SUMMARY
Remyelination is a regenerative process in the central nervous system (CNS) that produces new myelin sheaths from adult stem cells. The decline in remyelination that occurs with advancing age poses a significant barrier to therapy in the CNS, particularly for long-term demyelinating diseases such as multiple sclerosis (MS). Here we show that remyelination of experimentally-induced demyelination is enhanced in old mice exposed to a youthful systemic milieu through heterochronic parabiosis. Restored remyelination in old animals involves recruitment to the repairing lesions of blood-derived monocytes from the young parabiotic partner, and preventing this recruitment partially inhibits rejuvenation of remyelination. These data suggest that enhanced remyelinating activity requires both youthful monocytes and other factors, and that remyelination-enhancing therapies targeting endogenous cells can be effective throughout life.
INTRODUCTION
Remyelination is a spontaneous regenerative process in the adult CNS that restores saltatory conduction, prevents axonal degeneration, and promotes functional recovery (Duncan et al., 2009; Edgar and Nave, 2009; Smith et al., 1979). Stimulation of remyelination in demyelinating diseases such as MS could alleviate the major underlying causes of disability – impaired conduction by demyelinated neurons and axonal degeneration.
However, like most mammalian tissues, the CNS experiences declining efficiency of regeneration with increasing age (Sim et al., 2002). Reduced remyelination in aged animals occurs in part due to changes in the environmental signals regulating remyelination (Hinks and Franklin, 2000), but also reflects epigenetic changes within aging oligodendrocyte precursor cells (OPCs), which decrease their ability to differentiate into remyelinating oligodendrocytes (Shen et al., 2008; Tang et al., 2000). These age-dependent changes mirror, and may in part determine, a well-recognised feature of many chronically demyelinated MS lesions, which contain oligodendrocyte lineage cells that fail to differentiate into myelinating oligodendrocytes (Kuhlmann et al., 2008).
Recent data suggest that age-associated defects in neural stem cells can be reversed by reactivation of telomerase (Jaskelioff et al., 2010), suggesting that aged OPCs might, in principle, remain competent for efficient remyelination. We therefore investigated whether aged OPCs can indeed be rejuvenated by exogenous factors, reversing the typical age-associated decline in remyelination efficiency.
Using toxin-induced focal demyelination in the mouse spinal cord, together with heterochronic parabiosis (Conboy et al., 2005; Villeda et al., 2011), we tested whether exposure to a youthful systemic environment might improve remyelination by OPCs in the aged CNS. We chose this experimental system for several reasons. First, acute toxin-induced demyelination is better suited to studying the regenerative biology of demyelination than immune-mediated models that are complicated by autoimmunity.
Second, because effective remyelination is generally associated with acute demyelination, it is likely that chronic demyelination arises as a consequence of deficiencies in the regenerative response; thus, age-associated delays in remyelination in an acute experimental demyelination model reflect a possible basis for the evolution of chronic demyelination in MS (Confavreux and Vukusic, 2006; Goldschmidt et al., 2009). Finally, use of the parabiotic system uniquely tests the relevance of systemic factors to regeneration in the CNS by permitting exposure of aged tissues to blood-borne factors at physiologically relevant levels.
RESULTS
Exposure to a youthful systemic environment enhances remyelination in aged animals
To examine the impact of the systemic environment on remyelination efficiency after spinal cord demyelination, we surgically joined aged mice to isogenic or congenic young animals through heterochronic parabiosis. Three weeks after animals were parabiotically joined, demyelination was induced in the spinal cord of the old partner by focal injection of the demyelinating toxin lysolecithin. To control for possible effects of the parabiotic condition itself, heterochronic animals always were compared to isochronic pairs (young mice joined to young partners, or old mice joined to old partners).
