Summary
In the adult brain, continual neurogenesis of olfactory neurons is sustained by the existence of neural stem cells (NSCs) in the subependymal niche. Elimination of the cyclin-dependent kinase inhibitor 1A (p21) leads to premature exhaustion of the subependymal NSC pool, suggesting a relationship between cell cycle control and long-term self-renewal, but the molecular mechanisms underlying NSC maintenance by p21 remain unexplored. Here we identify a novel function of p21 in the direct regulation of the expression of pluripotency factor Sox2, a key regulator of the specification and maintenance of neural progenitors. We observe that p21 directly binds a Sox2 enhancer and negatively regulates Sox2 expression in NSCs. Augmented levels of Sox2 in p21-null cells induces replicative stress and a DNA damage response that leads to cell growth arrest mediated by increased levels of p19Arf and p53. Our results show a novel regulation of NSC expansion driven by a p21/Sox2/p53 axis.
Keywords: neurogenesis, senescence, DNA damage, DDR, p53, p19, replicative stress, transcription
Introduction
Stem cells persist in the adult brain in two discrete niches, the subependymal zone (SEZ), which generates olfactory interneurons and callosal oligodendrocytes, and the neurogenic subgranular zone (SGZ) of the hippocampus. In the SEZ, in particular, glial fibrillary acidic protein (GFAP)-expressing multipotential astrocytes with an elongated morphology and rather long cell cycles (B cells) appear to act as neural stem cells (NSCs). Activated, proliferative, B cells produce transit amplifying progenitor cells which primarily differentiate into neurons (Zhao et al., 2008). These activated B cells can be isolated and grown in culture as neurospheres with mitogens epidermal growth factor (EGF) and/or basic fibroblast growth factor (bFGF); under these conditions, they exhibit clonogenic capacity and the potential to clonally differentiate into neurons, astrocytes and oligodendrocytes (Andreu-Agulló et al., 2009). Lifelong neurogenesis and expansion of multipotent cultures in vitro are a reflection of NSC self-renewal, an essential property of stem cell populations that integrates cell proliferation with the maintenance of an undifferentiated state. Self-renewal is regulated by cell-extrinsic signals, produced within endogenous stem cell niches, and intrinsic regulators. Among intrinsic modulators, transcription factors that are important to sustain stem cell developmental potential as well as modulators of the cell cycle status appear especially relevant (Orford and Scadden, 2008).
SOX transcription factors are a family of proteins characterized by the presence of an SRY box, a motif of 79 amino acids encoding a high-mobility-group (HMG)-type DNA binding domain (Wegner, 2011). Closely related factors Sox1, Sox2, and Sox3 of the SoxB1 sub-family are expressed in most neural stem/progenitor cells and play a role in maintaining their undifferentiated state, albeit with some functional redundancy (Graham et al., 2003; Bylund et al., 2003; Bani-Yaghoub et al., 2006; Miyagi et al., 2008; Wegner et al., 2011). Sox2 is expressed at high levels in adult NSCs and a reduction in the levels of the gene results in deficient adult neurogenesis in the hippocampus, which is associated with the loss of GFAP+ progenitor cells (Ellis et al., 2004; Ferri et al., 2004; Favaro et al., 2009). Moreover, SOX2 hemyzygosity in humans also associates with neurological phenotypes and hippocampal malformation (Sisodiya et al., 2006). Altogether, these reports suggest that Sox2 sustains self-renewal of adult NSCs. In addition, human gliomas exhibit high levels of SOX2 expression and SOX2 silencing in glioblastoma tumor-initiating cells reduces their proliferation (Gangemi et al., 2009). Despite the relevant role of Sox2 in the regulation of normal and transformed NSCs, very little is known about the control of Sox2 expression in adult NSCs.
Sox2 is present in long-lived adult stem cells and is essential for the pluripotency of epiblast stem cells and embryonic stem (ES) cells (Wegner, 2011; Arnold et al., 2011). In line with this, Sox2 is one of the factors (together with Oct4/Klf4/c-Myc) that mediate the reprogramming of terminally differentiated somatic cells to a fully pluripotent state (Banito and Gil, 2010). Reprogramming is highly improved by the ablation of different senescence effectors, indicating that senescence acts as a barrier for the completion of this process. In this regard, it has been shown that ectopic expression of pluripotency factors in fibroblasts can trigger senescence by up-regulating the tumor suppressor p53 (also known as Trp53 in mice and TP53 in humans) and the cell cycle regulator p21 (also known as Cdkn1a and Cip1) (Banito and Gil, 2010; Blasco et al., 2011). Interestingly, cell cycling of adult murine NSCs is tightly regulated by p21, seemingly in a p53-independent manner (Kippin et al., 2005; Meletis et al., 2006). NSCs that are deficient in p21 exhibit increased cell cycle re-entry leading to subsequent exhaustion of the NSC pool (Kippin et al., 2005), albeit the mechanisms involved in p21-dependent regulation of self-renewal are not understood.
Here we show that p21 directly binds to the SRR2 enhancer downstream of the Sox2 gene and inhibits the expression of this gene in adult SEZ-derived NSCs. The loss of p21 results in increased levels of Sox2 leading to replicative stress that ultimately results in an arrest in stem cell growth that is dependent on the p53 and p19Arf tumor suppressors. Our results indicate that the modulation of Sox2 levels by p21 could be a regulatory mechanism to control the proliferation of NSC populations in the adult brain.
Results
Sox2-dependent growth arrest of p21-deficient NSCs
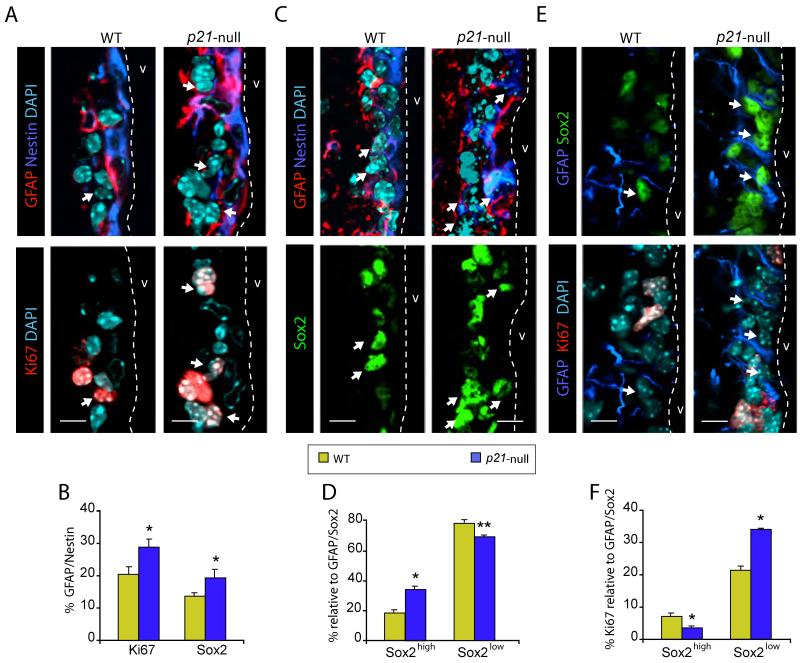
It has been shown that young mice lacking the p21 gene exhibit an increased number of long-term 2-bromo-5-deoxyuridine (BrdU)-retaining cells in the SEZ and yield more subependyma-derived clonal neurospheres than wild-type littermates (Kippin et al., 2005). In agreement with the proposed role of p21 as a cell cycle break in adult B-type NSCs, contained within the population of GFAP positive cells that also express the neural precursor marker Nestin, we could observe a higher proportion of GFAP/Nestin double positive (DP) cells in the SEZ of 2 month-old p21-null mice (10.0 ± 2.6 vs wild-type values of 3.5 ± 0.5 %; n = 3, p<0.05). Compared to the wild type, we also found an increase in the percentage of GFAP/Nestin DP cells that were also positive for the cell proliferation marker Ki67 in the SEZ of p21-null mice (Figures 1A, B).
Figure 1. Higher levels of Sox2 in the SEZ of p21-null mice negatively correlate B cells proliferation.
(A) Immunostaining for GFAP (red) and Nestin (blue) (top panels) and Ki67 (red) (bottom panels) in wild-type (WT) or p21-null mice. White arrows indicate triple positive cells. (B) Graph showing the percentage of Ki67-positive and Sox2-positive cells within the GFAP/Sox2 DP cell population. (C) Immunostaining for GFAP (red) and Nestin (blue) (top panels) and Sox2 (green) (bottom panels) in WT or p21-null mice. White arrows indicate triple positive cells. (D) Graph showing the percentage of Sox2high and Sox2low cells within the GFAP/Sox2 DP cell population. (E) Immunostaining for GFAP (red) and Sox2 (green) (top panels) and Ki67 (red) (bottom panels) in WT or p21-null mice. White arrows indicate triple positive cells. (F) Graph showing the percentage of Ki67-positive cells within the GFAP/Sox2high and GFAP/Sox2low cells. DAPI is used as nuclear counterstaining. The dashed white line indicates the lateral ventricle (v) limit. Data are shown as mean values ± s.e.m. (*p<0.05, **p<0.01). Scale bars: in A, C, E, 10 μm.
