Abstract
Handedness is most often measured by questionnaires that assess an individual’s preference for using a particular hand to perform a variety of tasks. While such assessments have proved reliable, they do not address the underlying neurobehavioral processes that give rise to the choice of which hand to use. Recent research has indicated that handedness associated with hemispheric specializations for different aspects of sensorimotor performance. We now hypothesize that an individual’s choice of which hand to use for a given task should result from an interaction between these underlying neurobehavioral asymmetries with task conditions. We test this hypothesis by manipulating two factors in targeted reaching movements: 1) Region of workspace and 2) visual feedback conditions. The first manipulation modified the geometric and dynamic requirements of the task for each arm, whereas the second modified the sensorimotor performance asymmetries, an effect predicted by previous literature. We expected that arm choice would be reflected by an interaction between these factors. Our results indicated that removing visual feedback improved the relative performance of the non-dominant arm and increased the choice to use this arm for targets near midline, an effect that was enhanced for targets requiring larger movement amplitudes. We explain these findings in the context of the dynamic dominance hypothesis of handedness and discuss their implications for the link between hemispheric asymmetries in neural control and hand preference.
1. Introduction
Previous research has established that some 90 percent of all humans prefer the right hand for most unimanual tasks (Annett, 1972; Corballis, 1997). Perhaps unsurprisingly, then, handedness has often been described as a preference to use a particular hand (Oldfield, 1971; Bryden, 1977). Moreover, it has been suggested that this preference might be based on early life experiences, such as parental modeling of right-handed behavior and asymmetric handling of infants (Hepper et al., 2005; see Sainburg, 2010 for review). Other research has emphasized a strong role of genetics in determining handedness in humans (Annett, 1972; Levy and Nagylaki, 1972; McMannus, 1985; Klar, 1996), and in primates (Hopkins et al., 1994). While the nature/nurture debate regarding the origin of handedness remains unresolved (see Schaafsma et al., 2009 for review), it has become clear that handedness is associated with specific hemispheric specializations that impart different, and complementary advantages in control to each arm. Specifically, we have provided evidence that the dominant arm is specialized for predictive control of limb and task dynamics, which can result in precise and energetically efficient coordination patterns (Sainburg and Kalakanis, 2000; Bagesteiro and Sainburg, 2002; Sainburg, 2002; Sainburg and Schaefer, 2004; Sainburg, 2005; Wang and Sainburg, 2007; Shabbott and Sainburg, 2008; Yadav and Sainburg, 2012; Przybyla et al., 2012). The non-dominant arm appears specialized for stabilizing performance through impedance mechanisms, a less efficient but more robust control strategy (Bagesteiro and Sainburg, 2003; Ghez et al., 2007; Duff and Sainburg, 2007; Schabowsky et al., 2007). Our understanding of these specializations has recently been extended to the hemispheres through studies in unilaterally brain-damaged adults (Schaefer et al, 2007; Haaland et al., 2009; Schaefer et al., 2009; Mutha et al, 2011; Mani et al., 2012). We now hypothesize that the choice of which hand to use for a particular task should reflect an interaction between these underlying specializations and task conditions. Therefore, one should not always be expected to use one arm, but rather individuals should tend to choose the arm that is more proficient for the task conditions, at hand. While previous research on handedness has either focused on identifying sensorimotor performance asymmetries or on limb selection choices, very few have assessed both of these phenomena in single reports. Several studies have focused on determining the neuromuscular control variables that lead to reliable interlimb performance asymmetries (Flowers 1975; Todor and Kyprie, 1980; Roy and Elliott, 1986; Carson et al. 1990; Carson et al., 1992; Elliott et al., 1994; Elliot et al., 1995; Carson et al., 1995). These studies have assumed, on the basis of previous research (e.g. Oldfield, 1971; Bryden, 1977), that right-handers use the right-hand in nearly all situations. Some support for this assumption has come from the finding that right-handers continue to use the dominant arm, even when doing so requires awkward postures (Bryden et al., 1994). The more recent research by our laboratory has used empirical and computational methods to dissociate two control mechanisms that are associated with dominant and non-dominant arm performance (for example Sainburg and Schaefer, 2004; Yadav and Sainburg, 2011). As mentioned above, the respective mechanisms can be described as predictive control of task dynamics and robust stabilization of performance, through impedance control. Research in stroke patients with specific unilateral lesions has confirmed that these processes are indeed specialized to different hemispheres (Schaefer et al., 2007; Haaland et al., 2009; Schaefer et al., 2009; Mutha et al., 2011; Mani et al., 2012).
A different line of research has focused on studying handedness through assessing the choices people make about which hand to use. For example, Annett (1970) asked subjects to indicate the hand (left, right, or either) with which they typically performed tasks such as writing, throwing, swinging a racquet, and hammering a nail. Subjects then used each hand to move a series of pegs from one row of holes to another as quickly as possible. Results showed that differences between hands in the mean time performance correlated with choice. More recent research has reported similar results using peg-moving and other tasks, such as finger-tapping and pen-dotting (Steenhuis and Bryden, 1989; Bishop et al., 1996; Bryden and Roy, 2005). These findings could be taken to suggest that hand choice depends in some way on performance differences between the hands. However, these studies may be limited by problems inherent to the subjective assessment of hand-preference. Such problems can stem from subjects misinterpreting the questions, having difficulty imagining themselves performing the tasks, or basing reports on faulty memories of how they perform common tasks (Brown et al., 2006). The focus of this study is to bring together these two lines of research, studies of arm selection and studies of arm performance in order to assess how arm selection might depend upon asymmetries in arm performance.
We reasoned that if these two aspects of handedness are related, then the choices that individuals make about which hand to use should reflect an interaction between performance asymmetries and task demands. Indeed, Stoloff et al. (2010) recently showed that reinforcements concerning the success of each hand can affect hand selection. Thus, the probability of task success (that was artificially manipulated) influenced the likelihood of choosing a particular hand for a reaching task. Our study is inspired by this result, but extends the question: Is limb selection dependent on an interaction between sensorimotor performance asymmetries and task demands? Our approach to manipulating sensorimotor performance asymmetries was to allow or prevent visual feedback during movements. We reasoned that the non-dominant left arm has been shown to have equal or greater accuracy compared to the dominant right arm without vision, but worse accuracy when vision is available (Guiard, et al., 1983; Carson et al., 1990; Imanaka, et al., 1995; Bagesteiro and Sainburg, 2002; Sainburg, 2002; Sainburg & Wang, 2002; Lenhard and Hoffmann, 2007; Wang and Sainburg, 2007; Goble and Brown, 2008;). Thus, manipulating visual feedback allowed us to manipulate the relative performance advantages between the arms. In fact, our findings confirmed an interaction between hand and vision conditions, such that the non-dominant left arm was more accurate under no-vision conditions, and the dominant right arm was more accurate under vision conditions. This success allowed us to ask whether limb choices are linked to these feedback-dependent differences in sensorimotor performance asymmetry.
We thus manipulated sensorimotor conditions (visual feedback) to assess the effects on reaching performance and then checked for corresponding changes in hand choice. We predicted that the relative choice to use the left non-dominant arm should increase under no-vision conditions, when this arm’s relative performance is enhanced. In addition, we expected this effect to be modulated by movement distance, which increases the index of difficulty of the task (Fitts, 1954). To test our predictions, we relied on one of the few well-established patterns of arm selection. It has repeatedly been shown that right-handers display an asymmetric distribution of dominant and non-dominant reaches across the workspace, preferring dominant reaches to targets located in the right and middle areas of the workspace and also to targets just left of the body-midline (Gabbard and Rabb, 2000; Bryden et al., 2000; Stins et al., 2001; Mamolo et al., 2004; Gabbard and Helbig, 2004; Helbig and Gabbard, 2004; Bryden and Roy, 2006). It has further been estimated that the threshold in the left workspace at which righthanders switch from using dominant reaches to non-dominant reaches (hereafter called the “switch-point”) is located approximately 20° left of body-midline (see Gabbard and Rabb, 2001 for review). The reliability of this finding provided an opportunity for us to test whether conditions that change the relative performance of the limbs may also change patterns of arm selection.