Chimerism in parabiotic pairs and accessibility of experimentally-induced lesions to circulating blood cells was evaluated using pairs in which one partner was transgenically marked by expression of green fluorescent protein (GFP). Cross-circulation was confirmed by flow cytometry at the time of sacrifice and revealed the expected mixing of GFP− and GFP+ cells in the spleens of each partner (Fig. S1A, B) (Wright et al., 2001). GFP+ cells also were detected in the lesions of old WT partners at multiple post-lesion timepoints (Fig. S1C). Together with the extravasation of fibrinogen and IgG at the lesion site (Fig S1D), these data point to disruption of the blood-spinal cord barrier in this model, as has been observed in MS. Thus, the spinal cord lesions of old animals in heterochronic pairings were exposed to both circulating cells and soluble factors derived from the young partner.
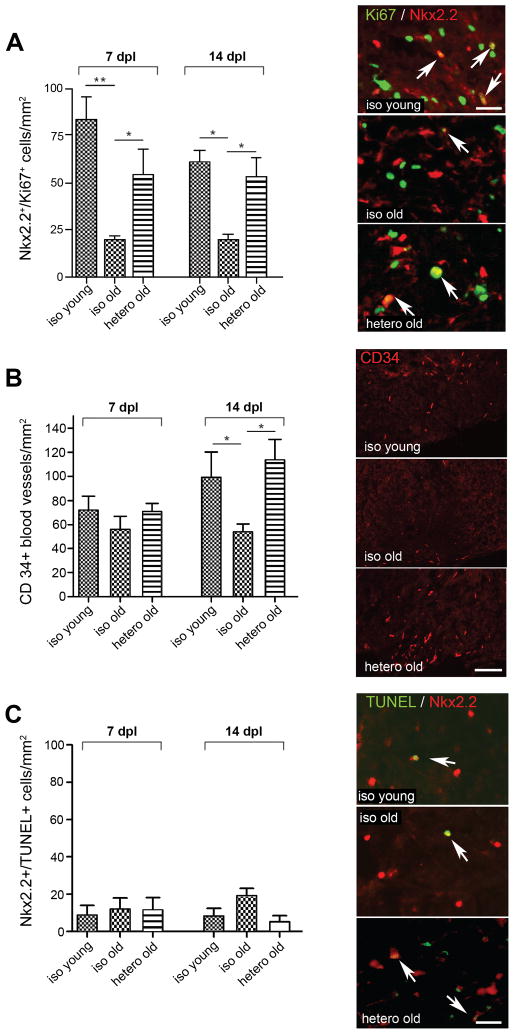
To ascertain the impact of exposure to youthful blood-borne factors for remyelination activity in aged partners, we first compared lesions at 7 days post lesion (dpl), when the lesions contain an expanding population of OPCs but no oligodendrocytes, and at 14 dpl when new OPC-derived oligodendrocytes appear. The numbers of proliferating OPCs were identified by co-expression of the OPC transcription factor Nkx2.2 and the proliferation marker Ki67. The density of proliferating OPCs was significantly increased in heterochronic-old animals compared to isochronic-old controls at 7 and 14 dpl (Fig. 1A). The enhanced OPC proliferation was associated with a significant increase in the density of CD34+ endothelial cells in heterochronic-old lesions at 14 dpl (Fig. 1B): vascular endothelial cells have previously been shown to induce OPC proliferation (Arai and Lo, 2009). Reactive astrocytosis was not different between the three parabiotic conditions (Fig. S1E). OPC apoptosis, measured by co-labeling of Nkx2.2+ cells with terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL), was not significantly different in heterochronic-old animals compared to isochronic-old controls at either 7 or 14 dpl (Fig. 1C).
Figure 1. Heterochronic parabiosis stimulates OPC proliferation and angiogenesis.
(A) Proliferating OPCs (Nkx2.2+/Ki67+), (B) CD34 blood vessels and (C) apoptotic OPCs (Nkx2.2+/TUNEL+) within lesions at 7 and 14dpl. Arrows indicate co-localisation. N ≥ 4 pairs were analyzed for each condition and timepoint. Quantified data are presented as means ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s post test. *denotes p < 0.05, **denotes p < 0.001. Scale bars: A,C 20 μm, B 125 μm. See also Fig. S1.