Sox2 is expressed by most long-lived stem cells that support tissue homeostasis in adult mice (Arnold et al., 2011). In the postnatal brain, it has been reported that the levels of Sox2 expression in individual cells are variable among the proliferating cells of the SEZ, including the GFAP/Nestin DP population (Ferri et al., 2004; Ellis et al., 2004). Accordingly, we could observe GFAP/Sox2 DP cells with different levels of Sox2 protein in wild-type mice (Figure 1C). In agreement with our previous findings of more B-type cells in the SEZ of p21-null mice, we also observed increased percentages of both GFAP/Sox2 DP cells (44.3 ± 2.0 vs wild-type values of 32.0 ± 2.7 %, n = 3, p<0.05) and GFAP/Sox2 DP cells that were also positive for Nestin, in p21 mutant mice (Figures 1B, C). Intriguingly, we observed that the lack of p21 altered the proportions of individual GFAP positive cells with high vs. low levels of Sox2 protein. Specifically, we found an increase in the percentage of GFAP-positive cells displaying high levels of Sox2 expression in the SEZ of p21-null mice when compared to the wild-type controls (Figures 1B-D), suggesting the possibility of a physiological regulation of Sox2 by p21 in NSCs.
Further analysis of the GFAP-positive population with different levels of Sox2 expression revealed a reduction by half in the proportion of cells with high levels of Sox2 (Sox2high) that were also positive for Ki67 in p21-null mice (5.4 ± 0.6 % vs. 10.3 ± 1.8 in wild-type littermates; n = 3, p<0.05), suggesting that increased levels of Sox2 in mutant cells might negatively correlate with proliferation. In this regard, we found that a 20 % of the GFAP positive cells with low levels of Sox2 (GFAP/Sox2low) were positive for Ki67, whereas only a 7% of the GFAP-positive population displaying high levels of Sox2 expression (GFAP/Sox2high) were also positive for this proliferative marker in wild-type mice. Interestingly, p21-null mice showed an increase in the percentages of Ki67-positive cells within the GFAP/Sox2low population and a decrease in the proportion of Ki67 positive cells among the GFAP/Sox2high population (Figures 1E, F). These data suggested that, whereas more GFAP positive B-type cells are cycling in the absence of p21, a fraction of cells with higher levels of Sox2 may be arresting.
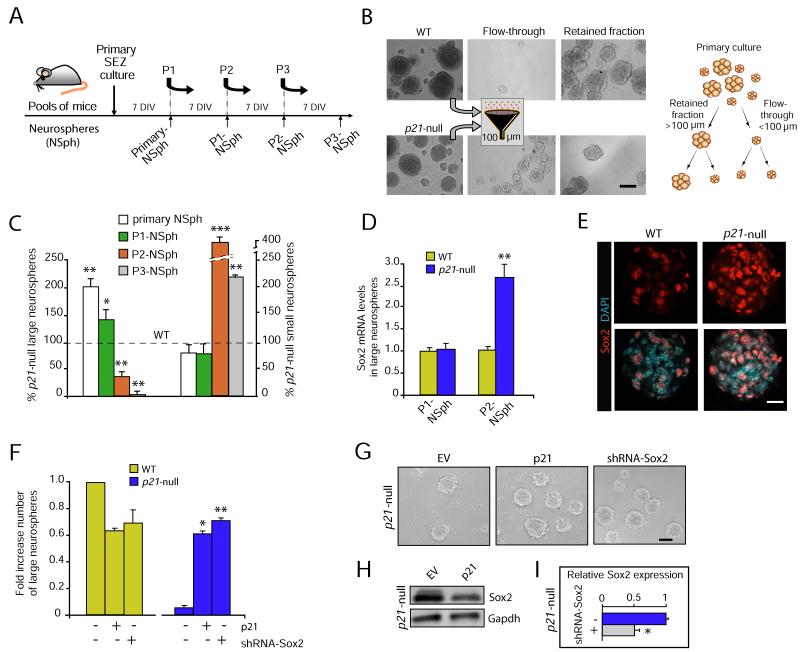
Expression of p21 is indispensable for the long-term self-renewal of adult NSCs and p21-null NSCs have a proliferative advantage at young ages that leads to their accelerated consumption during aging (Kippin et al., 2005). The exhaustion phenotype is readily manifested in vitro, when cells from the SEZ are grown and passaged as clonal neurospheres under mitogenic stimulation with EGF and FGF2. In fact, cells isolated from the subependymal region of p21-null mice yield higher numbers of neurospheres than wild-type controls, but the cultures exhibit an eventual loss of neurosphere-forming ability due to defects in self-renewal (Kippin et al., 2005). In agreement with these findings, we observed that, in comparison with the wild-type litter mates, cellular cultures derived from the SEZ of young p21 mutant mice yielded higher numbers of primary neurospheres which produced increased numbers of large neurospheres that incorporated more BrdU (Figures S1A, B), but less secondary neurospheres after passage (P) 2 (95 ± 3 out of 500 cells plated in wild type vs. 69 ± 8 in p21 mutant cultures; n = 4, p<0.01) when the cells were seeded at 2.5 cells/μl to preserve the clonality of the assay (Ferrón et al., 2007). The results indicated defects in self-renewal that could be observed at the level of a single neurosphere.
Since lack of p21 results in a significant increase in the size of primary neurospheres and because large neurospheres appear to be more self-renewing (Kippin et al., 2005; Andreu-Agulló et al., 2009), we focused our studies in those neurospheres that were above 100 μm in diameter after 7 days in vitro. To do so, we performed a selection by size, where only the neurospheres larger than 100 microns were subsequently passaged and analyzed (Figures 2A, B); in all the passages, these neurospheres produced both large and small clones, whereas further culture of the smaller neurospheres gave rise only to spheres below the 100 μm cut-off (data not shown). Relative to wild-type controls, primary cultures from p21-deficient mice yielded increased numbers of large P1-neurospheres (>100 μm). However, these gave rise to neurospheres that displayed reduced BrdU incorporation (45.6 ± 2.5 % compared to a wild-type value of 62.8 ± 1.5 % in P2-neurospheres, n = 2; Figures S1A, B), suggesting that the proliferation of p21-deficient NSCs was impaired during passaging. The number of p21-null secondary neurospheres >100 μm rapidly declined with passaging, and beyond the third passage no large neurospheres were recovered. Conversely, the number of secondary neurospheres <100 μm increased with the passage number relative to those in wild-type cultures (Figure 2C). Detrimental effects of the lack of p21 in large neurosphere recovery were not the result of increased apoptosis, as assayed by flow cytometry using propidium iodide (PI) and Annexin V staining of isolated large neurospheres 24 hours after their dissociation (Figure S1C). This type of analysis indicated a cell growth arrest of self-renewing NSCs in the absence of p21 and provided us with a system to examine the molecular changes underlying the exhaustion phenotype.
Figure 2. Growth arrest of p21-deficient NSCs is Sox2 dependent.
(A) Schematic of the neurosphere (NSph) culture along the different passages (P). (B) Phase contrast images taken at passage 2 of neurospheres from wild-type (WT) or p21-null NSCs (upper and lower panels, respectively) selected by size (left). Schematic depicting the size-based selection of the cultures (right). (C) p21-deficient NSC cultures undergo a premature growth arrest (left axis) that is associated with a decrease in the diameter of the neurospheres (right axis), relative to cultures from wild-type mice (100 %, dashed line) (n = 5). (D) Assessment in wild-type and p21-null NSCs of Sox2 mRNA levels by qPCR at P1 and P2, showing an increase of endogenous Sox2 expression in p21-deficient cells that correlates with reduced clonogenicity of large neurospheres. (E) Immunostaining for Sox2 (orange) in WT and p21-null NSCs. DAPI is used as nuclear counterstaining. (F) Expression of a full length p21 cDNA or knockdown of Sox2 levels rescues the growth arrest exhibited by p21-deficient NSCs (n = 3). (G) Phase contrast images of p21-deficient neurospheres infected with retroviruses carrying an empty vector (EV), a Sox2 shRNA or a full length p21 cDNA (p21). (H) Immunoblot for Sox2 protein in p21-null cells transduced with empty vector (EV) or p21. (I) Relative Sox2 expresion by qPCR in p21-null cells transduced with empty vector (EV) or a specific shRNA. Scale bars: in E, 20 μm; in B and G, 100 μm. Data are represented as the average ± s.e.m. of the indicated number of the experiments (n) (*p<0.05; **p<0.01; ***p<0.001).
Using this system, we investigated whether the expression of Sox2 was altered during passaging of the p21-deficient cultures. Interestingly, we observed a specific up-regulation of Sox2 mRNA (2.70 ± 0.12 fold increase, n = 3; p<0.01) in p21-null large neurospheres at passage 2, as assessed by quantitative real time-polymerase chain reaction (qPCR) (Figure 2D). Confocal and high-throughput quantitative fluorescence microscopy also revealed a higher proportion of p21-null cells positive for Sox2 protein relative to wild types (44.2 ± 9.4 compared to 27.5 ± 4.4 %, n = 5; p<0.05) and increased Sox2 protein levels in p21-deficient cells (mean fluorescence in arbitrary units per nucleus ± s.e.m.: 355.8 ± 7.5 in wild-type and 445.3 ± 5.3 in p21-knockout cells, n = 5; p<0.001) (Figures 2E & S1D). These results indicated that the up-regulation of Sox2 in a p21-null background correlates with impaired NSC expansion also in vitro.