2. Experimental Procedures
2.1 Subjects
Forty-eight (24 females) neurologically healthy, young (18–34 years of age) volunteers were recruited from the Pennsylvania State University community. Each subject signed a consent form approved by the Pennsylvania State University Institutional Review Board. The experiment was conducted in accordance with ethical guidelines set forth in the Declaration of Helsinki. We ensured that all our subjects were strongly right-handed by using an inclusion criterion of at least 90% score on the handedness questionnaire adapted from Hull (1936). This survey consists of 35 questions that ask about preferences for unimanual tasks similar to those used by Oldfield (1971), such as throwing, writing, and jar opening. Each question required the subjects to indicate for that task whether they preferred the right hand (score = 1), left hand (score = −1), or either hand (score = 0). Scores from all 35 questions were summed, and level of handedness, in terms of hand preference for a given task, was calculated as a percentage response. Thus, a score of 100% would have represented an “absolute” right-hander and a score of −100% would have represented an “absolute” left-hander.
2.2 Experimental Setup
Figure 1 shows the experimental setup. Subjects sat in a dental-type chair and wore a torso harness (not shown in Figure 1) that stabilized trunk position, ensuring symmetrical positioning of the arms relative to the task space. Each arm was supported against the effects of gravity and friction by an air sled to minimized possible effects of fatigue during the course of the task. Subjects faced an interactive 2D virtual workspace in which stimuli displayed on a 52” HDTV (Sony Electronic Inc.) were reflected onto a mirror that obscured vision of the arms. This interactive environment was programmed in REALbasic 2008 (REAL Software, USA). The distance between the TV and the parallel mirror was adjusted so as to give the illusion that the stimuli appeared in the plane of the fingertips.
Figure 1.
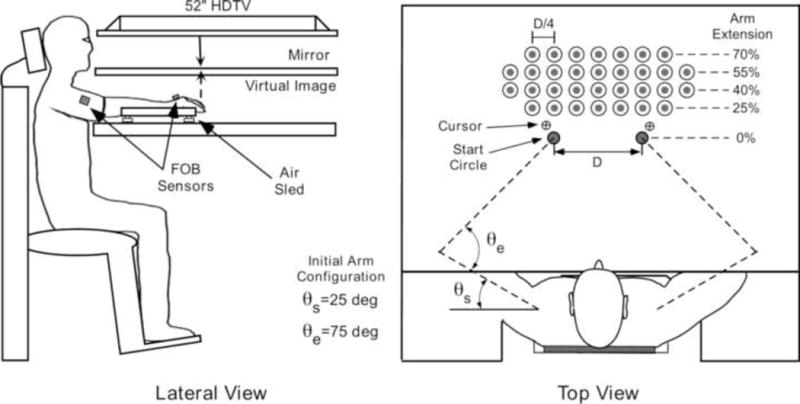
Experimental setup.
Figure 1 (top view) shows an overhead schematic of the initial arm configurations and the general setup of the visual stimuli. A six-degree-of-freedom (6-DOF) Flock of Birds tracking system (Ascension Technology, USA) recorded the position and orientation of the limb segments at 130 Hz sampling rate. One 6-DOF sensor was attached to the middle of each wrist and one was attached to the middle of each upper arm. Sensors remained firmly attached throughout experiment. Because we immobilized the fingers using a splint, this allowed us to record position of the tip of the index finger relative to the wrist sensor, and positions of the elbow and the shoulder joint relatively to upper arm sensor. These offsets were determined prior the experimental session. The 2-dimensional position of the tip of the index finger was used to project the cursor, one for the right hand and one for left hand, onto the mirror. The display of cursor projection was updated at a maximum available TV refresh rate in PC mode (60 Hz).
2.3 Experimental Design
Forty-eight subjects were assigned to one of six groups. Each group had eight subjects (four females). Four of the groups performed non-choice reaches. One group made left-arm reaches with full vision (age: 24 ± 4 years; M ± SD). The second group made left-arm reaches under no-vision conditions (age: 25 ± 5 years). The third group made right-arm reaches with full vision (age: 25 ± 5 years). The fourth group made right-arm reaches under no-vision conditions (age: 26 ± 4 years). This phase of our study was designed to identify interlimb differences (right vs. left arm) in sensorimotor performance and whether and how these differences are affected by visual feedback (vision vs. no-vision) across workspace. The final two groups performed choice reaches under either vision (age: 24 ± 2 years) or no-vision (age: 26 ± 5 years) conditions. This phase of our study was designed to determine pattern of reaching frequency across workspace. Each subject performed a total of 320 reaches (10 reaches to each of 32 targets) presented in pseudo-randomized order. We constrained the process of target randomization such that no two targets were presented consecutively in the same workspace region (left, middle, or right). The matrix of 32 targets (3.5 cm in diameter) is shown in Figure 1 (top view). This matrix was scaled according to the arm length of each individual. Target rows were set to 25%, 40%, 55% and 70% of arm length and target columns were set to 25% of the distance between left or right start positions from the body midline, symmetrically with respect to the left and right workspace regions. Two start positions (circles 2 cm in diameter), one for the non-dominant and dominant arm, were set for each individual with respect to the elbow joint internal angle, θe = 75°, and shoulder joint external angle, θs = 25° (see Figure 1, top view).
2.4 Experimental Task
Subjects were instructed to move as quickly and accurately as possible. In the non-choice condition, they were instructed to use only the right or left arm for the entire experimental session. In the choice condition, they were instructed to reach to chose an arm to reach on each trial. The target appeared prior to trial initiation. The onset of each trial was self-paced, so the subject had ample time to plan the movement and/or to decide which arm to use. We did not ask subjects to verbally report their arm choice. We recorded their movement as explained earlier in Experimental Setup (Section 2.2). Each participant was given one second to complete each reach. Each trial was initiated by an audio-visual go-cue that occurred after the participant maintained the right and left cursors in their respective start circles for 300 ms. For motivation, points were awarded on the basis of final position accuracy. However, these points were not translated into monetary reward at study completion. Final cursor regions that were within 1.75 cm, 3.5 cm, and 5.25 cm of the center of the target were awarded 10 points, 3 points, and 1 point, respectively. At the end of each trial, feedback about the accuracy score and cursor path were displayed for 1 second. This cursor path was shown in the form of small circles (1 cm in diameter) that corresponded to cursor locations sampled during the trial at 130 Hz. Note that the vision group saw the cursor and between trials, whereas for the no-vision group, the cursor disappeared after breaching the start circle and reappeared after trial completion.
2.5 Data Analysis
The Flock of Birds system recorded displacements of joints and limb segments with a maximum static and dynamic measurement error of approximately 2 mm3. These kinematic data were processed with custom software programmed in IgorPro (WaveMetrics, Inc., USA). The data were smoothed using an 8 Hz dual-pass Butterworth filter and differentiated to yield velocities and accelerations of limb joints and segments. Tangential hand velocities were used to identify movement onsets and terminations. Movement onset was defined by the last minimum prior to the 5% threshold of maximum tangential velocity. Likewise, movement termination was defined by the first minimum following 5% threshold of peak tangential velocity (Sainburg, 2002).
