Abstract
The risk of male to female transmission of HIV is impacted by baseline inflammation in the female genital tract, semen viral load and seminal plasma’s ability to induce specific patterns of cervical cytokine signalling and influx of immune cell populations. Disruption of the epithelial barrier during non-consensual intercourse may trigger further inflammation and initiation of cell-signalling pathways, thus facilitating transmission of HIV and expansion of local infection. Adolescent and pregnant women are at high risk for sexual violence and may exhibit alterations of genital mucosal immunity that promote immune activation, making them uniquely vulnerable to HIV acquisition.
Keywords: Adolescence, genital mucosal immunity, HIV, lactobacillus, pregnancy
Introduction
Transmission of HIV through heterosexual intercourse is relatively inefficient, with an estimated rate of male to female transmission of 0.08% per act (95% CI 0.06–0.11) and of 0.04% per act for female to male transmission (95% CI 0.01–0.14).1 However this transmission risk is impacted by numerous host and viral variables and may be significantly increased in the setting of non-consensual sexual intercourse. Disruption of the genital epithelial barrier, anal penetration, mucosal inflammation and concomitant sexually transmitted infection (STI) may all increase the risk of HIV acquisition.2 Higher semen viral load, which characterizes acute HIV infection, is associated with increased risk of transmission.3–5 Transmission of sexually transmitted infections is well described in rape victims6 and may increase both HIV infectiousness and susceptibility through disruption of mucosal barriers, target cell recruitment and activation, altered cytokine production, impaired HIV-specific cytotoxic T lymphocyte (CTL) function and enhanced HIV replication.7,8 Adolescents and pregnant women, who are highly vulnerable to sexual violence and non-consensual sex, may be at higher risk for HIV acquisition secondary to altered baseline concentrations of specific genital tract cytokines, chemokines, vaginal microbiota and antimicrobial peptides. The current review examines the genital mucosal factors that may make adolescent and pregnant women especially vulnerable to HIV acquisition in the setting of sexual violence.
The epithelial barrier and the impact of sexual intercourse
An intact genital mucosal epithelium is the first barrier to infection and is coated in protective genital tract secretions with an acidic pH. Microtrauma to this barrier may occur with consensual sex, but more severe breaches may occur with non-consensual sex,9,10 thus facilitating viral transmission across the epithelium and infection of submucosal target cells. Disruption of the epithelial barrier may also occur in response to seminal plasma cytokines or in response to cytokines released by genital tract epithelial cells following semen exposure. Tumour necrosis factor (TNF)-α and interleukin (IL)-1, for example, may disrupt epithelial cell tight junctions. In addition, epithelial trauma is likely to activate mucosal cytokine and chemokine signalling, resulting in an influx of HIV target cells.11 NF-κB activation following epithelial trauma may promote HIV replication through binding to the viral long terminal repeat sequence.12
The risk of HIV acquisition is also associated with specific properties of semen, independent of viral load. Semen buffers the protective acidity of the vagina.13 Several seminal peptides, including fragments of prostatic acid phosphatase and semenogelins, assemble into amyloid fibrils that may enhance HIV infection by facilitating virion attachment and entry.14,15 Seminal proteins may alter female genital tract cytokine and chemokine profiles to induce TNF-α, macrophage inflammatory protein (MIP)-3α, granulocyte macrophage-colony stimulating factor (GM-CSF), monocyte chemotactic protein (MCP-1), IL-7 and IL-8, therefore activating the cells that facilitate HIV infection;16–19 this inflammatory effect may be exaggerated when semen is HIV infected.17 Transforming growth factor (TGF)-β, which is highly abundant in seminal fluid, may play a key role in induction of cervical cytokine response following coitus. A recent study investigating the effects of pooled seminal fluid on immortalized and primary cervical cells found that TGF-β induced expression of GM-CSF and IL-6 from both human Ect1 cervical epithelial cells and primary cervical cells.20 Similar responses were not observed following stimulation of cells with a selection of other cytokines found in seminal plasma. TGF-β neutralizing antibodies, receptor antagonists and signalling inhibitors diminished seminal plasma induction of GM-CSF and IL-6, but did not affect production of IL-8, MCP-1, MIP-3α or IL-1α. Thus, seminal fluid TGF-β may influence cervical immune function after coitus.