To test whether augmented levels of Sox2 in the absence of p21 were indeed responsible for the observed NSC growth arrest, we knocked-down Sox2 expression in p21-deficient NSCs using short-hairpin RNAs (pRS-shRNA-Sox2; Banito et al., 2009). As a control, we performed parallel rescue experiments by reintroducing p21 using retroviruses generated with the transfer vector pMSCV2.2-GFP-IRES-p21 (pMigR1-p21). To do so, single cells obtained from P2-neurospheres of the two genotypes were infected 24 hours after dissociation and subjected to neurosphere forming assays 48 hours after infection. Re-expression of p21 in mutant cultures rescued the proportion of large P3-neurospheres to wild-type levels (Figures 2F, G), indicating that the observed deficits are likely the direct result of the loss of p21 in adult NSCs. Phenotypic rescue following restoration of p21 levels in p21-null cells also correlated with a reduction in Sox2 protein levels, as determined by Western blot (Figure 2H). This is in agreement with a previous report indicating that loss of p21 results in a stem cell phenotype in the SEZ of adult animals with no apparent effects during fetal/early postnatal development (Kippin et al., 2005). Retroviral delivery of shRNA-Sox2 to NSCs led to about 50% reduction of Sox2 mRNA levels (Figure 2I). Knock-down of Sox2 in wild-type cells slightly reduced the yield of large P3-neurospheres. Remarkably, the reduction of Sox2 in p21-null NSCs recued the numbers of large P3-neurospheres to wild-type levels (Figures 2F, G). Altogether, our data indicated that p21 maintains self-renewal by negatively regulating Sox2 expression.
The CKI p21 regulates Sox2 gene expression
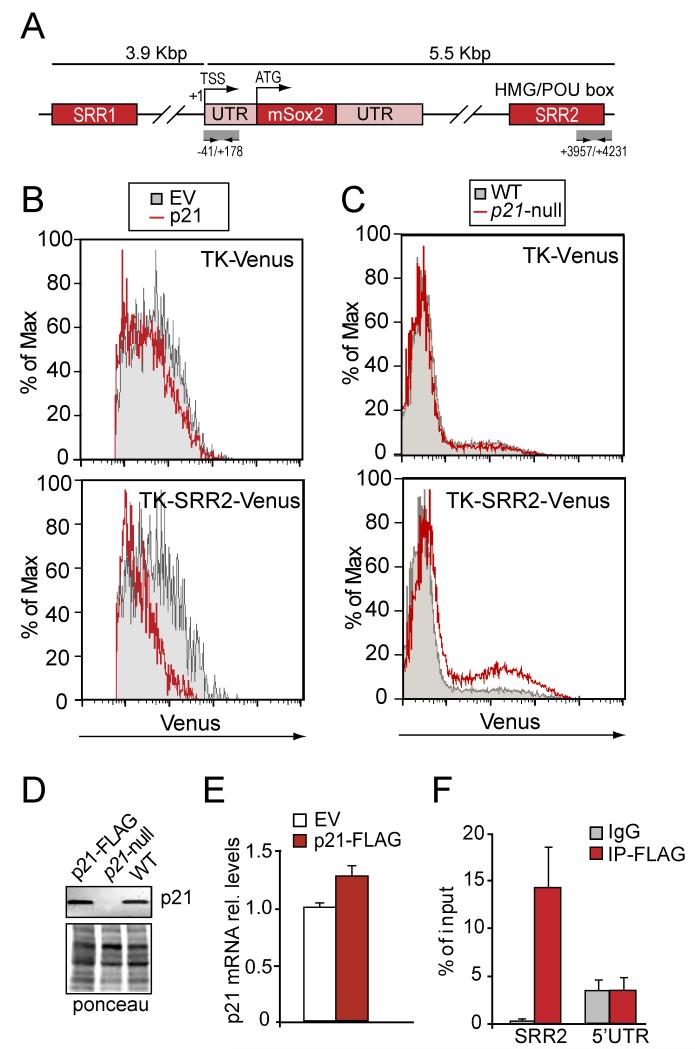
Consistent with the possibility that p21 might negatively regulate the expression of the Sox2 gene, we found that a 2.5-fold increase in the expression of p21 over the endogenous levels led to a 70% repression of the endogenous Sox2 gene, as measured by qPCR (Figure S2A). It has been shown that p21 can modulate transcription by direct association with transcription factors/coactivators at specific promoters in a cell cycle-independent way (Dotto et al., 2000; Devgan et al., 2005; Besson et al., 2008) and, therefore, we next evaluated the possibility that p21 might directly repress the expression of the Sox2 gene. Expression of Sox2 in embryonic and neural stem cells is under the positive control of two transcription enhancer regions, Sox-2 regulatory region 1 (SRR1) and SRR2 (see Figure 3A). Moreover, reporter analysis in vivo indicate that the SRR2 element (located 3 kbp downstream of the Sox2 gene) functions to up-regulate Sox2 expression in fetal NSCs and B cells of the adult SEZ (Miyagi et al., 2004; 2006). Therefore, we performed cytometry-based assays for the fluorescent product of the Venus reporter gene driven by a herpes simplex virus thymidine kinase (tk) basal promoter (positions 109 to 51) and the SRR2 region of the Sox2 genomic DNA (positions 3641 to 4023) (Miyagi et al., 2004) in p21 wild-type cells that were co-nucleofected with a pcDNA3.1 empty vector or a pcDNA3.1-p21 construct. Forced expression of p21 in NSCs inhibited transcription from the SRR2-TK-Venus reporter (45 ± 12 % Venus fluorescence intensity in p21-transduced relative to empty vector) whilst expression from the control TK-Venus reporter was unchanged (Figure 3B). In addition, although we did not find differences in the activity of the TK-Venus reporter between p21-null and wild-type NSCs, we observed a significant activation of the SRR2-Venus reporter in p21-deficient cultures when compared to the wild-type controls (2.51 ± 0.05-fold increase in the mean fluorescence intensity of the Venus-positive population in p21-null relative to wild-type cultures; Figure 3C), indicating that p21 can regulate the SRR2 enhancer activity.
Figure 3. p21 inhibits Sox2 gene expression.
(A) Diagram showing some of the regulatory regions in the murine Sox2 locus. (B) Flow cytometric analysis of Venus expression in NSCs transfected with the indicated reporters in the absence or presence of recombinant p21; a representative experiment is shown (n = 2). (C) Flow cytometric analysis of Venus expression in wild-type or p21-null NSCs; a representative experiment is shown (n = 2). (D) Immunoblot showing the expression levels of the p21 protein in p21-null NSCs (p21-null), or wild-type NSCs left untreated (WT) or transduced with p21-expressing viruses (p21FLAG). Ponceau staining of the membrane is shown as a loading control. (E) Levels of p21 mRNA expression in wild-type NSCs infected with GFP or GFP-p21-FLAG-expressing viruses (n = 3). (F) NSCs infected with FLAG-tagged p21-expressing viruses were subjected to ChIP assays with anti-FLAG or isotype control (IgG) antibodies. The graph shows the quantitation of p21 binding to the indicated regions of the Sox2 gene by qPCR (n = 2). Data are presented as the average ± s.e.m. of the indicated number of the experiments (n) (*p<0.05).
To test the possibility of a direct interaction of p21 with the SRR2 enhancer region, we performed chromatin immunoprecipitation (ChIP) assays with anti-Flag antibodies in chromatin isolated from neurospheres which had been infected with retroviruses bearing a GFP reporter, either empty (pMigR1) or containing a p21-FLAG cDNA (pMigR1-p21-FLAG). Ectopic levels of p21 mRNA and protein achieved by the retroviral infection of p21-deficient cells were similar to the physiological levels observed in wild-type cells (Figures 3D, E). Under these conditions, we observed specific binding of FLAG-tagged p21 to the SRR2 enhancer that was validated by real-time qPCR of immunoprecipitated chromatin fragments. Primers directed to a promoter region (−41/+178) were used to confirm the specificity of the binding of p21 to the SRR2 regulatory region (Figure 3F). Moreover, we performed ChIP analyses in cells of the c17.2 neural stem cell line with two different anti-p21 antibodies and found enriched specific binding of endogenous p21 to the SRR2 enhancer in contrast to the promoter region tested (Figure S2B). All together, these results indicate a direct role for p21 in the inhibition of the SRR2 enhancer in NSCs.
Elevated Sox2 levels induces senescence in neurosphere-forming cells
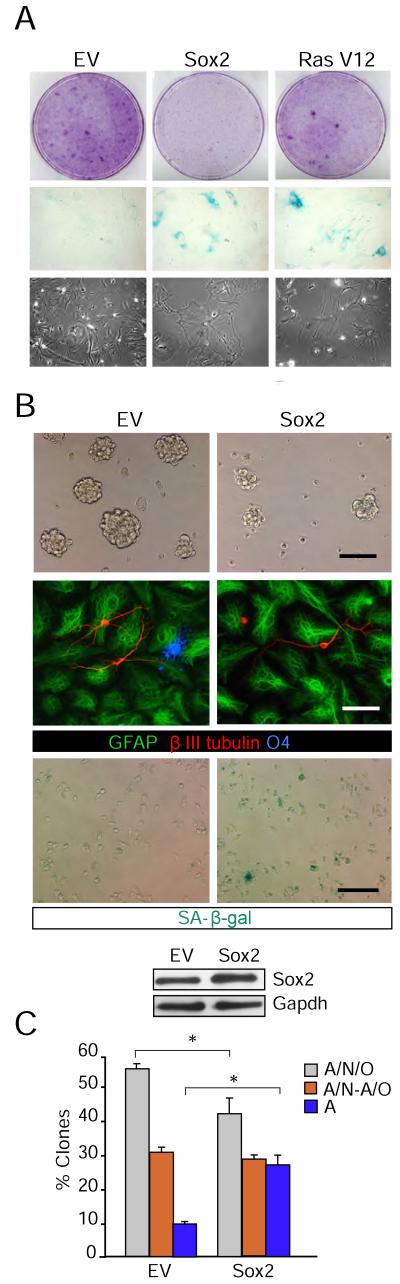
NSCs isolated from neonatal mice require Sox2 to grow and self-renew (Favaro et al., 2009). In contrast, our results suggested that up-regulation of Sox2 in adult NSCs might impair NSC self-renewal. The analysis of the physiological role of Sox2 in pluripotent ES cells indicates that either a reduction or an increase in Sox2 levels can impair pluripotency and self-renewal (Kopp et al., 2008), suggesting that Sox2 levels should be kept within strictly regulated levels in stem cells. Moreover, overexpression of reprogramming factors results in increased proportions of cells displaying senescent features (Banito et al., 2009; Hong et al., 2009; Kawamura et al., 2009; Marion et al., 2009; Li et al., 2009; Banito and Gil, 2010). In agreement with this, we observed that overexpression of Sox2 in wild-type mouse embryonic fibroblasts (MEFs) caused a drastic reduction in cell proliferation, along with a 2-fold increase in the proportion of SA-β-galactosidase positive cells (Figures 4A & S3A; oncogenic Ras V12 mutation was used as a positive control).
