated by two investigators who did not have knowledge of the atherosclerotic burden. Another investigator measured the aortic atherosclerotic burden. In addition to the parameters mentioned above, the patient's age and gender, indication for the CT scan, reasons for scanning, type of protocol (high-dose versus low-dose), existing implants, and existing teeth were recorded.
Statistical analyses
The descriptive statistics and simple algebraic calculations, as well as the minimum and maximum value analyses, were conducted using Microsoft Excel software (Microsoft, Seattle, WA, USA). The data were presented with graphs, and the simple statistical tests (distribution analyses, Mann-Whitney test, Kruskal-Wallis test, and Spearman's coefficient) were performed using the GraphPad Prism Software (GraphPad, La Jolla, CA, USA). The intraclass correlation coefficient (ICC) was used to assess the intra-rater reliability for the atherosclerosis measure (21). The qualitative data analysis was performed using the chi-square test. Because the majority of the quantitative data did not display a normal distribution in the d'Agostino and Pearson distribution analyses, the non-parametric Mann-Whitney test was used for the comparison of the two groups, and the Kruskal-Wallis test and Dunn's post-test were used for the comparison of several groups. The non-parametric Spearman's coefficient was used where appropriate for the correlation analyses. Irrespective of the previous hypotheses, these tests were two-tailed. Subsequently, a surrogate parameter was introduced that presented the quantitative data associated with the aortic atherosclerotic burden in qualitative terms: a value of 0 was assigned if no plaque was present, and a value of 1 was assigned if any amount of plaque was present. The logistical regression models were fitted to the target parameter “atherosclerosis present or not”, first by forcing the inclusion of all the data and then by implementing a forward step-wise selection procedure of the previously determined parameters. Parallel to this process, in the last step, the linear regression models were fitted to the target parameter “aortic atherosclerotic burden”. The subgroup analyses were conducted for the group of edentulous patients, as well as for the group of dentulous patients. A p<0.05 was considered significant.
ETHICS
Approval of the local ethics committee was obtained (AN4249; 297/4.16).
RESULTS
Distribution of caries and fillings
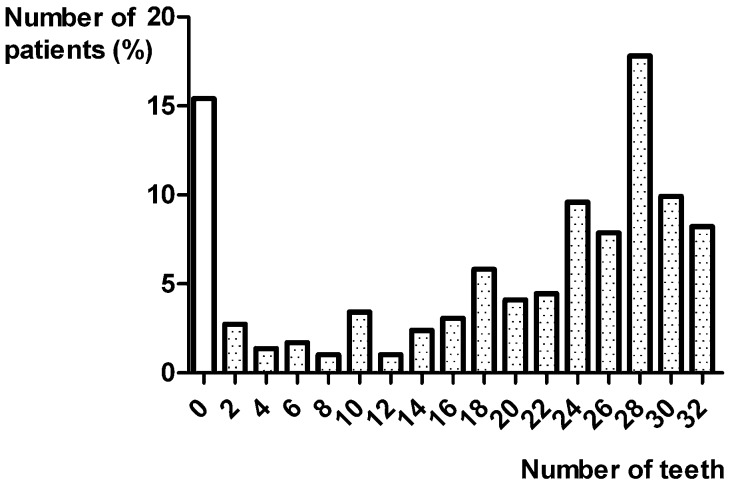
The number of existing teeth per patient, expressed as a percentage, is illustrated in Figure 2. The 10 impacted teeth were excluded.
Figure 2.
Existing teeth per patient (%).
Intra-rater variability of the atherosclerosis measure
The intra-rater variability in the quantification of the aortic atherosclerotic burden was extremely low, with an ICC of 0.9529 (p<0.0001; n = 87).