In order to quantify and statistically analyze the effects of visual feedback and region of workspace on sensorimotor performance of the dominant and non-dominant arm, we used non-choice reaches in order to have an equal number of reaches to all possible targets. We divided the workspace into three distinct regions: left (columns 1–4), middle (column 5) and right (columns 6–9) as shown in Figures 2–4. We quantified two kinematic variables to assess movement accuracy and quality under non-choice groups. Movement accuracy was quantified as final position error (FPE), which was defined as the shortest distance between the final position of the cursor center (index finger) and the center of the target: FPE = ((xe − xt)2 + (ye − yt)2)0.5, where (xe, ye) and (xt, yt) are the 2-dimensional Cartesian coordinates of the final position of the cursor (index finger) and the center of the target, respectively. Movement quality was indexed by hand path linearity deviation (HPLD), which was defined as the ratio between the minor and major axes of movement. The major axis was the farthest distance between any two points given on the hand path, and the minor axis was the farthest distance perpendicular to the major axis from any given point on the hand path. Note that HPLD reflects interjoint coordination because differences in HPLD vary as a function of the coordination between limb segments during reaching (Sainburg, 2002). Both FPE and HPDL were subjected to a 3-way mixed model ANOVA with arm (left/right) and condition (vision/no-vision) as between-subject factors, and workspace region (left, middle or right) as a within-subject factor. We used Tukey HSD for all post hoc tests.
Figure 2.
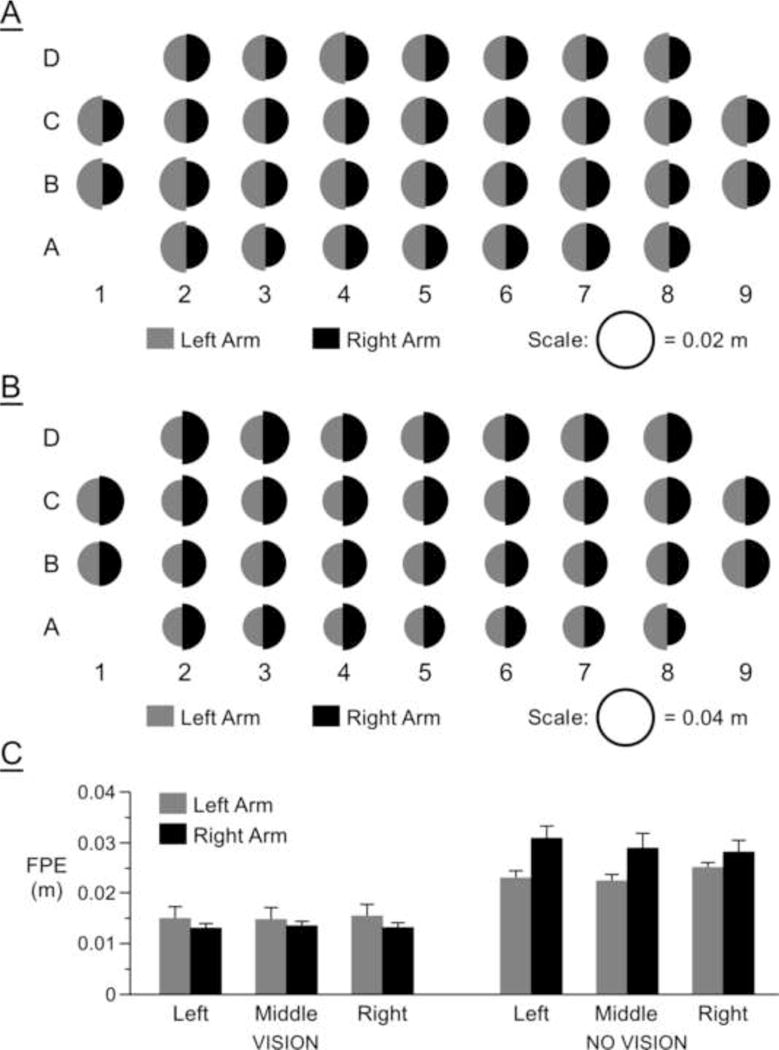
Final position error (FPE) averaged across subjects: (A) vision condition for each target; (B) no-vision condition for each target; (C) vision and no-vision conditions averaged across target regions.
Figure 4.
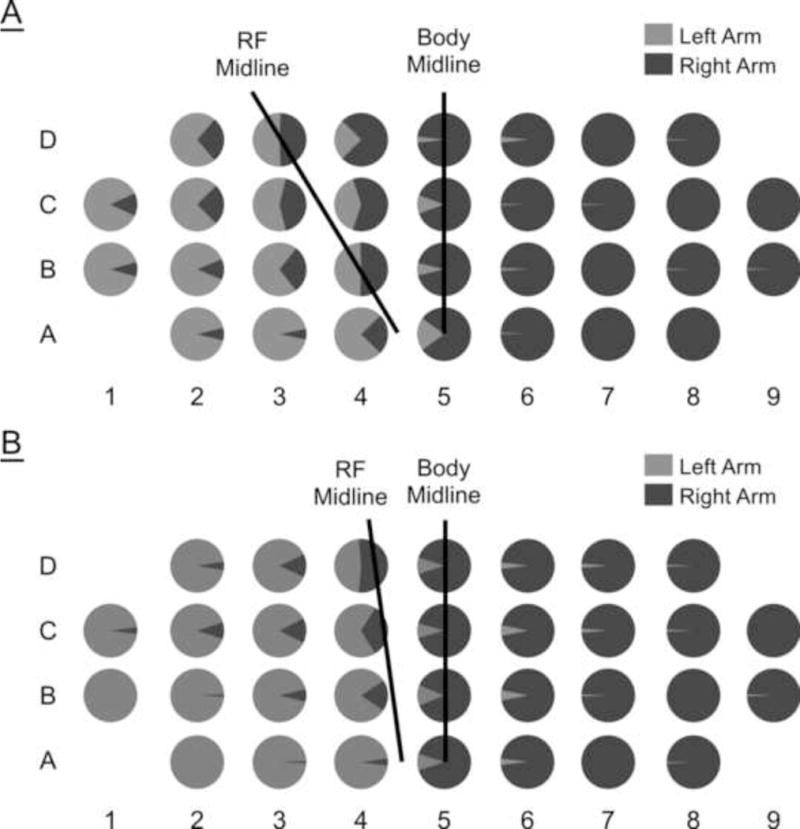
Reaching frequency averaged across subjects for each target: (A) vision condition; (B) no-vision condition. Note shift of the midline of reaching frequency (RF Midline) towards the midline of the body under no-vision condition.
In order to assess the effects of visual feedback and workspace region on arm choice, we used only choice reaches. We first computed the average frequency of dominant and non-dominant reaches to each target across subjects and identified the midline of reaching frequency (RF Midline) using a linear approximation to points in space that yielded 50% of right arm reaches at each row of targets (see Figure 4). This RF Midline divided the workspace into left and right regions of reaching frequency. Both the percentage of space reached with the dominant right arm (Right Arm Space) and the frequency of right arm reaches (Right Arm 3RF) were subjected to simple t-tests in order to determine the effects of visual feedback on arm choice. We quantified the offset of the RF Midline from the midline (RF Midline Offset) at each row of targets. This RF Midline Offset was computed as the percentage of the distance between the midline of the body and the extreme left or right target at each row. In order to quantify effects of both visual feedback and target distance amplitude, we subjected RF Midline Offset to 2-way ANOVA with row (A/B/C/D) as a within subjects factor and visual feedback condition (vision/no-vision) as a between-subject factor. We used Tukey HSD for all post hoc tests.