Figure 4. Sox2 overexpression in NSCs induces senescence.
(A) MEFs were infected with empty vector (EV) or constructs encoding the indicated factors. Cells were selected for 7 days and used for crystal violet (upper panels) or SA-β-galactosidase stainings (middle panels). Phase-contrast images of the cells at the end of the selection are shown (lower panels). Transduction with oncogenic Ras V12 has been included as a positive control. (B) NSCs were infected with empty vector (EV) or Sox2. Sorted cells were subjected to self-renewal, multipotency and senescence assays. From the top: Expression of Sox2 reduces the number and size of secondary neurospheres (phase-contrast images, upper panels), impairs multipotency (immunofluorescence images with the indicated antibodies) and induces senescence (SA-β-galactosidase stainings). Lower panel: Immunoblot showing the Sox2 protein levels in the NSCs were infected with empty vector (EV) or Sox2. (C) Quantitation of the differentiation potential of the infected NSCs in (B) (n = 3). Data are represented as the mean ± s.e.m. of the indicated number of the experiments (n) (*p<0.05). Scale bars: in B, 100 μm; in C, 30 μm; in D, 50 μm.
To investigate the role of increased levels of Sox2 in the regulation of adult NSCs features, wild-type cells were transduced with retroviruses containing pMY-GFP or pMY-GFP-IRES-Sox2 and FACS-sorted cells were then subjected to both self-renewal and clonal multipotency assays. Cultures over-expressing Sox2 (45% increase relative to controls, as determined by immunoblot) showed a 50% reduction in the number (53 ± 11 % fold decrease relative to empty vector; n = 5, p<0.05) and size (57 ± 6 vs. 96 ± 8 μm in diameter, respectively; n = 5, p<0.05) of secondary neurospheres when compared with the GFP controls (Figure 4B). Moreover, in multipotency assays, neurospheres formed under these conditions were individually picked and allowed to differentiate for 7 days and the clones derived were evaluated by immunofluorescence for the presence of the three neural lineages (oligodendrocytes, astrocytes and neurons); we found that over-expression of Sox2 impaired NSC potentiality, reflected by a significant increase in the proportion of unipotent (astrocyte-only) clones at the expense of tripotent clones (containing neurons, astrocytes and oligodendrocytes) (Figures 4B, C). Together, these results indicated that elevated levels of Sox2 restrain self-renewal of adult NSCs, reducing their neurosphere forming potential and multilineage differentiation.
The increment in Sox2 levels was not associated with apoptosis as measured by flow cytometry using Annexin V staining of GFP or GFP-Sox2 infected NSCs and by immunoblot detection of activated caspase 3 (Figures S3B, C). We did observe, however, a higher proportion of cells exhibiting senescence-associated (SA)-β-galactosidase staining in Sox2-overexpressing cultures (Figure 4B). These results indicated that reduced stem cell frequency and self-renewal potential following overexpression of Sox2 were likely the result of cell growth arrest/senescence in neurosphere-forming cells.
Increased levels of DNA damage in p21-null cells are associated with Sox2 overexpression
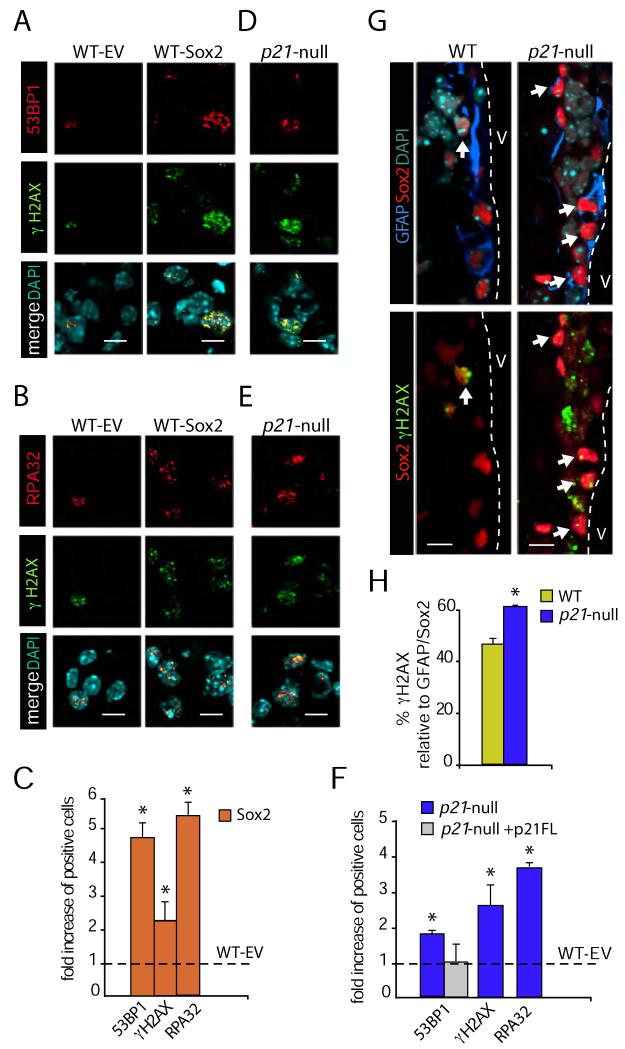
Senescence can be the result of replicative exhaustion or be elicited in response to stresses such as DNA damage (Collado et al., 2007). In this regard, we observed that overexpression of Sox2 in wild-type NSCs resulted in significantly higher percentages of cells immunopositive for the phosphorylated form of histone H2AX, a widespread marker of DNA damage and replication stress (Figures 5A-C). Since H2AX can be phosphorylated at stalled replication forks even in the absence of DNA breakage, we also analyzed the presence of nuclear foci of 53BP1, which specifically mark sites of chromosome breaks. High-content analysis of the immunofluorescence data (see Figure S4 for details) showed that forced expression of Sox2 led to a 2-fold and 5-fold increase in the percentage of γH2AX-positive or 53BP1 foci-containing cells, respectively, relative to the GFP-controls (36 ± 5.1 vs. 8 ± 3.4 for 53BP1 and 19 ± 6 vs. 10 ± 3 for γH2AX, n = 7; p<0.05) (Figure 5C). Oncogene-induced DNA breaks that promote senescence are thought to be initiated by replication stress which, if persistent, can lead to chromosomal breakage. To determine whether DNA replication stress could be involved, we examined Sox2 over-expressing cells for single-stranded DNA, which is typically associated with stalled DNA replication forks. Indeed, Sox2-transduced cultures contained more cells exhibiting ssDNA-binding replication protein A (RPA) foci (Figures 5B-C).
Figure 5. Genomic instability in p21-deficient NSCs is associated with Sox2 deregulation.
(A) Examples of Sox2-transduced NSCs showing increased immunostaining for γH2AX (green) and 53BP1 (red) relative to control empty vector (EV) cells. (B) Examples of Sox2-transduced NSCs showing increased immunostaining for γH2AX (green) and RPA (red) relative to control empty vector (EV) cells. (C) Bar diagram showing the quantitation of 53BP1, γH2AX and RPA stainings in NSCs overexpressing Sox2 relative to control vector vector (EV)-transduced cells (dashed line) (n = 3). (D) Examples of p21-null cells showing increased immunostaining for γH2AX (green) and 53BP1 (red) relative to control wild-type (WT) cells. (E) Examples of p21-null cells showing increased immunostaining for γH2AX (green) and RPA (red) relative to control wild-type (WT) cells. (F) Bar diagram showing the quantitation of 53BP1, γH2AX and RPA stainings in p21-null cells relative to control wild-type (dashed line, WT-EV) cells and p21-null NSCs re-expressing p21 (p21-null+p21FL) (n = 3). (G) Representative sections of the SEZ from wild-type or p21-null mice stained with the indicated antibodies. Triple positive cells are indicated by empty white arrowheads. (F) Bar diagram showing the proportions of GFAP/Sox2 DP cells that are also γH2AX-positive in the SEZ of p21-null and wild type mice (n = 3). Data are represented as the mean ± s.e.m. of the indicated number of the experiments (n) (*p<0.05). Scale bars: in A, B, D and E, 10 μm; in G, 20 μm.