Univariate analyses
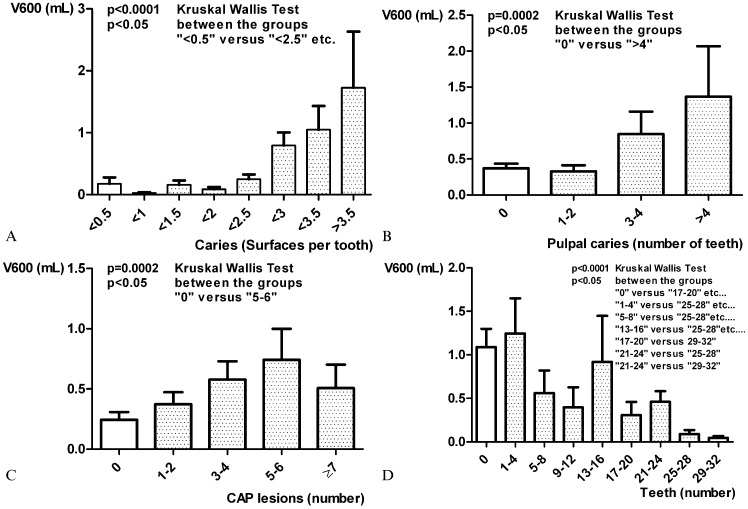
The patients with caries on more than one surface per tooth had a greater atherosclerotic burden (0.44±1 mL) than the patients with caries on less than one surface per tooth (0.13±0.61 mL; p<0.0001). As Figure 3A shows, in the patients with caries on more than 2.5 surfaces per tooth, the atherosclerotic burden increased exponentially. Above a threshold of the mean of two decayed surfaces per existing tooth, there was an inverse correlation between the atherosclerotic burden and the number of existing teeth (p<0.0001; Kruskal-Wallis test).
Figure 3.
A) Correlation between the aortic atherosclerotic burden (axis of ordinates, in milliliters) and caries (axis of abscissa, surfaces per tooth). B) Correlation between the aortic atherosclerotic burden and pulpal caries. C) Correlation between the aortic atherosclerotic burden and chronic apical periodontitis (CAP). D) Correlation between the aortic atherosclerotic burden and the number of teeth.
The patients with at least one tooth affected by pulpal caries had a greater atherosclerotic burden (0.37±0.89 mL) than the patients with no pulpal caries (0.53±1.18 mL; p = 0.0046). Figure 3B shows the increase in the atherosclerotic burden in relation to the number of teeth with pulpal caries.
The larger the sum of the distances between the incisal points and the alveolar crest, as a surrogate parameter for marginal periodontitis, the greater the aortic atherosclerotic burden (Spearman's rho = 0.6138; p<0.0001). The sum of the alveolar crest heights as a second parameter for periodontitis correlated inversely with the atherosclerotic burden (Spearman's rho = -0.3680; p<0.0001).
The patients with at least one tooth affected by CAP had a greater atherosclerotic burden (0.24±0.6 mL) than the patients with no CAP (0.51±1.13 mL, p<0.0001). The greater the number of teeth affected by CAP, the greater the atherosclerotic burden (Figure 3C). The correlation between the number of existing teeth and the atherosclerotic burden is shown in Figure 3D. Neither the reasons for scanning nor the type of protocol used correlated with the atherosclerotic burden.
Logistical regression analyses
Table 1 shows the results of the logistical regression analyses of the target variable “presence of aortic atherosclerosis”. In increasing order of significance, the parameters sum of the bony height (periodontitis), gender (male), decayed surfaces per tooth, and age were found to be independent risk factors for aortic atherosclerosis (Model 1). The Wald statistic can be considered a measure of the magnitude of a factor's effect in the model. The value of B, the estimate of the effect, indicates the direction and extent of a change in the value of the target parameter in the sense of an odds ratio. The value of 1.16 for age leads to the conclusion that the atherosclerotic burden increases every year, and the value of 0.973 (<1) for bony height indicates a lowering of the atherosclerotic burden for every millimeter of height of the alveolar crest. Models 2 and 3 focus on the superficial and pulpal caries. The omission of the 44 edentulous patients had no effect, and neither CAP nor pulpal caries were independent risk factors in the models.
Table 1.
Logistic regression analyses of the target variable “presence of aortic atherosclerosis”. First model: Superficial caries and pulpal caries are grouped together as one parameter. Second model: Superficial caries and pulpal caries are separated parameters. N = 248 (both models), Nagelkerke's R2 = 0.677 (first model) and 0.644 (second model). Pulpal caries appears in the models only if superficial caries is removed (model not shown).