Semen also impacts genital tract immune cell populations. In a cross-sectional study of healthy, HIV uninfected women in the United Kingdom, women who reported unprotected sexual intercourse within the past 3 days (n = 11) had a significantly greater proportion of CD4+ T lymphocytes in endocervical cytobrushes compared with women who were abstinent for at least 3 days (n = 20). No significant differences in density of T lymphocyte activation markers were identified between the two groups.21 In a more recent cross-sectional study of HIV uninfected women in Australia, biopsies were taken from the ectocervix 12 hr after unprotected vaginal coitus, after vaginal coitus with use of a condom or in the absence of coitus. A significant influx of macrophages, dendritic cells and memory T cells was observed after coitus.19 An ongoing study of cervical intraepithelial immune cells collected by cytobrush from healthy, HIV uninfected American women (all receiving hormonal contraception) after both barrier protected and unprotected sex suggests similar findings, with a significant increase in percentage of CD3+ lymphocytes identified by flow cytometry 10–14 hr after barrier unprotected vaginal intercourse (Nakra, Buckley and Herold, work in progress). All of these immune cell responses could increase the risk of HIV acquisition.
Special populations: adolescent and pregnant women
Adolescent women may be uniquely susceptible to HIV for several biological and behavioural reasons. Epidemiological studies in areas of high HIV prevalence, such as South Africa, have identified early sexual debut as an independent risk factor for HIV acquisition.22 Adolescent women are highly vulnerable to sexual violence; in a recent large, cross-sectional study, 18.7% of females in the United States ages 14–17 years reported a history of sexual assault.23 In a 2011 survey of 653 African American females ages 15–21 years who were participating in a STI prevention study, 24% of subjects reported a history of sexual abuse.24 Subjects reported an average of 8.4 lifetime sexual partners, and only 26% reported consistent condom use in the prior 60 days. Moreover, 10.6% of subjects reported engaging in anal sexual intercourse in the prior 60 days, which has been independently associated with a greater risk of STI in adolescents.25
High rates of STI in adolescents26–28 and the presence of cervical ectopy may result in a synergistic risk for HIV acquisition. The relationship between HIV acquisition and cervical ectopy is discussed in more detail by Venkatesh and Cu-Uvin in this issue of the Journal. Young women are more likely to have a greater degree of cervical ectopy or immature cervical epithelium, characterized by predominantly single-layer columnar epithelial cells. The degree of cervical ectopy may vary with tobacco and hormonal contraception or may increase in the setting of incident STI.29 The cervical transformation zone is especially dynamic during the first years post-menarche, when ovulation is irregular, making early sexual debut an especially vulnerable interval for STI acquisition and persistence.30–32 An increased proportion of cervical ectopy may increase STI and HIV acquisition risk by providing a larger ‘target’ with thinner epithelium. However, cervical ectopy may also impact the epithelial cytokine and chemokine network. A cross-sectional study comparing cervicovaginal lavage (CVL) concentrations of cytokines and chemokines in adolescent females in the United States with predominantly immature versus mature cervical epithelium identified significantly higher concentrations of IL-1α, IL-1β, IL-6, IL-8, MIP-1α, regulated upon activation, normal T cell expressed, and secreted (RANTES), TNF-β, IL-10, IL-12, and interferon (IFN)-γ in the group with a greater proportion of immature cervical epithelium.33
Alterations of mucosal antimicrobial peptides and microbiota in the genital tract may also impact susceptibility or response to genital tract pathogens in adolescent females.33–35 In a cross-sectional analysis of genital tract secretions collected by cervicovaginal lavage (CVL) at a single time point from 20 sexually active American adolescents [mean age ± standard deviation (SD) of 16.9 ± 0.2 years] and 54 adult females (mean age ± SD of 30.7 ± 1.1 years, P < 0.001) who had no evidence of incident genital tract infection, CVL from adolescents had higher concentrations of lactoferrin, lysozyme and human neutrophil peptides (HNP) 1–3, as well as modest increases in IL-6 and IL-1α. These findings suggest a state of genital mucosal immune activation in the adolescents sampled, which could also represent an influx of activated HIV target cells in the genital tract and thus a greater risk of HIV acquisition. Antimicrobial peptides such as HNP 1–3, lysozyme and lactoferrin have intrinsic antimicrobial activity and may contribute to the observation that genital tract secretions have variable antimicrobial activity against herpes simplex virus (HSV), HIV and bacterial pathogens ex vivo.36–44 In multiple studies of HIV uninfected adult women in the United States, Herold and Keller et al. observed that the concentrations of pro-inflammatory cytokines, lysozyme, lactoferrin and HNP 1–3 correlated with the ability of CVL to inhibit HSV plaque formation ex vivo.42,43,45 Consistent with the observation that the adolescents had higher levels of these immune mediators, adolescents also had greater anti-HSV activity relative to adults.34 Whether this finding translates to protection against HSV or is simply a biomarker of inflammation will require further studies.