We next evaluated DNA damage and replicative stress in our p21 mutant cells. Similarly to Sox2 overexpressing cells, we observed higher proportions of cells with 53BP1-positive foci and γH2AX in p21-null cultures than in wild-type controls (Figures 5D-F). We also observed increased numbers of cells with RPA-positive foci (6.9 ± 13 vs. 34.5 ± 2.3 % in wild-type and p21-knockout cells, respectively, n = 2; Figures 5E-F), an indication of augmented replicative stress in p21-deficient NSCs. Reintroduction of a full length p21 cDNA restored the increased percentages of 53BP1-positive cells found in p21-null cultures to the wild-type levels (Figure 5F). Since our results indicated that lack of p21 results in genomic instability, we next investigated whether this correlated with the deregulation of Sox2 expression. Indeed, we observed that 70 ± 15 % of the Sox2 positive p21-null cells were also positive for γH2AX staining, suggesting that increased Sox2 levels could be behind the replicative stress found on p21 deficient NSCs.
Analyses for DNA damage with specific markers by immunofluorescence in vivo showed a general increase in the proportion of GFAP-positive cells that were also positive for γH2AX (59.5 ± 3.9 % vs. wild-type values of 44.5 ± 2.9; n = 6, p<0.05) or RPA foci (56.1 ± 6.2 % vs. wild-type values of 30.4 ± 3.3; n = 3, p<0.05) in p21-null mice. Moreover, and in agreement with the in vitro data, we also observed an increased percentage of γH2AX positive cells within the GFAP/Sox2high population in the absence of p21 (62.3 ± 2.6 vs. wild-type values of 49.0 ± 3.2 %, n = 3, p<0.05; Figures 5G, H). It has been reported that H2AX can also be phosphorylated by kinases of the PIKK family in response to GABA signals that are naturally produced in the subependymal niche, suggesting that activation of a H2AX signalling pathway may represent one mechanism restricting NSC proliferation under physiological conditions (Fernando et al., 2011). However, we observed that 80.8 ± 3.8 % of the γH2AX/GFAP DP cells in the SEZ of p21-deficient mice presented additional evidences of DNA breakage such as 53BP1 foci, in contrast to a wild-type value of 37.3 ± 4.2 % (n = 3, p<0.001).
DNA damage checkpoint activation in p21-null and Sox2 overexpressing cells
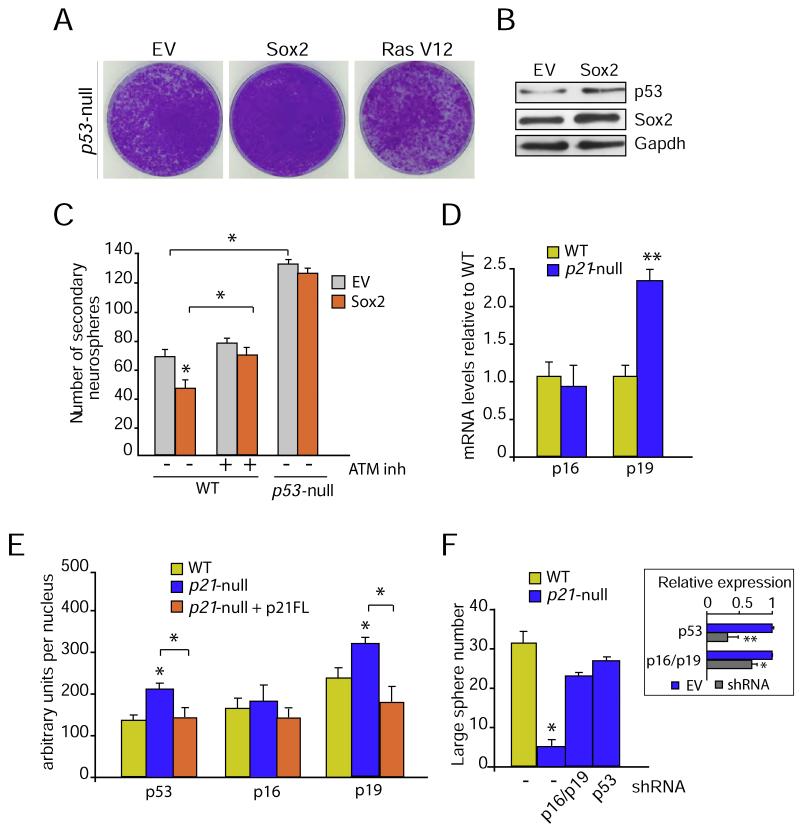
Our results therefore suggested that increased levels of Sox2 in p21-null cells were causing a cell growth arrest that eventually led to cellular senescence. Senescence is controlled by the tumor suppressor p53 and by p16Ink4a (p16) and p19Arf (both encoded by alternative reading frames of the Ink4a/Arf locus, also known as Cdkn2a locus) which operate as downstream effectors (Collado et al., 2007; Banito and Gil, 2010). Oncogene-induced stress results in increased production of p19Arf which, in turn, raises p53 levels by inhibiting MDM2, the E3-ubiquitin ligase mainly responsible for the degradation of the p53 protein (Collado et al., 2007). In this regard, it has been shown that forced expression of pluripotency-inducing factors, including Sox2, induce the accumulation of p53, p16 and/or p19Arf proteins in MEFs and that p19Arf, rather than p16, constitutes the main barrier to reprogramming by leading to the accumulation of p53 in murine cells (Li et al., 2009; Banito and Gil, 2010). In fact, we observed that overexpression of Sox2 in wild-type MEFs led to a cell growth arrest associated with an increase in the levels of the products of the Cdkn2a gene p16 and p19Arf (Figures S5A, B). Remarkably, MEFs deficient for p53 were refractory to the growth arrest induced by Sox2 overexpression, indicating that the blockade in cell growth was dependent on the presence of this tumor suppressor (Figure 6A).
Figure 6. DNA damage checkpoint activation in p21-null and Sox2 overexpressing cells.
(A) Crystal violet staining of murine p53-null MEFs transduced with empty vector (EV) or constructs encoding the indicated factors. Sox2 overexpression does not induce cellular senescence in the absence of p53. Transduction with oncogenic Ras V12 has been included as a positive control. (B) Cellular lysates from NSCs transduced with the indicated constructs were subjected to immunoblotting using the specified antibodies. Sox2 overexpression induced an increase in p53 protein levels. (C) Wild-type or p53-null NSCs were transduced with constructs encoding GFP or Sox2/GFP. Expression of Sox2 decreased the number of secondary neurospheres in the wild-type cells but did not affect the self-renewal of p53-null NSCs. Treatment of Sox2/GFP-transduced cells with the ATM inhibitor KU55933 restored neurosphere formation to the levels found in GFP-transduced cells (n = 3). (D) Assessment in wild-type or p21-null NSCs of p16, and p19Arf mRNA levels by qRT-PCR, showing an increase of endogenous p19Arf expression in p21-deficient cells (n = 3). (E) Levels of immunostaining for p16, p19Arf, and p53 in the nuclei of p21-wild-type and null cells, as measured by high content fluorescence microscopy (n = 3). (F) p21-deficient neurospheres were transduced with control (-), p53 or p16/p19Arf shRNAs. Graph showing the rescue of the growth arrest observed in p21-deficient NSCs by knocking down p16/19 or p53. The inset shows the knockdown efficiency of the indicated shRNAi constructs by qPCR analysis. Data are represented as the average ± s.e.m. of the indicated number of the experiments (n) (*p<0.05).
We also observed an accumulation of the p53 protein in NSCs in response to increased levels of Sox2, as assessed by both high-content immunofluorescence (see below) and immunoblotting analyses (Figure 6B). To test whether p53 was indeed required for the Sox2-induced growth arrest, wild-type or p53-null NSCs were transduced with viruses containing GFP or GFP-Sox2 and FACS-sorted cells were then subjected to neurosphere assays. Importantly, Sox2 overexpression did not affect the self-renewal of p53-null NSCs (Figure 6C). Together, these findings demonstrated that expression of Sox2 above physiological levels induces cell growth arrest in a p53-dependent manner.
It has been reported that oncogene-dependent replicative stress can induce a DSB-initiated DNA damage response (DDR) within a single cell cycle (Bartkova et al., 2006; DiMicco et al., 2006). In agreement with this, oncogene-induced senescence could be bypassed by depletion of the DSB-responsive kinase ATM (Shiloh et al., 2003). Since DSB markers such as 53BP1 foci are elevated on p21-deficient NSC, and to determine whether the DDR was involved in the growth arrest induced by Sox2 overexpression, we tested the effect of KU55933, a specific pharmacological inhibitor of ATM, in the ability of GFP and GFP-Sox2 overexpressing NSCs to form neurospheres. Importantly, and despite a limited toxicity of the drug, Sox2-overexpressing cultures treated with 1 μM KU55933 yielded neurosphere numbers similar to that of GFP controls (Figure 6C). Furthermore, and in agreement with the effects observed on Sox-overexpressing NSCs, permanent inhibition of the ATM kinase by continual treatment of the cultures with 1 μM KU55933 rescued the yield of P3-large neurospheres in p21-null cultures to wild-type values (wild-type: 33.1 ± 5.2; p21-null: 6.7 ± 1.2; p21-null treated with KU55933: 29.0 ± 0.1; n= 2-7).