| Model number | Wald | Direction of the Effect | Estimator | Lower CI | Upper CI | p-value | |
| 1 | Age | 49.32 | + | 1.16 | 1.113 | 1.209 | <0.001* |
| Caries | 26.345 | + | 6.237 | 3.1 | 12.547 | <0.001* | |
| Gender | 10.983 | Male | 4.624 | 1.869 | 11.436 | 0.001* | |
| Bony height | 8.563 | - | 0.973 | 0.956 | 0.991 | 0.003* | |
| Constant | 4.369 | 0.009 | 0.037* | ||||
| CAP | ns | ||||||
| Number of teeth | ns | ||||||
| Indication for CT scan | ns | ||||||
| CT protocol | ns | ||||||
| Fillings per tooth | ns | ||||||
| 2 | Age | 43.31 | + | 1.151 | 1.104 | 1.201 | <0.001* |
| Caries | 18.963 | + | 5.115 | 2.441 | 10.719 | <0.001* | |
| Gender | 10.229 | Male | 4.435 | 1.78 | 11.049 | 0.001* | |
| Bony height | 9.912 | - | 0.971 | 0.953 | 0.989 | 0.002* | |
| Incisal point to bone crest | 4.822 | + | 1.037 | 1.004 | 1.072 | 0.028* | |
| Constant | 7.476 | 0.001* | |||||
| CAP | ns | ||||||
| Pulpal caries | ns | ||||||
| Number of teeth | ns | ||||||
| Indication for CT scan | ns | ||||||
| CT protocol | ns | ||||||
| Fillings per tooth | ns |
Abbreviations: p = significance; CI = confidence interval; * = significant; CAP = chronic apical periodontitis; ns = not significant.
Linear regression analyses
In the linear regression analyses, age and the number of teeth with pulpal caries proved to be independent proatherogenic factors, while the number of fillings per tooth was an independent protective factor affecting atherosclerosis. The “number of teeth” and the sum of the distances between the incisal point and the alveolar crest played no role in the models (Table 2). The estimate of effect “B” can be understood to be an indication of the direction and extent of the effect of the corresponding parameter.
Table 2.
Linear regression analyses of different factors influencing the atherosclerotic burden. First model: all patients. Second model: Edentulous patients. N = 292 (first model) and n = 44 (second model). The R2 values for the first and second models were 0.276 and 0.194, respectively.
| Model number | Estimator | Direction of the effect | p-value | |
| 1 | Age | 0.378 | + | <0.001* |
| Pulpal caries | 0.331 | + | <0.001* | |
| Filling index (number of fillings per tooth) | 0.148 | - | 0.018* | |
| Gender | ns | |||
| CAP | ns | |||
| Bony height | ns | |||
| Incisal point to bone crest (distance) | ns | |||
| Superficial caries | ns | |||
| Number of teeth | ns | |||
| Indication for CT scan | ns | |||
| CT protocol | ns | |||
| 2 | Age | 0.441 | + | 0.003* |
| Bony height | ns | |||
| Indication | ns | |||
| CT protocol | ns | |||
| Gender | ns |
Abbreviations: p = significance; * = significant; ns = not significant; CAP = chronic apical periodontitis.
The analysis of the subset of the 44 edentulous patients showed that only the age of the patients remained an independent risk factor for the atherosclerotic burden (Table 2).
As in the logistical models, the omission of the 44 edentulous patients weakened the factor of age and strengthened the other factors.
DISCUSSION
The results of the study show for the first time that dental caries may be an independent risk factor for atherosclerosis, with a magnitude of risk comparable to that of periodontitis. Pulpal caries and chronic apical periodontitis leading to the spread of inflammation to endodontic structures are also associated with an increased atherosclerotic burden. The number of fillings is inversely correlated with the atherosclerotic burden. In the logistical and linear regression models, the magnitude of the effect of caries on atherosclerosis slightly exceeded that of male gender and was of the same order of magnitude as that of marginal periodontitis.
When interpreting the data, the limitations of this study should be considered. The retrospective study design that was necessary to avoid unnecessary exposure to radiation is a weakness because in retrospective studies, selection or sampling bias can lead to distortions. Pathologies of the teeth, jaws, or cardiovascular system were not prominent in any of the 292 patients, and it is not anticipated that the diagnosis of a tumor disease or an accident, which applied to most of the patients in our study, could have an effect on the status of the parameters studied. Neither the reasons for scanning nor the diagnoses made from the CT scan or the scan protocols have any correlation with the parameters studied, especially with caries or CAP. It is conceivable that among the 68 patients with suspected arthritis, there were also some with psoriasis, although this finding was not documented for any patient. Psoriatic arthritis is weakly associated with a lower radiographic bone level and a greater number of missing teeth (22), as well as with coronary heart disease (23). If psoriasis patients had been present in the study population, this factor would have led merely to the over-representation of periodontitis, while the assessment of caries would not have been affected.