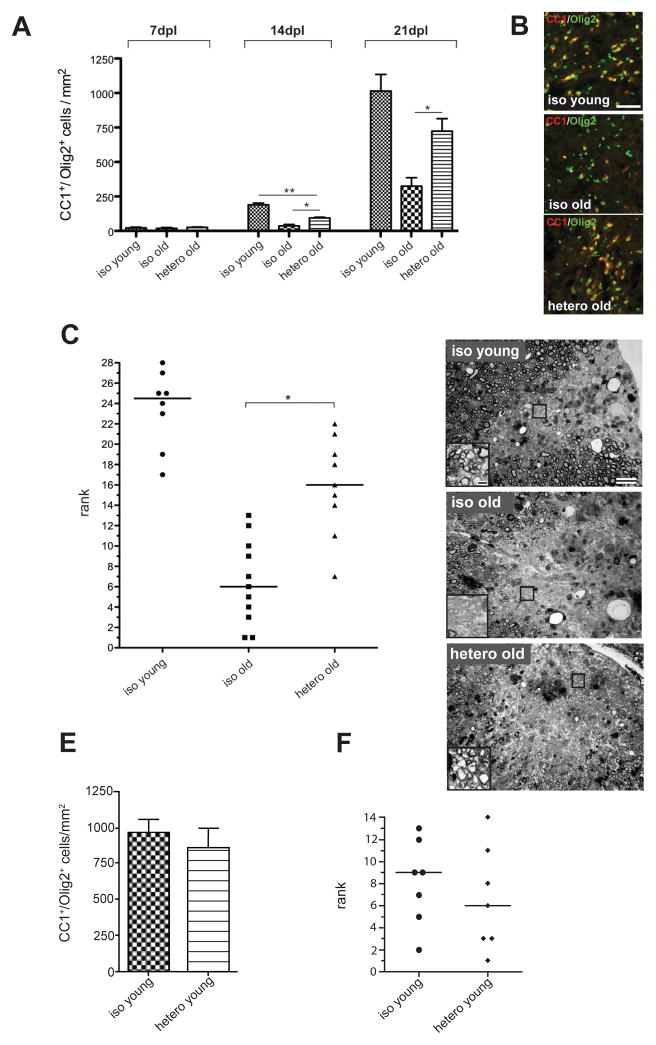
We next determined the density of Olig2+/CC1+ mature oligodendrocytes in heterochronic-old lesions compared to isochronic-old controls, and found it significantly increased at 14 and also 21 dpl, when in young animals remyelination is complete (Fig. 2A, B). At 21 dpl, the prevalence of mature oligodendrocytes in heterochronic-old lesions was equivalent to Olig2+/CC1+ cell densities in the isochronic-young group. These results were confirmed using antibodies to transferrin, an independent marker of oligodendrocytes (Connor and Fine, 1986)(Fig. S2A). To test whether the enhanced production of Olig2+/CC1+ mature oligodendrocytes in heterochronic-old lesions leads to increased remyelination, we used histological analysis of semi-thin resin sections to assess differences remyelination in all three groups at 21dpl. This technique reliably detects the characteristically thin myelin sheaths that identify remyelination, and revealed that remyelination improved significantly in heterochronic-old animals as compared to isochronic-old controls (Fig. 2C, D). Lesion volume in heterochronic animals did not differ from lesion volume in isochronic-old animals (Fig. S2B), indicating that the parabiotic enhancement of remyelination was not attributable to differences in lesion size. Together, these data demonstrate that exposure of aged animals to a youthful systemic environment increases angiogenesis at the site of demyelinated lesions, promotes OPC proliferation, and reverses the age-associated differentiation block of OPCs, restoring the ability of these cells to form mature, remyelinating oligodendrocytes to levels indistinguishable from young animals.
Figure 2. Heterochronic parabiosis stimulates OPC differentiation and remyelination.