3. Results
3.1 Effects of visual feedback on dominant and non-dominant arm performance
In order to test the effects of visual feedback on arm performance, subjects made reaches in one of 4 non-choice groups, which were formed by fully crossing our 2 levels of arm (left/right) with our 2 levels of feedback (vision/no-vision). Figure 2A shows final position error averaged across subjects for each arm and for each target in the vision condition. The diameter of each half circle (gray for left, black for right) represents the amplitude of the error averaged across subjects. The dominant right hand showed slightly smaller errors for approximately 60% of the targets across the workspace. There were no differences between the arms for one-third of the targets, and three targets showed slightly smaller errors for the left arm (D2, C4 and C6). Figure 2B shows the corresponding plot for final position error in the no-vision condition. Without visual feedback, the left arm showed substantially smaller errors for 90% of the targets, while the right arm showed slight advantages for only 1 target (3%). For the purpose of statistical analysis, we collapsed data across targets in three regions of space: left (columns 1–4), middle (column 5), and right (columns 6–9).
Figure 2C shows the average final position error for the left and right arms in each region for both vision and no-vision conditions. A 3 (regions) X 2 (arms) X 2 (conditions) ANOVA on final position error revealed a significant interaction between arm and condition, F(1,28) = 5.21, p = .03. Right arm accuracy depended more on the presence or absence of visual feedback, such that right arm movements became substantially less accurate under no-vision conditions relative to left arm movements (see Figure 2). These results are consistent with previous research reporting similar final position accuracy for the left and right arms under vision (Carson et al., 1990; Imanaka et al., 1995) and left-arm superiority under no-vision conditions (Guiard et al., 1983; Bagesteiro and Sainburg, 2002; Sainburg, 2002; Sainburg and Wang, 2002; Lenhard and Hoffmann, 2007; Goble and Brown, 2008).
We next quantified deviations from hand-path linearity as a reflection of intersegmental coordination (Sainburg et al, 1999). Figures 3A and 3B show linearity deviations averaged across subjects for each arm and for each target in vision and no-vision conditions, respectively. Figure 3C shows linearity deviations averaged across targets in each region (left, middle, and right) for both feedback conditions (vision and no-vision). Another 3-way mixed model ANOVA with region as a within-subject factor, and arm and condition as between-subject factors revealed a significant interaction between arm and region, F(2,56) = 17.20, p < .0001. This interaction reflects a larger left arm dependence on the region of space for linearity deviations: Left arm movements to the right region were substantially less straight for reaches irrespectively of visual feedback conditions. These findings are consistent with our previous studies (Sainburg and Kalakanis, 2000; Sainburg, 2002), which indicated that direction dependent curvatures of left arm movements reflect deficits in intersegmental coordination.
Figure 3.
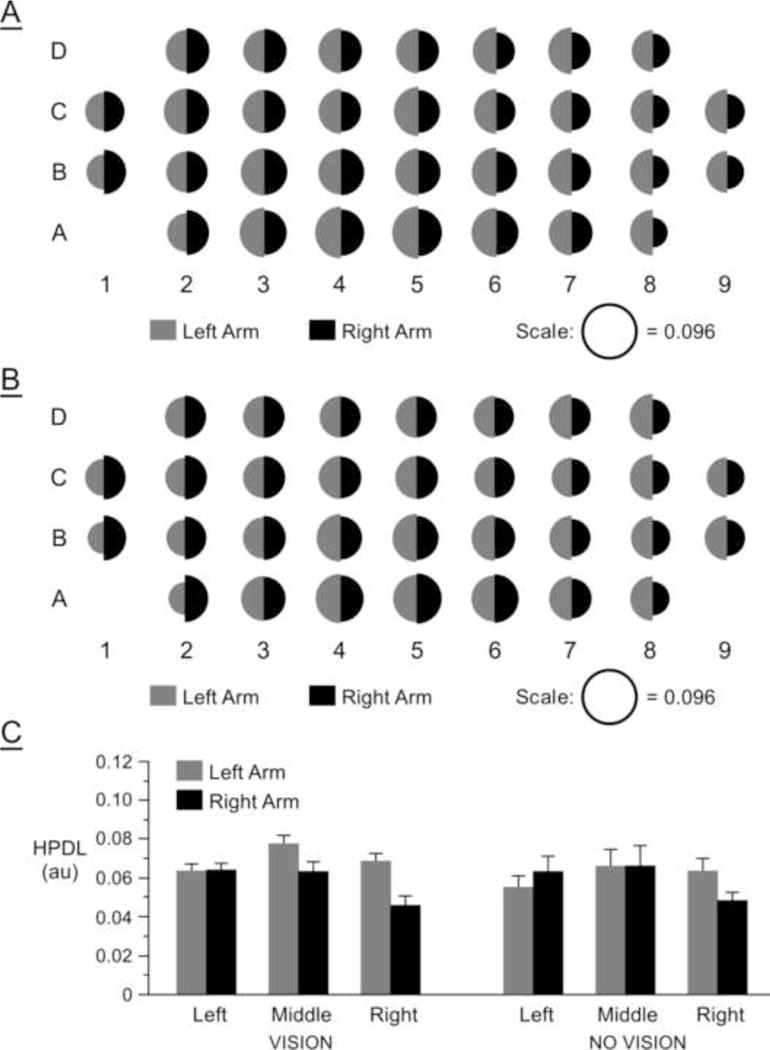
Hand path linearity deviation (HPDL) averaged across subjects: (A) vision condition for each target; (B) no-vision condition for each target; (C) vision and no-vision conditions averaged across target regions.
Overall, our findings for non-choice reaches toward targets across the workspace indicate that interlimb differences in both movement accuracy and coordination depend on visual feedback. In the case of accuracy, there were no interlimb differences for reaches made with visual feedback, but we found left arm advantages under no-vision conditions. In the case of linearity deviation, we showed a significant right arm advantage in the center region, which was not present under the no-vision condition. These performance differences in accuracy and curvature provided the basis for the dependent variables in the next phase of the study. We hypothesized that movement accuracy and coordination might inform the decisions that subjects make about which arm to use when reaching across the workspace.
3.2 Effects of visual feedback on arm selection
In order to test the effects of visual feedback on arm selection, subjects reached in one of two choice groups. The vision group saw a cursor that reflected position of the tip of the finger during each reach. The no-vision group did not see a cursor during the movement. Subjects were presented with one target on each trial and reached to that target with whichever hand they chose.
Figures 4A and 4B show the distributions of reaching frequencies, averaged across subjects for each target and for each hand under vision and no-vision conditions, respectively. Each pie chart reflects the percentages of total reaches made by either the left arm (light gray) or the right arm (dark gray). For reaches made with visual feedback, the dominant right arm was not only chosen exclusively for the targets in the right and center regions of the workspace but also was chosen for some of the left-region targets close to the midline (see Figure 4A). Subjects reached the right arm more to target D4 and to targets D3, C3, C4 and B4 with equal frequency, relative to the left arm. In general, the number of right arm reaches that subjects made into the left region of workspace increased with the distance of the target from the body. This pattern of reaching both supports and extends similar findings that have reported data sampled with fewer targets and, by extension, lower spatial resolution (Gabbard and Rabb, 2001; Stins et al., 2001; Gabbard and Helbig, 2004; Mamolo et al., 2004).