Another methodological weakness of the study is that the calcium score as the measure of the arterial atherosclerotic burden is only a surrogate parameter, albeit an objectively measurable parameter, for cardiovascular morbidity and mortality. Socioeconomic aspects were not taken into consideration, although they have a known effect, on oral health (24,25) and atherosclerosis (26,27). The inverse correlation between restorative measures and atherosclerosis could ultimately be at least partially based on the fact that patients who particularly value dental care also placed particular value on the prevention of caries and periodontitis.
The radiological methods for estimating the extent of periodontal disease are based on the assessment of the bone loss pattern and bone loss level. These measurements are inferior to surgical measurements of alveolar bone height and clinical attachment measurements because they underestimate the extent of periodontitis compared with other methods (28). Accordingly, the extent of periodontitis in this study was underestimated (6,29). The sum of the maximum distances from the selected incisal points to the alveolar crest at 10 representative locations for visualizing the horizontal bony defect in the upper and lower jaw (30) and the sum of the bone heights at these locations were significantly correlated with the extent of the atherosclerotic burden. However, the above mentioned measurement methods have disadvantages that diminish their objectivity. The use of an incisal point as the fixed coronal reference point could be less reliable than the normally used enamel/cement junction (28).
The CT evaluation and the missing clinical evaluation of caries and periodontitis is a weak point of the study, but it was the existence of CT scans of the entire body that made the study possible. Selecting an approach that allowed us to quantify the arterial atherosclerotic burden and evaluate the teeth and jaws retrospectively, without exposing the patients to additional radiation, was the only possible approach that would have been ethical. Because measurements of the tooth and jaws are impossible without CT scans, these scans were accepted as the source of data in this study.
Although the full extent of the resolution capacity of modern CT was used to obtain images of the teeth, CT resolution remains limited by the dimensions of the detectors. Considering the somewhat higher resolution of cone-beam CT images in the transverse plane compared with the resolution of CT scans (31), it would have been useful to conduct cone-beam CT scans (CBCTs) on the patients. However, because none of the patients had a dental, orthodontic, or maxillary surgery problem, this examination was not conducted on any patient. The resolution of the CT images used in the present study in the x and y axes was within the order of magnitude of the resolution of modern CBCTs (32), and CBCT and CT both have the advantage of yielding proportional 3D imaging. The “rapid volumetric image acquisition from a single low radiation dose scan”, thought to be an advantage of CBCT (33), has been overcome by the progress of transmission of the vast amount of data from the contact ring of the CT and of the processing time compared with CBCT. The radiation dosage of CT imaging is decreasing (34), but it is still higher than that of CBCT systems (35). Operating a CBCT system is more economical and requires less space (32). CBCT excites weaker beam-hardening artifacts (35), but the delineation of soft tissue is inferior to that with conventional CT. Regarding the teeth and jawbones, the information provided by both methods is identical (35), and the superiority of CBCT over other conventional methods in the detection and assessment of caries is under discussion (32).
Although the quality and resolution of the images were good, smaller or incipient carious lesions in the low sub-millimeter range may have escaped detection, and the total extent of caries in the patients may have been underestimated. Caries are visible clinically before they can be observed in radiographic images because a certain degree of demineralization of the enamel must occur before they are visible in an X-ray (36). The resolution of the CT scans used here was clearly higher than the resolution that allows more accurate detection of periapical lesions compared with the resolution of periapical radiographs (37).
The frequency of tooth decay found was consistent with epidemiological studies from central Europe (38). The distribution of caries and fillings was also consistent with data in the literature (39,40), indicating that while some incipient lesions may have been missed, the overall incidence of caries was reliably assessed.