Expression analysis by qPCR of the products of the Cdkn2a gene in NSCs derived from p21 wild-type or knockout mice, indicated that, while there was no change in the levels of p16 between the two genotypes, the levels of p19Arf mRNA went up by 2-fold in p21-null NSCs (Figure 6D). In addition, high-content microscopy analysis of the cultures revealed a higher amount of p19Arf and p53 proteins per nucleus in p21-null NSCs compared to wild-type controls (Figure 6E). Therefore, we next investigated whether p53 and/or p19 were responsible for the cell growth arrest by knocking down the expression of Cdkn2a or p53 in p21-deficient NSCs. Retroviral delivery of shRNAs targeting p16/p19Arf or p53 to NSCs at passage 2 resulted in a significant reduction of the mRNA levels of these effectors (Figure 6F). Knock-down of either p53 or p16/19Arf in p21-null NSCs did not modify the proportions of cells positive for γH2AX (38.3 ± 2.3 % in cells transduced with empty vector; 30.0 ± 4.0 % with shRNA-p16/19Arf; 32.6 ± 3.2 % with shRNA-p53; n = 3) but restored the yield of large neurospheres to levels that did not differ from wild-type controls (Figure 6F). Therefore, our results suggest that the overexpression of Sox2 found on p21-deficient NSC triggers a p53-dependent cell growth arrest that could be initiated by the DDR and/or the induction of p19Arf expression.
Genomic instability in p21-null cells can be restored by reduction of Sox2 levels
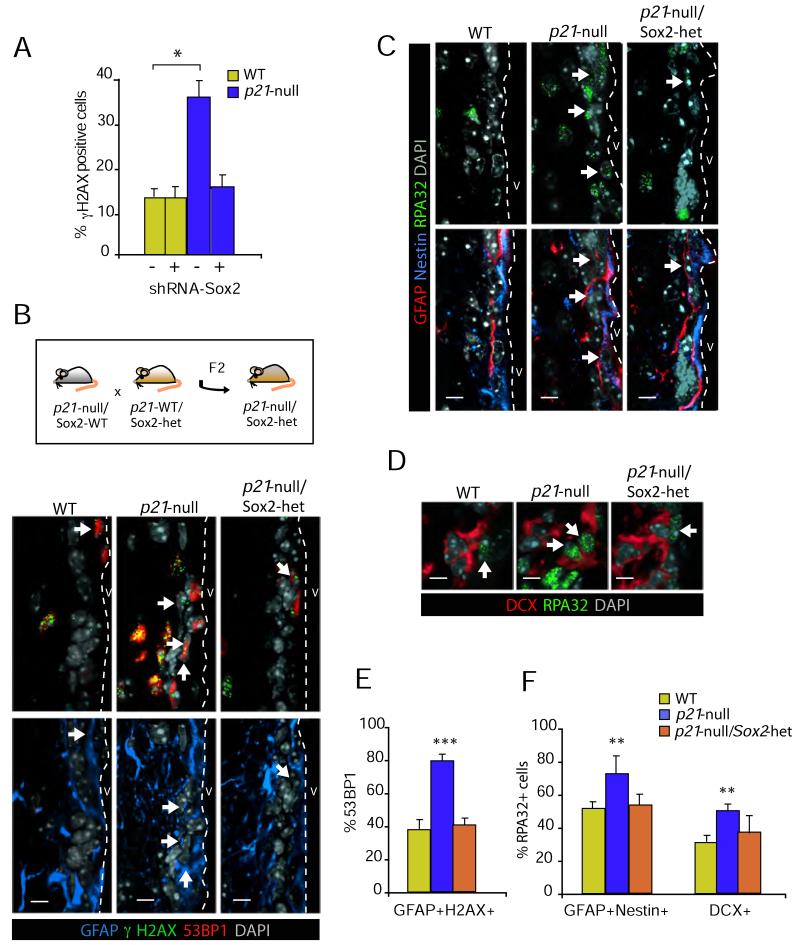
Our results suggested that increased levels of Sox2 in p21-deficient NSCs engage the DNA damage checkpoint leading to a cell growth arrest. We next sought to determine whether the increase in DNA damage markers was causally linked to Sox2 overexpression. For this we first evaluated the levels of γH2AX in p21-deficient cells transduced with the shRNA-Sox2, which restored neurosphere formation to wild-type levels in self-renewal assays (see Figure 2F). Remarkably, the proportion of γH2AX-positive cells was decreased to wild-type values upon reduction of Sox2 levels in vitro (Figure 7A), suggesting that increased levels of this Sox protein activate the DDR in a similar fashion than cells do in response to aberrant expression of oncogenes.
Figure 7. Genomic instability in p21-null cells can be restored by reduction of Sox2 levels.
(A) Lack of p21 leads to an increase in γH2AX staining, which is rescued by Sox2 knockdown in neurosphere cells (n = 3). (B) Immunostaining for GFAP (blue), γH2AX (green) and 53BP1 (red) in wild-type (WT), p21-null or p21-null/Sox2-het mice. White arrows indicate triple positive cells. (C) Immunostaining for GFAP (red) and Nestin (blue) and RPA (green) in wild-type (WT), p21-null or p21-null/Sox2-het mice. White arrows indicate triple positive cells. (D) Immunostaining for DCX (blue) and RPA (green) in wild-type (WT), p21-null or p21-null/Sox2-het mice. White arrows indicate DP cells. (E) Graph showing the proportions of γH2AX and GFAP DP cells that are also positive for 53BP1 in wild type (WT), p21-null or p21-null/Sox2-het mice (n = 3 animals per genotype). (F) Graph showing the proportions of Nestin and GFAP DP cells that are also positive for RPA (left bars) and of DCX-positive neuroblasts that are also RPA-positive in wild-type (WT), p21-null or p21-null/Sox2-het mice (n = 3). Data are represented as the average ± s.e.m. of the indicated number of the experiments (n) (*p<0.05; **p<0.01; ***p<0.001). Scale bars: in B and C, 10 μm; in D, 5 μm
We next set up to obtain physiological data by analyzing whether increased levels of Sox2 were at the basis of the phenotypes observed in the p21-null mice in vivo. To test this we crossed p21 knockout mice with Sox2 heterozygous mice and analyzed the offspring for DNA damage and replicative stress markers in the SEZ. Mice heterozygous for Sox2 and knockout for p21 (p21-null/Sox2-het) showed a clearly reduced expression of Sox2 when examined by immunofluorescence (Figure S7). To test our hypothesis, we decided to concentrate the analysis in the GFAP-positive and GFAP/Nestin DP cell populations. Remarkably, we could observe reduced levels of DNA damage markers and fewer GFAP-positive cells or GFAP/Nestin DP cells that were also positive for γH2AX or RPA foci just by visual examination when Sox2 levels were reduced in a p21-null background (Figure 7B). The signs of replicative stress in GFAP/Nestin cells observed in p21-null mice appeared also alleviated in a Sox2 heterozygous background (Figure 7C). Interestingly, DCX-positive neuroblasts derived from GFAP-positive B cells also displayed signs of replicative stress in p21-null mice, which were reduced to wild-type levels in p21-null/Sox2-het mice (Figure 7D).
Moreover, reduction of Sox2 levels in a p21-null background also decreased the percentages of GFAP cells presenting 53BP1 foci, to levels found in wild-type controls (Figure 7E). Reduction of Sox2 levels also restored the signs of replicative stress observed in p21 mutant mice, as the percentage of GFAP/Nestin DP cells and of DCX-positive neuroblasts that were also positive for RPA foci in p21-null/Sox2-het did not differ from those of wild-type mice (Figure 7F). In conclusion, the results presented here indicate that p21 preserves integrity of the NSC genome by finely modulating the levels of Sox2.
Discussion
The Cip/Kip p21 is a well-known cell cycle regulator and acts as a molecular break inducing a permanent cell cycle arrest (senescence) in response to DNA damage and p53 activation in most primary cells (Besson et al., 2008). Interestingly, p21 appears to mediate a distinct cellular response in stem cell populations. In NSCs and hematopoietic stem cells (HSCs), cell cycle restriction regulated by p21 appears to be critical for self-renewal, as p21-deficient NSCs and HSCs proliferate more actively but become consumed over time (Kippin et al., 2005; Orford and Scadden, 2008). Still, the molecular mechanisms by which p21 regulates stem cell pools have remained largely unknown. Our data support a model in which p21 can regulate NSC self-renewal, at least in part by repressing Sox2 gene expression.
We show that p21 associates to the Sox2 enhancer SRR2 and represses Sox2 gene expression. It has been reported that p21 does not bind directly to regulatory elements but can act as a repressor through binding to transcription factors, such as E2F1, c-Myc or STAT3 (Dotto, 2000; Devgan et al., 2005). Recent data indicated that p27, another member of the Cip/Kip subfamily of CKIs, also acts as a transcriptional repressor through binding to p130-E2F4 complexes; bound p27 is needed for the subsequent recruitment mSin3A, a core protein of a co-repressor HDAC1/HDAC2 histone deacetylase complex (Pippa et al., 2012). Although p21 can interact with E2F-containing complexes, expression of p130 and E2F4 is very low in our proliferating neurospheres and, moreover, p21 does not appear to be required for the binding of mSin3a to the SRR2 enhancer (data not shown), suggesting that p21 actions on Sox2 transcription in NSCs depend on a different molecular mechanism. The specific activity of SRR2 in fetal NSCs is specified mainly by the Sox2 protein itself with the contribution of POU-domain proteins such as Brn-1 and Brn-2 or Oct6, in a similar way to the regulation of this enhancer in embryonic cells by Oct-3/4–Sox-2 complexes (Miyagi et al., 2004). Further work will be necessary to address whether interactions of p21 with any of these transcriptional regulators underlie its role in the repression of Sox2 in murine NSCs.