Our data also indicate that the no-vision group displayed a different pattern of reaches, when compared to the vision group (see Figure 4B). Both groups reached almost exclusively with the right arm to the targets in the right and the center regions of the workspace. However, under the no-vision condition the number of right arm reaches to left-region targets was equal or greater than left arm reaches. Thus, the right-arm reaching frequency was at least fifty percent for only one target in the left workspace (4D), whereas it reached or exceeded fifty percent for four targets in the left workspace under visual conditions (B4, C4, D4, and D3).
In order to quantify these patterns of reaching between vision and no-vision conditions, we computed the following parameters: (1) percentage of reaches made with the right arm (Right Arm RF); (2) percentage of targets reached more frequently by the right arm (Right Arm Space); and (3) the offset between the midline of reaching frequency and the midline of the body (RF Midline Offset) for each row of targets (see Methods). Figure 5 shows the mean and standard errors for these measures across subjects and targets. Right Arm RF was significantly lower for the no-vision group (59.2% ± 3.8%, M ± SE) compared to the vision group (68.0% ± 5.1%), t(1,15) = 15.36, p = .02 (see Figure 5A). Right Arm Space was also significantly lower in the no-vision group (61.8% ± 1.6%) relative to the vision group (68.2% ± 2.4%), t(1,15) = 5.06, p = .04 (see Figure 5B). These differences were driven by a modulation of reaching frequencies between vision and no-vision groups for targets located farther from the body (see Figures 4A and 4B). In Figure 5C, we show the average RF Midline Offset computed for each row of targets, separately. A mixed model ANOVA on RF Midline Offset with feedback condition as a between-subject factor and target row (A–D) as a within-subject factor revealed a main effect of condition, F(1,7) = 23.76, p < .0001, such that the RF Midline Offset was greater for the vision group. There was also a main effect for target row, F(3,7) = 20.96, p < .0001; RF Midline Offset increased as the target rows moved farther from the body. These main effects were qualified by an interaction between row and condition, F(3,42) = 3.99, p < .014. Post-hoc analysis showed that this interaction was driven by the fact that there were no significant differences in RF Midline Offset between visual feedback conditions for the two target rows closest to the body (row A: p > .99; row B: p = .13, Tukey HSD), but there were significant differences between feedback conditions in the two target rows farthest from the body (row C: p = .011; row D: p = .0002, Tukey HSD).
Figure 5.
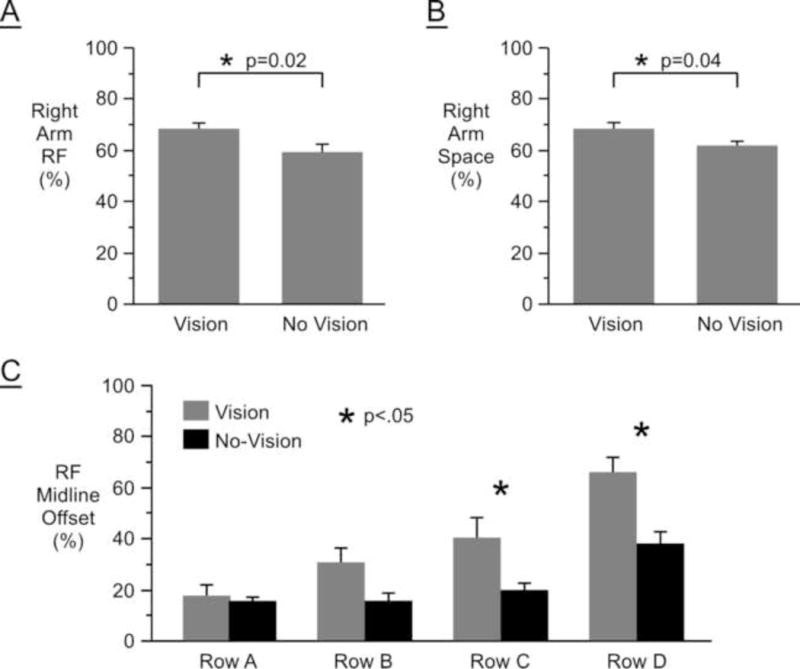
(A) Reaching frequency of the right arm (Right Arm RF) averaged across targets and subjects; (B) Percentage of the workspace reached with the right arm more frequently (Right Arm Space) averaged across targets and subjects; (C) Offset of the midline of reaching frequency (RF Midline Offset) from the midline of the body under vision and no-vision conditions.
Our main finding was that occlusion of visual feedback substantially affected which arm was chosen during the choice reaching task. The left arm was chosen more frequently under no-vision compared to vision conditions. In addition, the point in the workspace where subjects transitioned from primarily left arm reaches to primarily right arm reaches shifted to the right under no-vision conditions.
4. Discussion
We tested the hypothesis that arm selection choices should depend upon an interaction with sensorimotor performance asymmetries associated with handedness and with the demands of the given task. We tested this hypothesis in a targeted reaching paradigm by manipulating the region of workspace of presented targets and by manipulating visual feedback conditions. The first manipulation modified the geometric and dynamic requirements of the task for each arm, increasing the index of difficulty as the targets were presented further from the starting hand position. The two visual feedback conditions modified sensorimotor performance asymmetries, an effect predicted by previous literature (Guiard, et al., 1983; Carson et al., 1990; Imanaka, et al., 1995; Bagesteiro and Sainburg, 2002; Sainburg, 2002; Sainburg and Wang, 2002; Lenhard and Hoffmann, 2007; Wang and Sainburg, 2007; Goble and Brown, 2008). We predicted that arm choice would be reflected by an interaction between these two manipulations. Indeed, the relative performance of the non-dominant arm improved under no-vision conditions. These findings extend previous research by showing that the relative accuracy advantage of the left arm under no-vision conditions is present across the entire reachable horizontal workspace. Furthermore, our findings indicated that the rate of left-arm reaches increased in accordance with these left-arm performance advantages. In the sections that follow, we address these findings in turn. We conclude by discussing their implications for models of hand selection.
4.1 Removing vision affects relative limb performance
Our results confirm that removing visual feedback modulates the final position accuracy of both left- and right-arm reaches. While the accuracy of both arms was attenuated in no-vision conditions, this effect was more severe for the right arm. This interaction resulted in clear left-arm accuracy advantages for reaches made without visual feedback. This pattern contrasted the statistically equivalent left- and right-arm accuracy we observed when visual feedback was available (see Figure 2C). Taken together, these outcomes indicate that removing visual feedback improves the relative sensorimotor performance of the left arm.
These findings are important contributions to our understanding of motor lateralization. We have previously shown evidence that the two hemisphere/limb systems might be specialized for distinct motor control process — the dominant system for coordinating limb and task dynamics and the non-dominant system for postural stabilization (Sainburg, 2002; Sainburg, 2012). A key feature of this model is that the dominant system achieves better coordination by predictive control of limb and task dynamics. Such predictions have been shown to depend on visual information from previous movements (Ghez et al, 1995; Sainburg, 2002; Yadav and Sainburg, 2011). We have also provided evidence that the non-dominant system relies on impedance-control mechanisms that are largely mediated by proprioceptive mechanisms (Barnett and Harding, 1955; Gottlieb, 1996; Gottlieb, 1998; Ghez et al., 2007; Schabowsky et al., 2007). In accordance with this idea, recent studies have demonstrated a non-dominant arm advantage in the absence of visual feedback in tasks that require matching movement distance (Yamauchi et al., 2004) or position (Goble et al., 2006).