(A) Quantification of oligodendrocytes shows few CC1+/Olig2+ cells present in the lesion at 7dpl, independent of age. Higher oligodendrocyte densities at 14dpl indicates the generation of new oligodendrocytes, with significantly more differentiation occurring in heterochronic-old animals than in isochronic-old controls at 14 and 21dpl. Data are means ± SEM. (B) Oligodendrocytes in the lesion at 14dpl. Representative fields are shown for each parabiotic condition. (C) Ranking analysis of remyelination at 21dpl (rank 1 equals least remyelination). N ≥ 8 per group, horizontal line indicates median for each data set. Data analyzed by Kruskal-Wallis test followed by Dunn’s post test (D) Toluidine blue stained resin sections of lesions: representative images for each parabiotic condition at 21dpl. Widespread remyelination is evident in lesions in isochronic-young animals, contrasting with the relative paucity of remyelination in lesions of isochronic-old animals. Heterochronic-old animals showed increased remyelination compared to isochronic-old controls. Insets show magnification of boxed area. (E) Quantification of oligodendrocyte (Olig2+/CC1+) density in lesions of isochronic-young and heterochronic-young animals at 21dpl (n=4 animals per group). Data are means ± SEM and were analyzed with an unpaired two-tailed t test. No significant difference between the two groups was found. (F) Ranking analysis of remyelination at 21dpl (n=7 animals per group). Rank 1 equals least remyelination. The median is indicated by a horizontal line for each data set. Data were analyzed with a Mann-Whitney test, which did not detect a significant difference between the two groups. *denotes p < 0.05, **denotes p < 0.001, ns = not significant. Scale bars: B 50 μm, D 20μm (insets 2 μm)). See also Fig. S2.
The young systemic environment enhances the remyelinating function of endogenous, aged OPCs
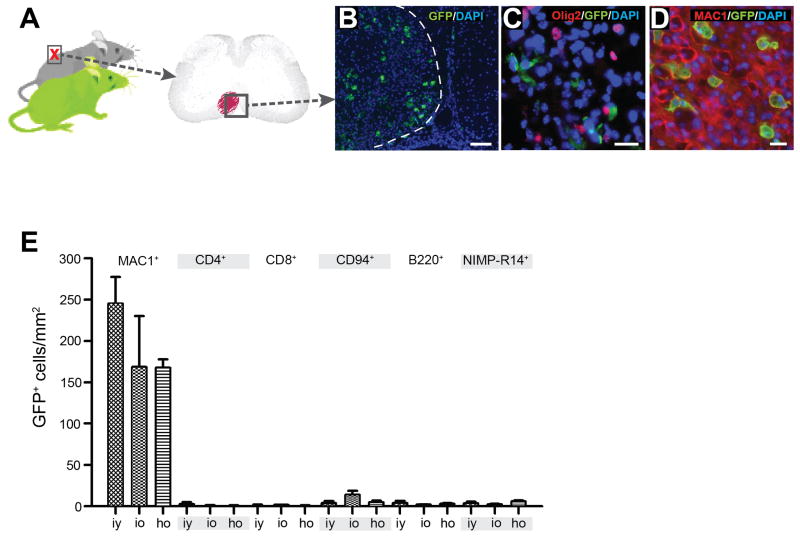
In heterochronic GFPyoung/WTold pairs, GFP+ cells were concentrated within the lesioned area of the spinal cord (Fig. 3A, B). We found no co-localisation of the oligodendrocyte lineage marker Olig2 with GFP+ cells in the lesions of old partners (Fig. 3C), indicating that enhanced remyelination did not result from engraftment of young OPCs, but rather was mediated by endogenous old OPCs, whose differentiation capacity was restored by exposure to a young systemic environment. In addition, to clarify whether enhancement of OPC differentiation in old animals exposed to a young systemic environment reflects a positive influence of the young environment, or dilution of negative inputs from the aged environment, we next asked whether OPC differentiation might be impaired in a young mouse exposed to an old circulation. Demyelinating spinal cord lesions in young mice parabiotically joined to old partners revealed no impairment of OPC differentiation (Fig. 2E) or remyelination (Fig. 2F) compared to isochronic-young control mice. Thus, in contrast to a recent report indicating the existence of age-upregulated systemic factors that actively suppress neurogenesis in the CNS (Villeda et al., 2011), exposure of young OPCs to an aged systemic environment does not dominantly impair regenerative function in young mice.