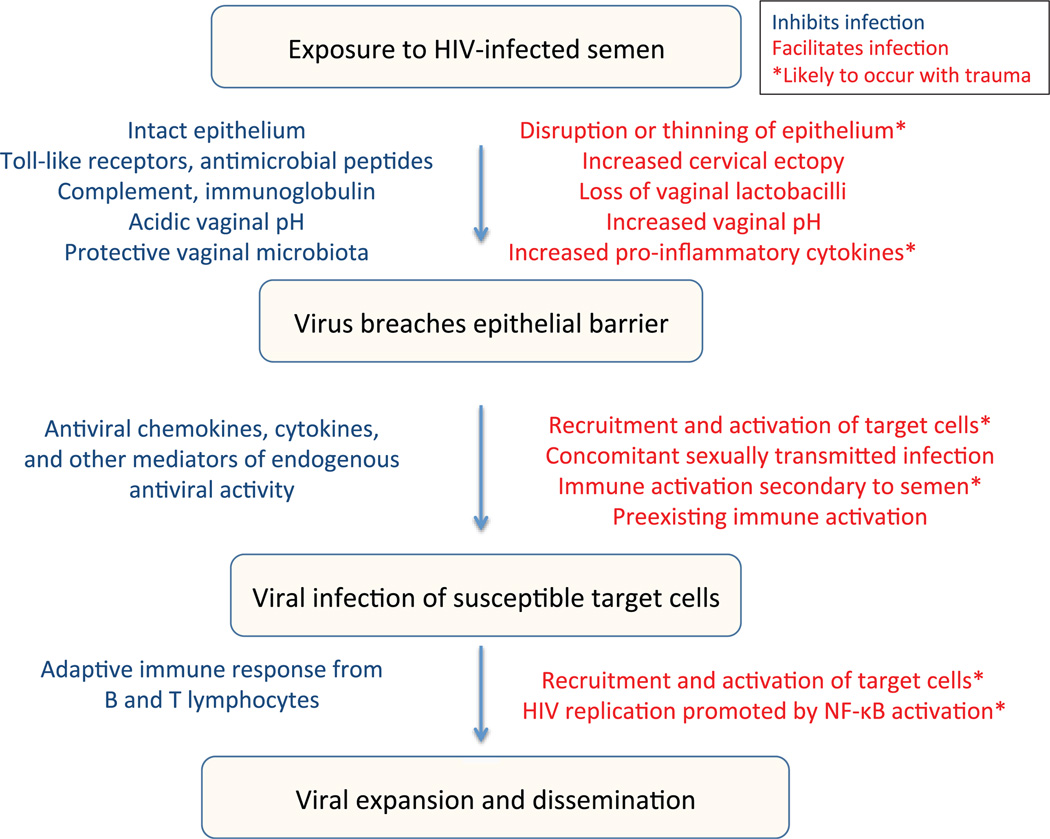
The in vivo protective effects of pro-inflammatory antimicrobial peptides remain unclear. For example, despite their ex vivo antiviral activity, higher genital HNP 1–3 levels have been independently associated with HIV acquisition, possibly reflecting an influx of HIV immune target cells.46 HNP 1–3, lysozyme and lactoferrin are released by degranulating neutrophils recruited to the genital tract in response to chemokines, including IL-8, MIP-3α and other stimuli.47 This cascade of immune activation, while augmenting host defence against some pathogens, could also recruit and activate HIV target cells,11 thus conferring a greater risk of HIV acquisition. These complex findings highlight the delicate balance of mucosal immunity (Fig. 1).
Fig. 1.
Factors in blue may inhibit HIV infection, and factors in red may facilitate infection. Asterisk denotes factors likely to occur with trauma.
While adolescents displayed increased levels of inflammatory mediators and anti-HSV activity relative to adults, they exhibited lower CVL levels of secretory leucocyte protease inhibitor (SLPI), IgG and IgA.34 Lower concentrations of vaginal SLPI have been associated with increased risk of HIV acquisition,48 and diminished cervicovaginal IgA could contribute to the high prevalence and recurrence rate of chlamydia observed in adolescents.49–53 Adolescent subjects were also noted to have a significantly higher average vaginal pH relative to reproductively mature women (mean ± SD 4.8 ± 0.08 versus 4.5 ± 0.07, P = 0.005). A paucity of Lactobacillus jensenii was observed in vaginal swabs collected from adolescents, and 30–45% of adolescents were found to be colonized with bacterial vaginosis associated bacterium (BVAB) 1, 2, 3 and Megasphaera, organisms found to be specific markers of bacterial vaginosis (BV) in older populations of women. This observed shift towards BV-associated microbiota could represent a significant risk factor for HIV acquisition,54 possibly by increasing concentrations of activated endocervical CD4+ T cells.55 Thus, a net increased inflammatory state in the genital tract of sexually active adolescents may synergize with behavioural factors to increase HIV acquisition risk.