Furthermore, some previous reports showed the non-dominant arm advantage in the final position accuracy in reaching movements without visual feedback (Bagesteiro and Sainburg, 2002; Sainburg 2002; Lenhard and Hoffmann, 2007). Our current findings are not only consistent with these results, but they also imply that the relative accuracy advantage of the non-dominant arm under no-vision conditions persists across the workspace. Interestingly, it also varies with workspace location, may account for some reported inconsistencies in previous studies that have compared interlimb differences in reaching accuracy under vision and no-vision conditions for movements to more restricted regions of the workspace (see Carson et al., 1990 for review).
4.2 Arm selection depends on visual feedback conditions
Previous research has shown that people generally prefer ipsilateral reaches to contralateral reaches, using the left arm to reach to the left workspace and the right arm to reach to the right workspace; see Peters (1996) and Gabbard and Rabb (2000) for reviews. However, this tendency is asymmetric across the workspace. For example, right-handers make more contralateral reaches to targets close to the body midline with their dominant arms (Gabbard and Rabb, 2000; Stins et al., 2001; Gabbard and Helbig, 2004; Mamolo et al., 2004). Our current results confirmed this pattern across a much larger range of horizontal workspace, but also found that the pattern is modulated by both visual-feedback conditions and distance of the target from the body.
The critical difference between the limb selections that individuals made under vision and no-vision conditions was the location in the left workspace at which our right-handed subjects switched from using mostly dominant reaches to mostly non-dominant reaches. This “switch-point” migrated closer to the body-midline under no-vision compared with vision conditions. The fact that the switch-point shifted under no-vision condition is important because the relative accuracy of the left arm also improved when we removed visual feedback (see Figures 2C and 3C). This correspondence between left-arm choices and left-arm performance is consistent with our hypothesis that asymmetries in interlimb performance might inform the decision to use one arm or the other. Moreover, it suggests that arm selection may be influenced by consideration of interlimb performance differences.
These results indicate an interaction between hand choice and visual feedback conditions, such that the non-dominant arm is selected more under no-vision conditions than it is under vision conditions. However, it should be stressed that in both cases, the dominant arm is actually selected more than the non-dominant arm. Feedback conditions simply modulate these choices in accord with the relative performance advantages of each arm. Thus, removing vision did not reverse the overall tendency of right-handers to prefer the right hand for this task. Instead, the magnitude of this general right-hand choice decreased when we removed vision, and this decrease appeared to be driven by an increase in left-hand reaches to targets left of the body midline. We therefore take our findings to suggest that arm selection is modulated by the relative sensorimotor performance between the arms. In addition, arm choice was modulated by movement distance, a substantial task demand that altered the index of difficulty of the required movement. In fact, the horizontal “switch-point” between right and left arms depended on the distance of the reach from the body. Previous studies on arm choice have typically used a limited number of targets placed across the workspace at equal distances from the body (Gabbard and Rabb, 2000; Stins et al., 2001; Gabbard and Helbig, 2004; Mamolo et al., 2004). Our current findings confirm the influence of target distance on hand selection choices: The degree of offset between the workspace midline and the reaching frequency midline was greatest for the two target rows farthest from the body. Moreover, our full array of targets provided enough resolution to determine that the differences in reaching frequency offset between the vision and no-vision groups was also greatest for the farthest two target rows (see Figure 5C). These results indicate that larger target amplitudes drove the “switch-point” migration toward the body-midline.
4.3 Models of hand selection
Based on evidence that damage to the left hemisphere impairs various goal-directed movements, Liepmann (1905) proposed a left-hemisphere dominance for motor planning in right-handers. This led to the view that individuals should prefer the dominant arm for performing all unimanual tasks. Recent research has updated this view, suggesting instead that circuits in both hemispheres contribute to the planning and control of reaches with each arm (Sainburg, 2002). This perspective has motivated exploration of plausible neuromuscular variables that might distinguish the dominant and non-dominant motor control systems.
Earlier attempts to find such variables have largely yielded equivocal results. Some work has suggested that the dominant and non-dominant systems differ mainly in terms of the facility with which they exploit sensory feedback for making movement corrections. Consistent with this idea, some studies have reported dominant-arm advantages for error correction based on visual feedback (Flowers, 1975; Elliott et al., 1994; Elliot et al., 1995). However, other studies have not been able to substantiate those results (Roy and Elliott, 1986; Carson et al., 1990; Carson et al., 1992; Shabbott and Sainburg, 2008). Studies that have tested other dominant-system specializations like motor planning, initiation, and sequencing, have also yielded inconsistent findings (Todor and Kyprie, 1980; Carson et al., 1990; Carson et al., 1993; Elliott et al., 1994; Carson et al., 1995). Yet other researchers have continued to characterize handedness mainly in terms of choice, a tradition established much earlier by Oldfield (1971) and Bryden (1977). The current study links these performance- and preference-based views by relating accuracy and coordination to arm selection in the same experimental paradigm.
Recent work from our lab has provided evidence that handedness arises from hemispheric specializations of the dominant and non-dominant systems for dynamic coordination and control of limb impedance for postural stabilization, respectively (see Sainburg 2002; Sainburg and Eckhardt, 2005; Sainburg 2012 for reviews). Our current findings indicate that hand selection results from an interaction between asymmetries in sensorimotor performance (here manipulated by visual feedback conditions) and task demands. This implies rather efficient action-selection process that takes into account a variety of intrinsic and extrinsic task conditions. This idea is consistent with the recent finding that right-handers can be induced to use the right hand less when the chances of task success are systematically manipulated in a computer-based task (Stoloff et al., 2011). Interestingly, Oliveira et al (2010) reported that temporarily disrupting activity in posterior parietal cortex using rTMS can increase ipsilateral reaches, a finding that was taken to suggest that hand choice might reflect a “competitive” decision process. Our current findings, indicating a link between arm performance and arm selection, confirm and extend these results by indicating that limb selection clearly takes account of both performance asymmetries and task demands.
Previous explanations for arm choice have attributed selection patterns to the proximity of the hand to an intended target (Helbig and Gabbard, 2004) or to attentional biases due to the fact that the visual target stimuli in each hemifield are initially processed in the hemisphere that controls the ipsilateral limb (Verfaellie et al., 1984; Verfaellie and Heilman, 1990). It has also been suggested that the bias for ipsilateral reaches may be driven by reaction-time advantages predicted by the well-known Simon effect. in which shorter times are associated with responses (arm choices in this case) that are spatially compatible with the target (Hommel, 1993). However, these explanations do not predict the asymmetry of limb selection across the workspace, nor do they account for the feedback-induced changes in selection patterns we observed here. In fact, previous work has shown that performance advantages of ipsilateral reaches persist when visual stimuli are hemifield-reversed using a mirror, suggesting that advantages for ipsilateral reaches arise from movement-dependent factors rather than intrahemispheric attentional processing benefits (Carey et al., 1996).
Overall, the above explanations of hand selection do not account for the strong bias to reach across the midline with the dominant arm. Moreover, these studies have often accounted for this bias by saying that it simply results from a habitual pattern of arm use, developed through parental modeling early in life (see Sainburg, 2010 for review). However, our current findings indicate that hand choices reflects a process that accounts for current sensorimotor and task conditions. Nevertheless, it should be stressed that the failure of visual feedback conditions to reverse the bias in selecting the dominant arm for midline targets does suggest that other factors also affect arm selection choices. Indeed, this might be as simple an explanation as a habitual bias for using the dominant arm. However, the research cited above by Stoloff et al. (2011) and Oliveira et al. (2010) suggest that the pattern of choices could be reversed if the performance advantages for the non-dominant arm were larger. Further research is necessary to test this hypothesis.
Highlights.