Figure 3. Engrafted cells in the spinal cord lesions of parabionts are chiefly young-partner derived macrophages.
(A) Parabiotic pairings in which cells of the young partner were transgenically labelled by GFP expression were established. In the old partner lesion from each GFPyoung/WTold pairing, GFP+ cells were confined to the lesion (B). (C) No GFP+ cells in old partner lesions were found to co-localize with Olig2+ nuclei. (D) Almost all GFP+ cells co-localized with the macrophage marker MAC1. (E) Characterization of the GFP+ inflammatory infiltrate at 5dpl in isochronic-young (iy) (n=3), isochronic-old (io) (n=2) and heterochronic-old (ho) animals (n=4). No statistically significant differences were found amongst the parabotic groups. The overwhelming majority of GFP+ cells were MAC1+ macrophages. Very few CD4+ T cells, CD8+ T cells, CD94+ natural killer cells, B220+ B cells and NIMP-R14+ neutrophils were present in the lesions. Data are means ± SEM. Scale bars: B 100 μm, C,D 20 μm.
Monocytes from the young partner are critical for rejuvenation of remyelination
Analysis of the GFP+ cells within the lesions of old partners in heterochronic pairings revealed them to be almost exclusively MAC1+ macrophages (Fig. 3D, E). Very small numbers of GFP+/CD4+ and GFP+/CD8+ T lymphocytes, GFP+/CD94+ natural killer cells, GFP+/B220+ B cells and GFP+/NIMP-R14+ neutrophils were also present (Fig. 3E). The inflammatory infiltrate derived from the parabiotic partner did not differ in extent or composition (Fig. 3E) among isochronic-young, isochronic-old and heterochronic-old animals at 5 dpl. In addition, the mean densities of partner-derived GFP+ macrophages within the lesions (~200 cells/mm2) were comparable to those reported in a parabiosis model of autoimmune spinal cord demyelination (Ajami et al., 2011).
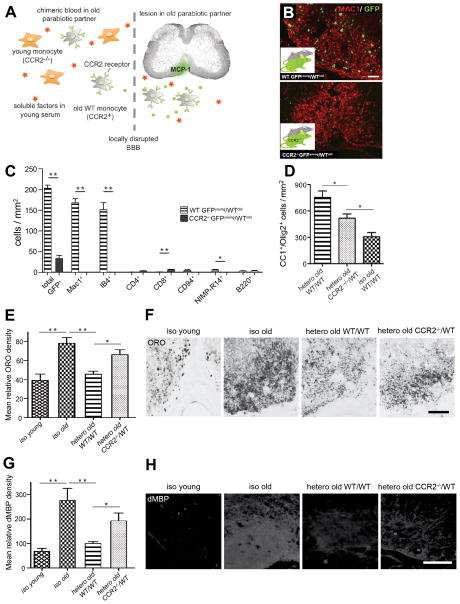
Because the innate immune system (principally macrophages) has been suggested to stimulate remyelination (Kotter et al., 2006; Shechter et al., 2009), we hypothesized that the young blood-derived macrophages recruited to the lesion might play a role in the rejuvenation of remyelination via heterochronic parabiosis. Monocytes/macrophages are recruited from the circulation to sites of inflammation by chemotaxis in response to gradients of MCP-1, which binds the chemokine receptor CCR2 (Charo and Ransohoff, 2006). Given this critical role of CCR2 in macrophage recruitment (Boring et al., 1997; Kurihara et al., 1997), we examined remyelination in single young CCR2-deficient mice and found a significant reduction in CC1+/Olig2+ cells at 21 dpl (Fig. S3A), thereby confirming a role for recruited monocytes in remyelination. We next used CCR2-deficient, GFP-expressing mice as young partners in heterochronic parabiotic pairings. This system specifically blocked the recruitment of young macrophages to lesions in old partner animals (Fig. 4A, B), but did not affect cross-circulation, as measured by splenic chimerism, in either isochronic or heterochronic pairs (Fig. S3B). Recruitment of GFP+ non-macrophage cells, which was very low to begin with, was largely unaffected by CCR2-deficiency of the young partner (Fig. 4C), and aged CCR2+ monocytes were still recruited to the lesion from the chimeric circulation.