This study did not distinguish between primary and secondary decayed surfaces because this differentiation was irrelevant for the analysis. The exact localization of the caries was not taken into consideration in the analysis for the same reason. The severity of the caries was considered only to the extent that the number of decayed surfaces per tooth was counted, and any visible opening of the pulpal cavity was assumed to be pulpal decay. If the pulpal cavity was not opened, neither the depth nor size of the carious lesions was taken into consideration. The reasons for a missing tooth could not be determined retrospectively with sufficient certainty. The loss of a tooth was not considered to be due to caries (41), and it was treated as a separate parameter. The categorical differentiation of the DMFS score with respect to fillings was not reconstructed because this distinction was not important for the purpose of the study. These modifications of the assessment of the caries status compared with the DMFS score were made to enable execution of the complex statistical analysis performed to address the research question without misinterpreting the absence of a tooth as caries-related (41). Considering the significance of periodontal disease as a cause of tooth loss in elderly patients (19), this method would have led to unacceptable skewing of the results, in view of the mean age of the patients examined in this study.
Neither the original DMFS score nor another established method known as the composite score (12) was used for these calculations. Neither of these scores, because of their association with criteria that can be better attributed to periodontitis, would have allowed a differentiation to be made between the effect of caries and that of periodontal disease on the aortic atherosclerotic burden. In this context, it is remarkable that not even the horizontal bone loss in the subgroup of edentulous patients was significant; age was the only factor that correlated significantly with the atherosclerotic burden in these patients. The analysis performed without taking this subset of data into consideration did not yield different results. The inclusion of the impacted teeth not considered in the DMFS score had no effect on the results.
The result showing that not only CAP but also caries with pulpal decay or no visible pulpal decay were associated with a greater atherosclerotic burden was somewhat surprising. Superficial caries is an inflammatory process localized in the oral cavity that does not affect the pulpal cavity or the bone, indicating that a lesser extent of association of the superficial caries with the atherosclerotic burden was expected than with CAP and pulpal caries. Although the extent of the association between pulpal caries and CAP was not less than that between caries and the atherosclerotic burden in the univariate analyses, only the factor “number of decayed surfaces per tooth” proved to be an independent risk factor in the logistical regression models. Pulpal decay remained a factor in the logistical models only when the factor “number of decayed surfaces per tooth” was removed. One obvious explanation for this finding may be the covariance of these factors, as pulpal caries and CAP occur primarily in patients with extensive tooth decay. The initial carious lesion and caries not yet affecting the pulpal cavity exist for a longer period compared with the pupal decay, which can precede pulpal decay by a number of years. An explanation other than disease lasting many years is that even forms of caries not yet involving the pulp are not merely local inflammatory lesions, but rather disease affecting the entire body.
As tooth loss is a strong measure of the history of oral diseases, such as caries, CAP, and marginal periodontitis, this factor was accorded particular weight. Tooth loss was significantly correlated with the aortic atherosclerotic burden. The explanation for the fact that the tooth loss factor (15) was not retained as an independent risk factor in the multivariate models may be that its cause is usually found in CAP, caries, and marginal periodontitis included in these models. The loss of the affected tooth generally means that the inflammation subsides. The caries itself is no longer present; the residual cyst is closed, and gingivitis and osteitis are no longer present. The loss of the tooth marks the start of the resolution of the inflammatory process.
There are questions as to the possible causality of the inflammatory processes associated with atherosclerosis that cannot be answered with epidemiological cross-sectional studies. This finding applies to this cross-sectional study that utilized retrospective data, with evidence level IIb of the Oxford Centre for Evidence-Based Medicine and level III of the Agency for Healthcare Research and Quality. Only a longitudinal study, optimally with a prospective design based on the initial evidence provided here, could ultimately clarify the question of causality. For an investigation of that nature, it would be necessary to have clinical and radiological baseline findings for a sufficiently large patient population. It would be necessary to observe these patients over a period of many years to determine the outcomes of these patients, including cardiovascular morbidity and mortality.
The hypotheses proposed in this study, namely whether caries are a cause of or a co-factor for developing atherosclerosis and whether restorative measures not only protect the tooth from further decay but also protect the patient from acceleration of atherosclerosis, can be investigated only prospectively. Both hypotheses are very speculative, and the data obtained in this study do not prove them. However, further study is warranted based on the findings presented here.