The work presented here also indicates that: 1) in response to mitogenic stimuli that promote NSC proliferation, p21-deficient neurosphere cultures undergo a cell growth arrest and exhibit signs of DNA damage that are reminiscent of replication stress (RS); 2) Sox2 expression in NSC is repressed by p21 and this correlates with a direct binding of p21 to the Sox2 enhancer; and 3) Sox2 overexpression in NSC leads to an activation of the DNA damage response and, in contrast, Sox2 depletion limits the damage that is observed in p21-deficient NSC, both in vitro and in vivo. The results presented here suggest a model whereby the repression of Sox2 by p21 is essential for maintaining a proper regulation of cell-cycle transitions in NSCs. In the absence of p21, Sox2 overexpression would allow a promiscuous entry in S-phase and therefore RS, which would ultimately limit NSC clonogenic potential. The mechanism by which NSCs undergo p53-mediated senescence in the absence of p21 is still unclear. It has been reported that p53 can induce cell growth arrest in the absence of p21 by increasing the expression of the genes encoding for Gadd45 and 14-3-3 sigma proteins (Taylor and Stark, 2001). However, we could not detect an increase in the expression of these genes in p21-null NSCs (data not shown), suggesting that p53 may act in combination with yet other pathways to induce a growth arrest in biological settings lacking p21.
Normal stem cells are known to accumulate DNA damage during successive replication rounds that in the end compromises stem cell function (Rossi et al., 2007; Nijnik et al., 2007). Moreover, several lines of evidence suggest that stem cells demand an especially tight control of replication to prevent replicative damage. First, embryonic stem cells and iPS cells accumulate copy number variations (CNV) upon serial culture (Narva et al., 2010; Laurent et al., 2011), a type of genomic aberrations that are hallmarks of RS. Second, previous evidences exist to suggest that enhanced expression of stemness factors, including Sox2, in somatic cells generates RS and the activation of a p53- and p21-dependent cell cycle arrest (Marion et al., 2009; Banito and Gil, 2010). Finally, several genomic analyses identified CNV events that were generated de novo during reprogramming, arguing that reprogramming factors can be inducers of RS (reviewed in Blasco et al., 2011). Hence, stemness is a state that is particularly prone to RS, and stemness factors have been shown to actively induce RS in embryonic cells. We here show that deregulated expression of Sox2 is also a potent inducer of RS in NSCs. The presence of H2AX phosphorylation and the accumulation of single stranded DNA observed upon Sox2 overexpression are strong indicators of RS (Toledo et al., 2011).
Regarding the question as to how Sox2 induces RS, it is worth mentioning that Sox2 is a known pro-oncogene (Gangemi et al., 2009) and is highly expressed in brain tumors (Phi et al., 2008). Given that oncogenes are known to induce RS (Bartkova et al., 2006; Di Micco et al., 2006), Sox2-induced RS might easily be related to its oncogenic role. In agreement with this notion, oncogene-induced RS is exacerbated in the context of a deregulated G1/S checkpoint, such as in the absence of p53 (Toledo et al., 2011). Hence, the findings reported here could be explained by a pro-proliferative role of Sox2 in NSC, which generates RS by facilitating promiscuous S-phase entry, and which is limited by p21. Importantly, γH2AX is also seen in vivo in p21 deficient brains, and can be alleviated by the depletion of Sox2. We propose that these observations constitute the first documented in vivo case of pluripotency-induced RS and senescence.
One important difference of our findings with oncogene-induced DNA damage is that the RS observed in NSC is p21-dependent in a p53-independent manner (Besson et al., 2008). We believe that this could be explained by a limited role of p53 in the regulation of the G1/S transition on NSC. In support of this view, and in contrast to what is observed in p21-deficient NSCs, lack of p53 in NSCs does not result in their exhaustion (Meletis et al., 2006). Moreover, whereas p53 deficiency is synthetic lethal with the deletion or hypomorphism of ATR at the organism level (Murga et al., 2009; Ruzankina et al., 2009), this synthetic lethal effect is not seen on neural progenitors of the fetal brain (Lee et al., 2012). Hence, p53 seems to play a limited role in suppressing RS on NSC. Noteworthy, deletion of p21 in leukemic stem cells has also been shown to exacerbate DNA damage independently of p53, which, similarly to our observation on NSC, also ends up limiting the self-renewal of these cancer cells (Viale et al., 2009). In this context, p21 would paradoxically be working as an oncogene, since it extends the proliferative lifespan of cancer stem cells by limiting replication-born genomic damage. Whether this role on leukemic stem cells is also related to the abnormal expression of pro-proliferative stemness factors including Sox2 remains unknown, but in favor of this view, SOX2 amplifications and altered expression have been found in human cancers that are not only of a brain origin and Sox2 expression has been associated with cancer stem cells from the lung (Bass et al., 2009; Maier et al., 2011). New oncogenic roles for cell cycle inhibitors are now being proposed (Besson et al 2008) and, for example, PDGF-induced gliomagenesis is reduced in mice lacking p21 (Liu et al., 2007). To what extent p21 might be particularly important in safeguarding the genome of cancer stem cell in Sox2 overexpressing tumors is an interesting avenue for future research.
Experimental Procedures
Animals
Mice of the p21 (Brugarolas et al., 1995), p53 (Donehower et al., 1992), and Sox2 (Avilion et al., 2003) mutant strains were crossed and maintained at the University of Valencia animal core facility in accordance with Spanish regulations (RD1201/2005).
Cell culture and transduction
Isolation and culture conditions of adult NSCs were performed as described (Kippin et al., 2005; Ferrón et al., 2007). Large neurospheres were selected using a 100 μm cell strainer (BD Falcon). Assessment of self-renewal and differentiation potentials was carried out as described (Andreu-Agulló et al., 2009). Vectors (pRetro Super puro) containing shRNAs for mouse Sox2, and their respective controls, have been described (Banito et al., 2009). shRNAs for p53 and p16/p19Arf were encoded in MLP vectors (Thermofisher). Vectors encoding for mouse Sox2 were gifts from Dr. Kinichi Nakashima. Reporter plasmids TK-Venus and SRR2-TK-Venus were a gift from Dr. Okuda Akihiro and have been previously described (Miyagi et al., 2004). Methods used for retrovirus production, isolation of MEFs and their infection have been described (Banito et al., 2009; Barradas et al., 2009). Transfected 293T cells were washed twice with PBS and then incubated overnight in NSC medium for retroviral production. NSCs were seeded at a 10 cell/μl density the day before infection and were then incubated with a 1:4 dilution of the viral supernatants for 6 hours in the presence of 4 μg/ml of polybrene. After incubation with the retroviral solution, NSCs were washed once with NSC medium, re-plated at the same concentration and cultured for 2 more days before passaging or being used for cell sorting. The ATM inhibitor KU55933 (Calbiochem; White et al., 2008) was used at 1 μM. Incubation for 6 hours with adriamycin (Pfyzer) at 0.5 μg/ml was used as a positive control for γH2AX staining induction. SA-β-galactosidase activity was carried out as described (Banito et al., 2009). NSCs were nucleofected using a Nucleofector (II) (Amaxa Biosystems) following the manufacturer’s recommendations. Briefly, 5×105 NSCs were nucleofected with 2 μg of TK-Venus or SRR2-TK-Venus reporter plasmids together with 2 μg of pcDNA3.1 or pcDNA3.1-p21. The expression of Venus was measured by flow cytometry (see below) 48 hours post-nucleofection.
Flow cytometry and cell sorting
For the infection experiments, transduced GFP positive cells were isolated using a MoFlo cell sorter (Dako). For the reporter assays, 3×103 live cells were collected and only the Venus positive population (about 15 % of the collected cells) was analyzed. For the analysis of apoptosis, NSCs were resuspended in annexin-binding buffer, and stained with 488- or 647- conjugated annexin V and propidium iodide (both from Invitrogen) for 15 minutes in the dark at room temperature. Annexin V/PI and Venus protein fluorescences were measured in a BD FACSCanto II (BD Biosciences) flow cytometer and analyzed using FlowJo V7.6.1 (Tree Star Inc.) software.
Immunocytochemistry
Cells were PFA-fixed for 20 minutes at room temperature and incubated in blocking buffer (PBS containing 10% normal goat serum and 0.2% Triton X-100) for 30 min. Cells were then incubated overnight at 4 °C with the following primary antibodies: rabbit anti-GFAP (1:200; Dako), anti-53BP1 (1:100; Abcam), anti-Sox2 (1:100; Abcam), and anti-p53 (1:100; Novocastra), mouse anti-βIII-tubulin (clone Tuj1; 1:300; Covance) and anti-γH2AX (1:200; Millipore), and rat anti-O4 (1:3; DSHB) and RPA32 (1:200; Cell Signalling). After several washes with PBS, immunoreactivity was detected with Cy3- or Cy2-conjugated appropriate secondary antibodies (1:500) from Jackson InmunoResearch Laboratories; or with biotinylated antibodies (1:300, Vector Laboratories) followed by Cy2-labeled streptavidin (1:200; Jackson InmunoResearch Laboratories). Cells were counterstained with 4′,6′,-diamidino-2-phenylindole (DAPI), washed and mounted with Fluorsave (Calbiochem). For γH2AX, RPA and 53BP stainings, cells were fixed with 1% formaldehyde for 10 minutes and treated with methanol (2 minutes at −20 °C) before incubation with the primary antibodies. Brains from the indicated animals were obtained and sectioned as described (Andreu-Agulló et al., 2009). Sections containing the SEZ were incubated with the following primary antibodies: goat anti-DCX (1:200; Santa Cruz Biotech.) or Sox2 (1:50; R&D), rabbit anti-Ki67 (1:50; Abcam) or 53BP1 (1:100; Abcam), chicken anti-GFAP (1:500; Chemicon), mouse anti-γH2AX (1:100; Millipore) or Nestin (1:150; Abcam), and rat anti-RPA32 (1:200; Cell Signalling). Immunoreactivity was detected as above. High-throughput immunofluorescence was analyzed with an InCell Analyzer 1000 (GE). Image processing and quantification was performed using InCell Investigator software (GE) and at least 1,000 cells were counted in each acquisition. Confocal immunofluorescence images were taken using an Olympus Fluoview FV10i automated scanning confocal system and analyzed with the FV10-ASW 2.1 viewer (Olympus) or the public domain Java image processing program ImageJ. For quantitation of Sox2 expression levels in the GFAP positive cells of the SEZ, the immunofluorescent signal corresponding to Sox2 immunostaining in each GFAP/Sox2 double positive cell was measured as mean pixel (px) density above background (5 px); Sox2 high cells ≥ 50 px; Sox2 dim cells = 4–50 px; Sox2 negative cells ≤ 4 px.