Figure 4. Young macrophages play a central role in the rejuvenating effect of heterochronic parabiosis.
(A) Experimental rationale: in CCR2−/− young/WTold parabionts, only old monocytes are recruited to the old partner lesion in response to MCP-1 signalling. Soluble factors retain access to the lesion by virtue of local disruption of the blood-spinal cord barrier (BBB). (B) Immunostaining for GFP and MAC1 in heterochronic WTyoung/WTold and CCR2−/− young/WTold pairs demonstrates the absence of donor-derived macrophages in CCR2−/− young/WTold lesions. (C) Comparison of the inflammatory infiltrate at 5 dpl shows that recruitment of donor-derived MAC1+ IB4+ macrophages predominates in old WT mice paired with young WT mice (n=3) and is abolished in old WT mice paired with young CCR2−/− mice (n=4, white bars). There are small numbers of CD4+ T cells, CD8+ T cells, CD94+ natural killer cells, B220+ B cells and NIMP-R14+ neutrophils in the lesions of both pairings. (D) Oligodendrocyte (CC1+/Olig2+) density at 21 dpl in CCR2−/− young/WTold pairs was significantly reduced compared to heterochronic old WT pairs, but elevated compared to isochronic-old controls (n=4 pairs per condition). Data analyzed by one-way ANOVA followed by Dunnett’s post test (E,F) Oil Red O staining revealed significantly more lipid-rich myelin debris in the lesions of isochronic-old pairings than in isochronic-young pairings, consistent with the notion that myelin debris have an inhibitory effect on remyelination. Oil Red O staining in heterochronic old WT lesions was significantly lower than in isochronic old controls. This enhanced myelin debris clearance observed in heterochronic pairings was attenuated when the young partner was CCR2-deficient. Note that the staining appears less diffuse in the isochronic young and the heterochronic groups, suggesting that more myelin debris has been taken up by phagocytic cells. (G,H) Similar observations were made for intralesional degenerated myelin basic protein accumulation. *denotes p<0.05; ** denotes p<0.001. Data are means ± SEM. Scale bars: B 50 μm E,G 100 μm. See also Fig. S3 and Fig. S4.
We found that the density of mature oligodendrocytes was decreased significantly in lesions of old WT animals paired to young CCR2-deficient animals, as compared to old animals paired with young WT partners (Fig. 4D). However, compared to isochronic-old controls, heterochronic-old animals, in which the recruitment of young macrophages was abolished by CCR2 deficiency (Fig. 4C), exhibited only partial inhibition of the rejuvenating effect of heterochronic parabiosis on OPC differentiation (Fig. 4D). Thus, this strategy represents a useful “cell deletion system” that provides clarification of the role of young monocytes, the major population of recruited young-partner cells, in the rejuvenation of remyelination.