This study shows for the first time that periodontitis and dental caries may both be associated with atherosclerosis. This finding and the inverse association between restoration and atherosclerosis must be confirmed in prospective studies with a longitudinal design. Only in this way can a possible causality between odontogenic inflammation and atherosclerosis be verified or ruled out.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–8. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 3.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 4.Söder PO, Söder B, Nowak J, Jogestrand T. Early carotid atherosclerosis in subjects with periodontal diseases. Stroke. 2005;36(6):1195–200. doi: 10.1161/01.STR.0000165916.90593.cb. [DOI] [PubMed] [Google Scholar]
- 5.Schillinger T, Kluger W, Exner M, Mlekusch W, Sabeti S, Amighi J, et al. Dental and periodontal status and risk for progression of carotid atherosclerosis: the inflammation and carotid artery risk for atherosclerosis study dental substudy. Stroke. 2006;37(9):2271–6. doi: 10.1161/01.STR.0000236495.82545.2e. [DOI] [PubMed] [Google Scholar]
- 6.Arbes SJ, Jr, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an Analysis of NHANES III data. J Dent Res. 1999;78(12):1777–82. doi: 10.1177/00220345990780120301. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Pizzo G, Guiglia R, Lo Russo L, Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21(6):496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Piconi S, Trabattoni D, Luraghi C, Perilli E, Borelli M, Pacei M, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. 2009;23(4):1196–204. doi: 10.1096/fj.08-119578. [DOI] [PubMed] [Google Scholar]
- 10.Nakano K, Inaba H, Nomura R, Nemoto H, Takeda M, Yoshioka H, et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol. 2006;44(9):3313–7. doi: 10.1128/JCM.00377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoke M, Schillinger T, Mlekusch W, Wagner O, Minar E, Schillinger M. The impact of dental disease on mortality in patients with asymptomatic carotid atherosclerosis. Swiss Med Wkly. 2011;141:w13236. doi: 10.4414/smw.2011.13236. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander AH, Sung EC, Chung EM, Garrett NR. Radiographic quantification of chronic dental infection and its relationship to the atherosclerotic process in the carotid arteries. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):615–21. doi: 10.1016/j.tripleo.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Glodny B, Nasseri P, Rehder P, Unterholzner V, Plaikner M, Koppelstätter C, et al. Reduced glomerular filtration rate due to loss of nephron mass may be an independent risk factor for atherosclerosis. Nephrol Dial Transplant. 2011;26(6):1882–7. doi: 10.1093/ndt/gfq678. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Gomes MS, Chagas P, Padilha DM, Caramori P, Hugo FN, Schwanke CH, et al. Association between self-reported oral health, tooth loss and atherosclerotic burden. JB Braz Oral Res. 2012;26(5):436–42. doi: 10.1590/s1806-83242012005000019. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Geneva: 1987. Oral Health Surveys - Basic Methods. 3rd Edition. [Google Scholar]
- 17.Bödecker CF. The modified dental caries index. J Am Dent Assoc. 1939;26:1453–60. [Google Scholar]
- 18.Low KM, Dula K, Bürgin W, von Arx T. Comparison of periapical radiography and limited cone-beam tomography in posterior maxillary teeth referred for apical surgery. J Endod. 2008;34(5):557–62. doi: 10.1016/j.joen.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Hämmerle CH, Ingold HP, Lang NP. Evaluation of clinical and radiographic scoring methods before and after initial periodontal therapy. J Clin Periodontol. 1990;17(4):255–63. doi: 10.1111/j.1600-051x.1990.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 20.Grimard BA, Hoidal MJ, Mills MP, Mellonig JT, Nummikoski PV, Mealey BL. Comparison of clinical, periapical radiograph, and cone-beam volume tomography measurement techniques for assessing bone level changes following regenerative periodontal therapy. J Periodontol. 2009;80(1):48–55. doi: 10.1902/jop.2009.080289. [DOI] [PubMed] [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Preus HR, Khanifam P, Kolltveit K, Mørk C, Gjermo P. Periodontitis in psoriasis patients. A blinded, case-controlled study. Acta Odontol Scand. 2010;68(3):165–70. doi: 10.3109/00016350903583678. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb AB, Dann F. Comorbidities in patients with psoriasis. Am J Med. 2009;122(12):1150.e1–9. doi: 10.1016/j.amjmed.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Bastos JL, Gigante DP, Peres KG, Nedel FB. Social determinants of odontalgia in epidemiological studies: theoretical review and proposed conceptual model. Cien Saude Colet. 2007;12(6):1611–21. doi: 10.1590/s1413-81232007000600022. [DOI] [PubMed] [Google Scholar]