RNA isolation and qRT-PCR analysis
Total RNA was extracted using TRIzol reagent and cDNA synthesised using SuperScript III reverse transcriptase kit (both from Invitrogen). cDNA products were amplified using an Applied Biosystems StepOne plus Fast Real-Time PCR system. Taqman probes for mouse Sox2, p21, p53, Gadd45, Sigma14-3-3, and Gapdh were purchased from Applied Biosystems. Primers used for qPCR assessment of p16, p19Arf, and control β2 microglobulin expressions were: p16F 5′-GTGTGCATGACGTGCGGG-3′, p16R 5′-GCAGTTCGAATCTGCACCGTAG-3′; p19ArfF 5′-GCCGCACCGGAATCCT-3′, p19ArfR 5′-TTGAGCAGAAGAGCTGCTACGT-3′; β2-mglobF 5′-CACCCCCACTGAGACTGATAC-3′, β2-mglobR 5′-TGGGCTCGGCCATACTG-3′.
ChIP analysis and immunoblotting
Chromatin immunoprecipitation (ChIP) procedure on neurospheres has been previously described in detail (Andreu-Agulló et al., 2009). Cells (2×107) of the c17.2 neural stem cell line (Snyder et al., 1992) were fixed in 1% formaldehyde solution in DMEM D5671 medium. After 13 min-incubation, the cross-linking was stopped by the addition of glycine to a final concentration of 0.125 M. Cells were lysed with three cell packed volumes of ChIP buffer (0.5% NP40, 1% Triton X-100, 5 mM EDTA, 50 mM Tris pH 7,4, 150 mM NaCl) containing the protease inhibitors phenylmethylsulfonyl fluoride (1 mM) and cocktail inhibitors (Sigma). Cell lysates were then sonicated to yield chromatin fragments between 500-1000 bp, and pre-cleared chromatin (150μg) was IP with the appropriate antibodies overnight. To elute the immunoprecipitates and reverse the formaldehyde crosslinks, the samples were then incubated overnight at 65 °C with 100 μl of elution buffer (1%SDS, 0.1 M NaHCO3). DNA was purified with the Wizard SV Gel and PCR Clean-Up System. For IPs, 3-5 μg of the following antibodies were used: anti-FLAG clone M2 (Sigma), anti-p21 (ab7960, Abcam), anti-p21 C-19 (SCBT), Quantitative PCR analysis for the ChIP experiments was carried out using the Applied Biosystems StepOne plus Fast Real-Time PCR system and SYBR green Ex-Taq Master Mix (TaKaRa). Primer pairs gave a single product as confirmed by dissociation curve analysis. The primers used to amplify the UTR and SRR2 regions were: R1fwd: 5′-CCCATTTATTCCCTGACAGC-3′ and R1rv: 5′-TGTGATTAGTTTTTGGAAAGG-3′; SRR2fwd: 5′-ATTTATTCAGTTCCCAGTCCAAGC-3′ SRR2rv: 5′-CCCTCTCCCCCCACGC-3′. For immunoblotting, neurospheres were rinsed with PBS, incubated in lysis buffer (20 mM PBS, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM NaF, 1 mM PMSF and Complete Protease Inhibitor Cocktail (Roche) for 15 min and centrifuged at 20,000 g for 15 min at 4 °C. Supernatants were subjected to immunoblotting using the following primary antibodies: rabbit anti-p53 (1:100; Novocastra) and activated caspase 3 (1:100; Cell Signalling), mouse anti-Gapdh (1:1000; Millipore) and goat anti-Sox2 (1:100; R&D).
Statistical analysis
All values are represented as the mean ± SEM. For each experiment, the number of independent assays is indicated as “n”. Differences among means were calculated by two-way Student’s t-test. For relative values, the arcsin transformation was applied to the data to obtain normally distributed values. Significance was set at: *p<0.05, **p<0.01, ***p<0.001. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) software.
Supplementary Material
Acknowledgements
We thank R. Lovell-Badge for kindly providing the Sox2 mouse strain and K. Nakashima and O. Akihiro for generously providing DNA constructs. We are grateful to M.J. Palop for help with the mice colonies and to A. Flores, A. Martínez and D. Martínez for their assistance with flow cytometry and high content cellular analysis. We thank L. Sevilla and J.M. Morante-Redolat for critical reading of the manuscript and valuable discussions. We also thank members of the I.F. lab for helpful discussions. This work was supported by grants from Spanish Ministerio de Ciencia e Innovación (MICINN) (Programa de Biomedicina, CIBERNED, and RETIC Terapia Celular), and Generalitat Valenciana (Programas Prometeo and ACOMP) to I.F.; MICINN and Xunta de Galicia to A.V.; MICINN/Fondo de Investigación Sanitaria to J.T, and Medical Research Council, Cancer Research UK and the Association for International Cancer Research to J.G.; J.T. is a Ramón y Cajal investigator and J.G is a EMBO Young Investigator. M.A.M-T. was funded by a Spanish FPI fellowship of the Ministerio de Educación y Ciencia. E.G-I. is supported by a predoctoral fellowship from Xunta de Galicia. A.B. is funded by the Portuguese Fundaçao para Ciência ea Tecnologia.
Footnotes
Author information
The authors declare no competing financial interests.
References
- Andreu-Agulló C, Morante-Redolat JM, Delgado AC, Fariñas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nature Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram M, Polo J, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banito A, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes & Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banito A, Gil J. Induced pluripotent stem cells and senescence: learning the biology to improve the technology. EMBO Rep. 2010;11:353–359. doi: 10.1038/embor.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani-Yaghoub M, et al. Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Bass AJ, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Serrano M, Fernandez-Capetillo O. Genomic instability in iPS: time for a break. EMBO J. 2011;30:991–993. doi: 10.1038/emboj.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nature Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes & Dev. 2005;19:1485–1495. doi: 10.1101/gad.341405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Dotto GP. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim. Biophys. Acta. 2000;1471:43–56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Ellis P, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Favaro R, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nature Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andäng M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri AL, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Gangemi RM, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisúa-Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes & Dev. 2005;19:756–67. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small increases in the level of Sox2trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- Laurent LC, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Shull ER, Frappart PO, Katyal S, Enriquez-Rios V, Zhao J, Russell HR, Brown EJ, McKinnon PJ. ATR maintains select progenitors during nervous system development. EMBO J. 2012;31:1177–1189. doi: 10.1038/emboj.2011.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Somatic cell type specific gene transfer reveals a tumor-promoting function for p21(Waf1/Cip1) EMBO J. 2007;26:4683–4693. doi: 10.1038/sj.emboj.7601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Wilbertz T, Braun M, Scheble V, Reischl M, et al. SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum Pathol. 2011;42:1078–1088. doi: 10.1016/j.humpath.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Marión RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Miyagi S, et al. The Sox-2 regulatory regions display their activities in two distinct types of multipotent stem cells. Mol. Cell Biol. 2004;24:4207–4220. doi: 10.1128/MCB.24.10.4207-4220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi S, et al. The Sox2 regulatory region 2 functions as a neural stem cell-specific enhancer in the telencephalon. J. Biol. Chem. 2006;281:13374–13381. doi: 10.1074/jbc.M512669200. [DOI] [PubMed] [Google Scholar]
- Miyagi S, et al. Consequence of the loss of Sox2 in the developing brain of the mouse. FEBS Lett. 2008;582:2811–2815. doi: 10.1016/j.febslet.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Murga M, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nature Genet. 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Närvä E, et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nature Biotechnol. 2010;28:371–377. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nature Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Phi JH, et al. Sox2 expression in brain tumors: a reflection of the neuroglial differentiation pathway. Am. J. Surg. Pathol. 2008;32:103–112. doi: 10.1097/PAS.0b013e31812f6ba6. [DOI] [PubMed] [Google Scholar]
- Pippa R, et al. p27(Kip1) represses transcription by direct interaction with p130/E2F4 at the promoters of target genes. Oncogene. 2012;31:4207–4220. doi: 10.1038/onc.2011.582. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, Brown EJ. Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nature Genet. 2009;41:1144–1149. doi: 10.1038/ng.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM, et al. Role of SOX2 mutations in human hippocampal malformations and epilepsy. Epilepsia. 2006;47:534–542. doi: 10.1111/j.1528-1167.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Toledo LI, Murga M, Fernandez-Capetillo O. Targeting ATR and Chk1 kinases for cancer treatment: a new model for new (and old) drugs. Mol. Oncol. 2011;5:368–73. doi: 10.1016/j.molonc.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.