Young macrophages enhance remyelination via myelin debris clearance
Given the importance of youthful macrophages in stimulating remyelinating activity in aged animals, we next asked how these cells might accomplish this effect in old lesions. We first examined whether young macrophages might produce factors that directly affect OPC function by analysing the expression of growth factors previously implicated in OPC proliferation and maturation (Mason et al., 2003; Vana et al., 2007). However, analysis of bone marrow-derived macrophages harvested from young-isochronic, old-isochronic, or heterochronic animals revealed no apparent differences in the production of a number of key OPC-regulatory growth factors (including IGF-1 and PDGF-1A) in any of the experimental conditions (Fig. S4A, B). Similarly, because immune-modulatory cytokines previously have been implicated as systemic regulators of tissue repair in the skeletal muscle and CNS (Brack et al., 2007)(Villeda et al., 2011), we undertook an array-based comparison of 97 cytokines in the serum of isochronic-old, heterochronic-old (WT/WT) and heterochronic-old paired with young CCR2-deficient animals (CCR2−/−/WT). This comparison yielded surprisingly few differences (Fig. S4C-G), suggesting that modulation of factors within the local microenvironment of the lesion exert a greater influence on remyelination than alterations in levels of specific circulating factors in the bloodstream.
We next considered whether differences in the phagocytic activity of young versus old macrophages might contribute to their differences in stimulating OPC function. Myelin contains proteins that inhibit OPC differentiation, and previous studies have highlighted the importance of macrophage-mediated removal of myelin debris in remyelination (Baer et al., 2009; Kotter et al., 2006). The efficiency of lipid-rich myelin debris clearance is reflected by the amount of detectable lipid within the lesion area. Staining of lesions with Oil-Red O revealed that lipid levels in the lesions of old partners involved in heterochronic parabiosis were similar to those in young isochronic pairs, and significantly reduced when compared to those in old isochronic pairs (Fig. 4E, F). The enhancement of myelin debris clearance after heterochronic pairing was substantially attenuated in pairings involving CCR2-deficient young partners (Fig. 4E, F). Similar results were obtained using antibodies to a degenerate form of the myelin protein myelin basic protein (MBP) (Fig 4G, H). Together, these data are consistent with a model in which young macrophages facilitate OPC differentiation and remyelination by augmenting the clearance of inhibitory myelin debris.
DISCUSSION
These experiments provide proof-of-principle that, despite their intrinsic alterations, aged OPCs remain responsive to exogenous pro-differentiation signals and retain their competence for efficient repair. These data further indicate that signals from the systemic environment can override age-related, intrinsic changes in OPCs, suggesting that these changes are predominantly epigenetic. This finding has fundamental implications for strategic approaches to remyelination-enhancing therapies, validating endogenous OPCs – even when aged, as they are likely to be in many MS patients – as a pharmacological target to stimulate remyelination.
Although the full cast of characters involved in the rejuvenation of aged OPCs has yet to be identified, our data implicates a cell-based mechanism, in which young macrophages recruited during the early phase of remyelination alter OPC survival, proliferation, and differentiation to promote a more youthful remyelination response. Central to this function is the greater capacity of young macrophages than old macrophages for efficient clearance of myelin debris. This finding is consistent with prior observations that transplanted young macrophages are more effective than old macrophages at improving wound repair in old animals (Danon et al., 1989). Moreover, this work demonstrates that the CNS maintains its responsiveness to age-regulated circulatory factors, such that age-dependent deficiencies in repair of these tissues can, in part, be reversed by circulating factors.
EXPERIMENTAL PROCEDURES
Animal procedures
C57BL/6(‘wildtype’, WT) mice, C57BL/6-UBC-GFP transgenic mice and CCR2−/− mice on the C57BL/6 background were obtained from Jackson Laboratories (US). Parabiotic pairs with a GFP+ partner were generated with either C57BL/6-UBC-GFP transgenic mice or C57BL/6-β-actin-EGFP transgenic mice. CCR2−/− GFP+ mice were generated by crossing the CCR2−/− with ubc-GFP transgenic mice. For chimerism analysis, CCR2−/− mice were joined to B6.SJL mice (a congenic strain on the C57BL/6 background, carrying the CD45.1 allele). Parabiotic pairs were joined as previously described (Wagers et al., 2002). A focal demyelinating spinal cord lesion was induced as previously described (Woodruff et al., 2004). See supplementary methods for details.