We proposed that handedness is based on hemispheric differences in neural control
We examined if sensorimotor performance asymmetries give rise to active hand choices
We modulated sensorimotor performance asymmetries by occlusion of visual feedback
We found hand preference to change respectively to performance asymmetries
We concluded that sensorimotor performance asymmetries predict hand choice
Acknowledgments
This research was supported by the following grants: National Institutes of Health, National Institute for Child Health and Human Development (R01HD059783) to Robert L. Sainburg.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Annett M. The distribution of manual asymmetry. Br J Psychol. 1972;63:343–358. doi: 10.1111/j.2044-8295.1972.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol. 2002;88:2408–2421. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol. 2003;90:1503–1513. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett CH, Harding D. The activity of antagonist muscles during voluntary movement. Ann Phys Med. 1955;2:290–293. doi: 10.1093/rheumatology/2.8.290. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Ross VA, Daniels MS, Bright P. The measurement of hand preference: a validation study comparing three groups of right-handers. Br J Psychol. 1996;87(Pt2):269–285. doi: 10.1111/j.2044-8295.1996.tb02590.x. [DOI] [PubMed] [Google Scholar]
- Brown SG, Roy EA, Rohr LE, Bryden PJ. Using hand performance measures to predict handedness. Laterality. 2006;11:1–14. doi: 10.1080/1357650054200000440. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Measuring handedness with questionnaires. Neuropsychologia. 1977;15:617–624. doi: 10.1016/0028-3932(77)90067-7. [DOI] [PubMed] [Google Scholar]
- Bryden MP, Singh M, Steenhuis RE, Clarkson KL. A behavioral measure of hand preference as opposed to hand skill. Neuropsychologia. 1994;32:991–999. doi: 10.1016/0028-3932(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Bryden PJ, Pryde KM, Roy EA. A performance measure of the degree of hand preference. Brain Cogn. 2000;44:402–414. doi: 10.1006/brcg.1999.1201. [DOI] [PubMed] [Google Scholar]
- Bryden PJ, Roy EA. A new method of administering the Grooved Pegboard Test: performance as a function of handedness and sex. Brain Cogn. 2005;58:258–268. doi: 10.1016/j.bandc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bryden PJ, Roy EA. Preferential reaching across regions of hemispace in adults and children. Dev Psychobiol. 2006;48:121–132. doi: 10.1002/dev.20120. [DOI] [PubMed] [Google Scholar]
- Carey DP, Hargreaves EL, Goodale MA. Reaching to ipsilateral or contralateral targets: within-hemisphere visuomotor processing cannot explain hemispatial differences in motor control. Exp Brain Res. 1996;112:496–504. doi: 10.1007/BF00227955. [DOI] [PubMed] [Google Scholar]
- Carson RG, Chua R, Elliott D, Goodman D. The contribution of vision to asymmetries in manual aiming. Neuropsychologia. 1990;28:1215–1220. doi: 10.1016/0028-3932(90)90056-t. [DOI] [PubMed] [Google Scholar]
- Carson RG, Chua R, Goodman D, Byblow WD, Elliott D. The preparation of aiming movements. Brain Cogn. 1995;28:133–154. doi: 10.1006/brcg.1995.1161. [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, Chua R, Elliott D. Asymmetries in the regulation of visually guided aiming. J Mot Behav. 1993;25:21–32. doi: 10.1080/00222895.1993.9941636. [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, Elliott D. Asymmetries in the discrete and pseudocontinuous regulation of visually guided reaching. Brain Cogn. 1992;18:169–191. doi: 10.1016/0278-2626(92)90077-y. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychol Rev. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Elliott D, Chua R, Pollock BJ. The influence of intermittent vision on manual aiming. Acta Psychol (Amst) 1994;85:1–13. doi: 10.1016/0001-6918(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Elliott D, Lyons J, Chua R, Goodman D, Carson RG. The influence of target perturbation on manual aiming asymmetries in right-handers. Cortex. 1995;31:685–697. doi: 10.1016/s0010-9452(13)80020-2. [DOI] [PubMed] [Google Scholar]
- Flowers K. Handedness and controlled movement. Br J Psychol. 1975;66:39–52. doi: 10.1111/j.2044-8295.1975.tb01438.x. [DOI] [PubMed] [Google Scholar]
- Gabbard C, Helbig CR. What drives children’s limb selection for reaching in hemispace? Exp Brain Res. 2004;156:325–332. doi: 10.1007/s00221-003-1792-y. [DOI] [PubMed] [Google Scholar]
- Gabbard C, Rabb C. What determines choice of limb for unimanual reaching movements? J Gen Psychol. 2000;127:178–184. doi: 10.1080/00221300009598577. [DOI] [PubMed] [Google Scholar]
- Gabbard C, Rabb C. Imagined and actual limb selection: a test of preference. Brain Cogn. 2001;46:139–144. doi: 10.1016/s0278-2626(01)80052-x. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The apraxias: neural mechanisms of disorders of learned movement. Am Sci. 1975;63:188–195. [PubMed] [Google Scholar]
- Ghez C, Gordon J, Ghilardi MF. Impairments of reaching movements in patients without proprioception. II. Effects of visual information on accuracy. J Neurophysiol. 1995;73:361–372. doi: 10.1152/jn.1995.73.1.361. [DOI] [PubMed] [Google Scholar]
- Ghez C, Scheidt R, Heijink H. Different learned coordinate frames for planning trajectories and final positions in reaching. J Neurophysiol. 2007;98:3614–3626. doi: 10.1152/jn.00652.2007. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Upper limb asymmetries in the matching of proprioceptive versus visual targets. J Neurophysiol. 2008;99:3063–3074. doi: 10.1152/jn.90259.2008. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Brown SH. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res. 2006;168:307–311. doi: 10.1007/s00221-005-0280-y. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Accuracy of planar reaching movements. I. Independence of direction and extent variability. Exp Brain Res. 1994;99:97–111. doi: 10.1007/BF00241415. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. On the voluntary movement of compliant (inertial-viscoelastic) loads by parcellated control mechanisms. J Neurophysiol. 1996;76:3207–3229. doi: 10.1152/jn.1996.76.5.3207. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. Muscle activation patterns during two types of voluntary single-joint movement. J Neurophysiol. 1998;80:1860–1867. doi: 10.1152/jn.1998.80.4.1860. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. Rejecting the equilibrium-point hypothesis. Motor Control. 1998;2:10–12. doi: 10.1123/mcj.2.1.10. [DOI] [PubMed] [Google Scholar]
- Guiard Y, Diaz G, Beaubaton D. Left-hand advantage in right-handers for spatial constant error: preliminary evidence in a unimanual ballistic aimed movement. Neuropsychologia. 1983;21:111–115. doi: 10.1016/0028-3932(83)90106-9. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Schaefer SY, Knight RT, Adair J, Magalhaes A, Sadek J, Sainburg RL. Ipsilesional trajectory control is related to contralesional arm paralysis after left hemisphere damage. Exp Brain Res. 2009;196(2):195–204. doi: 10.1007/s00221-009-1836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey JM, Liederman J, Geschwind N. Handedness is not a unidimensional trait. Cortex. 1986;22:33–53. doi: 10.1016/s0010-9452(86)80031-4. [DOI] [PubMed] [Google Scholar]
- Helbig CR, Gabbard C. What determines limb selection for reaching? Res Q Exerc Sport. 2004;75:47–59. doi: 10.1080/02701367.2004.10609133. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Wells DL, Lynch C. Prenatal thumb sucking is related to postnatal handedness. Neuropsychologia. 2005;43(3):313–315. doi: 10.1016/j.neuropsychologia.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Hommel B. Inverting the Simon effect by intention: Determinants of direction and extent of effects of irrelevant spatial information. Psychological Research. 1993;55:270–279. [Google Scholar]