- 25.Sisson KL. Theoretical explanations for social inequalities in oral health. Community Dent Oral Epidemiol. 2007;35(2):81–8. doi: 10.1111/j.1600-0528.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts CB, Couper DJ, Chang PP, James SA, Rosamond WD, Heiss G. Influence of life-course socioeconomic position on incident heart failure in blacks and whites: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2010;172(6):717–27. doi: 10.1093/aje/kwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franks P, Tancredi DJ, Winters P, Fiscella K. Including socioeconomic status in coronary heart disease risk estimation. Ann Fam Med. 2010;8(5):447–53. doi: 10.1370/afm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tugnait A, Clerehugh V, Hirschmann PN. The usefulness of radiographs in diagnosis and management of periodontal diseases: a review. J Dent. 2000;28(4):219–26. doi: 10.1016/s0300-5712(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 29.Jayakumar A, Rohini S, Naveen A, Haritha A, Reddy K. Horizontal alveolar bone loss: A periodontal orphan. J Indian Soc Periodontol. 2010;14(3):181–5. doi: 10.4103/0972-124X.75914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebolt CF, Vannier MW, Shrout MK, Pilgram TK. ROC analysis of observer-response subjective rating data — application to periodontal radiograph assessment. Am J Phys Anthropol. 1991;84(3):351–61. doi: 10.1002/ajpa.1330840310. [DOI] [PubMed] [Google Scholar]
- 31.Ito K, Gomi Y, Sato S, Arai Y, Shinoda K. Clinical application of a new compact CT system to assess 3-D images for the preoperative treatment planning of implants in the posterior mandible A case report. Clin Oral Implants Res. 2001;12(5):539–42. doi: 10.1034/j.1600-0501.2001.120516.x. [DOI] [PubMed] [Google Scholar]
- 32.Park YS, Ahn JS, Kwon HB, Lee SP. Current status of dental caries diagnosis using cone beam computed tomography. Imaging Sci Dent. 2011;41(2):43–51. doi: 10.5624/isd.2011.41.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lascala CA, Panella J, Marques MM. Analysis of the accuracy of linear measurements obtained by cone beam computed tomography (CBCT-NewTom) Dentomaxillofac Radiol. 2004;33(5):291–4. doi: 10.1259/dmfr/25500850. [DOI] [PubMed] [Google Scholar]
- 34.Fanucci E, Fiaschetti V, Ottria L, Mataloni M, Acampora V, Lione R, et al. Comparison of different dose reduction system in computed tomography for orthodontic applications. Oral Implantol (Rome) 2011;4(1-2):14–22. [PMC free article] [PubMed] [Google Scholar]
- 35.Carrafiello G, Dizonno M, Colli V, Strocchi S, Pozzi Taubert S, et al. Comparative study of jaws with multislice computed tomography and cone-beam computed tomography. Radiol Med. 2010;115(4):600–11. doi: 10.1007/s11547-010-0520-5. [DOI] [PubMed] [Google Scholar]
- 36.Stenlund H, Mejàre I, Källestål C. Caries incidence rates in Swedish adolescents and young adults with particular reference to adjacent approximal tooth surfaces: a methodological study. Community Dent Oral Epidemiol. 2003;31(5):361–7. doi: 10.1034/j.1600-0528.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 37.Jorge EG, Tanomaru-Filho M, Gonçalves M, Tanomaru JM. Detection of periapical lesion development by conventional radiography or computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(1):e56–61. doi: 10.1016/j.tripleo.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Splieth Ch, Schwahn Ch, Bernhardt O, Kocher T, Born G, John U, et al. Caries prevalence in an adult population: results of the Study of Health in Pomerania, Germany (SHIP) Oral Health Prev Dent. 2003;1(2):149–55. [PubMed] [Google Scholar]
- 39.Demirci M, Tuncer S, Yuceokur AA. Prevalence of caries on individual tooth surfaces and its distribution by age and gender in university clinic patients. Eur J Dent. 2010;4(3):270–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Carlos JP, Gittelsohn AM. Longitudinal studies of the natural history of caries. II. A life-table study of caries incidence in the permanent teeth. Arch Oral Biol. 1965;10(5):739–51. doi: 10.1016/0003-9969(65)90127-5. [DOI] [PubMed] [Google Scholar]
- 41.Broadbent JM, Thomson WM. For debate: problems with the DMF index pertinent to dental caries data analysis. Community Dent Oral Epidemiol. 2005;33(6):400–9. doi: 10.1111/j.1600-0528.2005.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]