Flow cytometry analysis of cross-circulation
Spleens from both partners of GFP+/WT parabiotic pairs were removed and homogenised, and red blood cells lysed. Cells were mixed with propidium iodide (PI) solution to label dead cells, which were excluded from analysis. Samples were subjected to flow cytometry and analyzed using FlowJo software (TreeStar, US).
Immunohistochemistry and quantification of immunolabelling
For staining with the anti-Olig2, anti-Ki67 and anti-NKx2.2 antibodies, antigen retrieval was performed. For staining with the mouse primary antibody anti-Nkx2.2, the Mouse-on-Mouse immunodetection kit (Vector laboratories, BMK-2202) was used with additional labelling with other antibodies or TUNEL. For CD34 immunostaining, slides were pretreated with ice-cold methanol for 10min before blocking. Nuclei were counterstained with DAPI. Cells of interest were counted in three different sections for each animal. To account for the varying size of the lesion in different sections, cell densities were calculated. A list of the antibodies used is provided in the supplementary methods.
Ranking analysis of remyelination
Semi-thin sections (1 μm) cut from resin-embedded tissue were stained with Toluidine Blue and blindly ranked according to the extent of remyelination as previously described (Arnett et al., 2004). Lesion volume measurements are described in the supplementary methods.
Cytokine Array
Blood samples were collected from each partner of the parabiotic pair directly before sacrifice. The RayBio Mouse Cytokine Antibody Array 6 (RayBiotech, Inc) was used to detect the presence or absence of 97 cytokines in each serum sample.
Statistics
All statistical analysis was performed in GraphPad Prism (GraphPad Software, Inc.) and differences were considered significant at p<0.05 (see supplementary methods for details).
Supplementary Material
Acknowledgments
We thank G. Buruzula and J. LaVecchio at Joslin’s HSCI/DERC Flow Cytometry Core for their excellent technical support. This work was supported in part by grants from the European Leukodystrophy Association (ELA) (to RJMF and AJW), Research into Ageing, the UK Multiple Sclerosis Society and National Multiple Sclerosis Society (to RJMF), and the Burroughs-Wellcome Fund, Harvard Stem Cell Institute, British Consulate General Science and Innovation Network, and National Institutes of Health 1 DP2 OD004345-01 (to AJW). PvW was supported by the National Health and Medical Research Council of Australia. JMR was supported by a PhD studentship from the Medical Research Council, UK.
References
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FMV. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett HA, Fancy SPJ, Alberta JA, Zhao C, Plant SR, Raine CS, Rowitch DH, Franklin RJM, Stiles CD. The bHLH transcription factor Olig1 is required for repair of demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Baer AS, Syed YA, Kang SU, Mitteregger D, Vig R, ffrench-Constant C, Franklin RJM, Altmann F, Lubec G, Kotter MR. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain. 2009;132:465–481. doi: 10.1093/brain/awn334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129:595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- Connor JR, Fine RE. The distribution of transferrin immunoreactivity in the rat central nervous system. Brain Res. 1986;368:319–328. doi: 10.1016/0006-8993(86)90576-7. [DOI] [PubMed] [Google Scholar]
- Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A. 1989;86:2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci U S A. 2009;106:6832–6836. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, Nave KA. The role of CNS glia in preserving axon function. Curr Opin Neurobiol. 2009;19:498–504. doi: 10.1016/j.conb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Goldschmidt T, Antel J, Konig FB, Bruck W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJM. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16:542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2010;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJM. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss V, Li J, Dupree J, Franklin RJM, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors: a critical determinant of remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–396. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Raff MC. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol. 2000;148:971–984. doi: 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vana AC, Flint NC, Harwood NE, Le TQ, Fruttiger M, Armstrong RC. Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J Neuropathol Exp Neurol. 2007;66:975–988. doi: 10.1097/NEN.0b013e3181587d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, Franklin RJM. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.