- Hopkins WD, Bales SA, Bennett AJ. Heritability of Hand Preference in Chimpanzees (PAN) Int J Neurosci. 1994;74(1–4):17–26. doi: 10.3109/00207459408987225. [DOI] [PubMed] [Google Scholar]
- Hull CJ. A study of laterality test items. J Exp Educ. 1936;4:287–290. [Google Scholar]
- Imanaka K, Abernethy B, Yamauchi M, Funase K, Nishihira Y. Hemispace asymmetries and laterality effects in arm positioning. Brain Cogn. 1995;29:232–253. doi: 10.1006/brcg.1995.1280. [DOI] [PubMed] [Google Scholar]
- Klar AJ. A single locus, RGHT, specifies preference for hand utilization in humans. Cold Spring Harb Symp Quant Biol. 1996;61:59–65. [PubMed] [Google Scholar]
- Lenhard A, Hoffmann J. Constant error in aiming movements without visual feedback is higher in the preferred hand. Laterality. 2007;12:227–238. doi: 10.1080/13576500701203891. [DOI] [PubMed] [Google Scholar]
- Levy J, Nagylaki T. A model for the genetics of handedness. Genetics. 1972;72:117–128. doi: 10.1093/genetics/72.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepmann H. The left hemisphere in action. Republished in 1908 in Drei Aufsatze aus den Apraxegebeit. Berlin: Krager; 1905. Translated in 1980 by D. Kimura, Translations from Liepmann’s essays on apraxia (Research Bulletin No. 506). Department of Psychology, University of Western Ontario, London, Canada. [Google Scholar]
- Mamolo CM, Roy EA, Bryden PJ, Rohr LE. The effects of skill demands and object position on the distribution of preferred hand reaches. Brain Cogn. 2004;55:349–351. doi: 10.1016/j.bandc.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Mamolo CM, Roy EA, Rohr LE, Bryden PJ. Reaching patterns across working space: the effects of handedness, task demands, and comfort levels. Laterality. 2006;11:465–492. doi: 10.1080/13576500600775692. [DOI] [PubMed] [Google Scholar]
- Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain. 2012 doi: 10.1093/brain/aws283. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus IC. Handedness, language dominance and aphasia: a genetic model. Psychol Med Monogr Suppl. 1985;8:1–40. [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Critical neural substrates for correcting unexpected trajectory errors and learning from them. Brain. 2011;134(Pt 12):3647–61. doi: 10.1093/brain/awr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, Diedrichsen J, Verstynen T, Duque J, Ivry RB. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc Natl Acad Sci U S A. 2010;107(41):17751–6. doi: 10.1073/pnas.1006223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M. Hand Preference and Performance in Left-Handers. In: Elliott D, Roy EA, editors. Manual Asymmetries in Motor Performance. Boca Raton: CRC Press; 1996. pp. 99–122. [Google Scholar]
- Przybyla A, Good DC, Sainburg RL. Dynamic dominance varies with handedness: reduced interlimb asymmetries in left-handers. Exp Brain Res. 2012;216:419–431. doi: 10.1007/s00221-011-2946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy EA, Elliott D. Manual asymmetries in visually directed aiming. Can J Psychol. 1986;40:109–121. doi: 10.1037/h0080087. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev. 2005;33(4):206–13. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Lateralization of goal-directed movement. In: Elliott D, Khan M, editors. Vision and goal-directed movement: neurobehavioral perspectives. Champaign: Human Kinetics; 2010. pp. 219–237. [Google Scholar]
- Sainburg RL. Handedness. In: Mooren FC, Skinner JS, editors. Encyclopedia of Exercise Medicine in Health and Disease. New Jersey: Springer; 2012. [Google Scholar]
- Sainburg RL, Eckhardt Optimization Through Lateralization: The Evolution of Handedness. Behavioral and Brain Sciences. 2005;28(4):611–612. [Google Scholar]
- Sainburg RL, Ghez C, Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol. 1999;81:1045–1056. doi: 10.1152/jn.1999.81.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol. 2000;83:2661–2675. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Lateiner JE, Latash ML, Bagesteiro LB. Effects of altering initial position on movement direction and extent. J Neurophysiol. 2003;89:401–415. doi: 10.1152/jn.00243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Mutha P. Applying Principles of Motor Control to Neurorehabilitation. In: Dietz V, Rymer Z, Nef T, editors. Neurorehabilitation Technology. New Jersey: Springer; 2011. [Google Scholar]
- Sainburg RL, Schaefer SY. Interlimb differences in control of movement extent. J Neurophysiol. 2004;92(3):1374–83. doi: 10.1152/jn.00181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res. 2002;145:437–447. doi: 10.1007/s00221-002-1140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma SM, Riedstra BJ, Pfannkuche KA, Bouma A, Groothuis TG. Epigenesis of behavioural lateralization in humans and other animals. Philos Trans R Soc Lond B Biol Sci. 2009;364:915–927. doi: 10.1098/rstb.2008.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabowsky CN, Hidler JM, Lum PS. Greater reliance on impedance control in the nondominant arm compared with the dominant arm when adapting to a novel dynamic environment. Exp Brain Res. 2007;182:567–577. doi: 10.1007/s00221-007-1017-x. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130(Pt 8):2146–58. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009;47(13):2953–66. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbott BA, Sainburg RL. Differentiating between two models of motor lateralization. J Neurophysiol. 2008;100:565–575. doi: 10.1152/jn.90349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhuis RE, Bryden MP. Different dimensions of hand preference that relate to skilled and unskilled activities. Cortex. 1989;25:289–304. doi: 10.1016/s0010-9452(89)80044-9. [DOI] [PubMed] [Google Scholar]
- Stins JF, Kadar EE, Costall A. A kinematic analysis of hand selection in a reaching task. Laterality. 2001;6:347–367. doi: 10.1080/713754421. [DOI] [PubMed] [Google Scholar]
- Stoloff RH, Taylor JA, Xu J, Ridderikhoff A, Ivry RB. Effect of reinforcement history on hand choice in an unconstrained reaching task. Front Neurosci. 2011;5(23):41. doi: 10.3389/fnins.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todor JI, Doane T. Handedness classification: preference versus proficiency. Percept Mot Skills. 1977;45:1041–1042. doi: 10.2466/pms.1977.45.3f.1041. [DOI] [PubMed] [Google Scholar]
- Todor JI, Kyprie PM. Hand differences in the rate and variability of rapid tapping. J Mot Behav. 1980;12:57–62. doi: 10.1080/00222895.1980.10735205. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Bowers D, Heilman KM. Hemispheric asymmetries in mediating intention, but not selective attention. Neuropsychologia. 1988;26:521–531. doi: 10.1016/0028-3932(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Heilman KM. Hemispheric asymmetries in attentional control: implications for hand preference in sensorimotor tasks. Brain Cogn. 1990;14:70–80. doi: 10.1016/0278-2626(90)90061-r. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Interlimb transfer of novel inertial dynamics is asymmetrical. J Neurophysiol. 2004;92:349–360. doi: 10.1152/jn.00960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp Brain Res. 2007;178:565–570. doi: 10.1007/s00221-007-0936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Imanaka K, Nakayama M, Nishizawa S. Lateral difference and interhemispheric transfer on arm-positioning movement between right and left handers. Percept Motor Skills. 2004;98:1199–1209. doi: 10.2466/pms.98.3c.1199-1209. [DOI] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Motor lateralization is characterized by a serial hybrid control scheme. Neuroscience. 2011;196:153–167. doi: 10.1016/j.neuroscience.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