Multiple studies have described a higher risk of HIV acquisition during pregnancy, which is not fully explained by behavioural variables.56 As with adolescents, few studies have described the genital mucosal immune environment in pregnant women. However, the available data suggest significant mucosal immune alterations in the setting of pregnancy relative to non-pregnant women, which may provide a rationale for the observed risk of HIV acquisition. Vaginal swabs collected at a single time point from 70 pregnant women in the United States at 35–37 weeks gestation had significantly higher concentrations of IL-1α, IL-1β, IL-1 receptor antagonist (ra), IL-8 and RANTES45 relative to non-pregnant women. These higher chemokine and cytokine concentrations may reflect a heightened state of immune activation to prevent ascending infection to the foetus but may also promote an influx of activated HIV target cells. Another study comparing mucosal immune mediators in CVL from pregnant or non-pregnant women also reported higher absolute concentrations of RANTES, IL-1α and IL-1ra in pregnant subjects,57 although protein-corrected CVL levels of IL-6, MCP-1, MIP-1α, elafin, human β defensin-2 and MIP-3α were lower in pregnant subjects. Of note, in this study, the in vitro ability of CVL to prevent HIV infection of TZM-bl cells was similar between pregnant and non-pregnant women, although it remains unclear if this activity translates to in vivo protection against HIV acquisition.
Bactericidal anti-E. coli activity of female genital tract secretions: harmful or protective?
The notion that the pregnant female genital tract may be modified to prevent perinatal infection is supported by the observation that vaginal swabs from pregnant women had significantly greater bactericidal E. coli activity relative to swabs collected from non-pregnant women [median percentage inhibition 65% (range 17–99) versus 26.2% (−39 to 96), respectively; P < 0.001].45 The bactericidal activity was inversely correlated with E. coli colonization, suggesting that this activity may protect against pathogens. This hypothesis is further supported by earlier studies demonstrating that women with BV exhibited reduced E. coli bactericidal activity58 and by proteomic work demonstrating that, in addition to host factors, proteins specific to Lactobacillus crispatus and jensenii may contribute to the E. coli bactericidal activity.59
However, as with the anti-HSV activity of genital tract secretions, the host and/or bacterial factors that contribute to E. coli bactericidal activity may differ between populations, and their impact on HIV risk may be complex. The increased activity observed in pregnant women could reflect an increased net state of immune activation and thus, paradoxically, a greater risk of HIV acquisition. Two studies conducted with samples from African women suggest that increased E. coli bactericidal activity may be a biomarker of increased risk of HIV acquisition. In a small sub-study that took advantage of vaginal swabs collected during the HIV Prevention Trials Network 035 study, women who acquired HIV during the trial had significantly increased bactericidal E. coli activity pre-seroconversion than women who remained HIV seronegative, even after adjusting for multiple other variables [OR 1.25 (1.04–1.49)].60 Similarly, higher levels of bactericidal E. coli activity were observed in South African women who acquired HIV during the CAPRISA 002 Acute Infection Study (R. P. Madan, J. Tugetman, L. Roberts, L. Werner, A. Grobler, S. S. Abdool Karim, J. A. Passmore, B. C. Herold, manuscript in progress). The high prevalence of BV in these cohorts suggests that mediators other than those produced by lactobacilli contributed to this activity. Thus, whether this activity serves as a biomarker of vaginal health and intact host defence or as a biomarker of inflammation and increased HIV risk (reflecting recruitment and/or activation of immune target cells) may depend on the population being studied. Larger, prospective studies are needed in diverse populations, including victims of sexual violence, to define the host and bacterial mediators of bactericidal E. coli activity in genital tract secretions and to elucidate the association between this activity, vaginal health and HIV acquisition risk.
Conclusions and future directions
The risk of HIV acquisition depends upon multiple, dynamic viral and host variables, which may be adversely impacted by sexual violence. Larger prospective studies that focus on the epithelial barrier, immune cell populations, concentrations of inflammatory and protective immune mediators, genital tract microbiota and the antimicrobial activity of genital tract secretions should promote the identification of factors that facilitate or prevent HIV infection. These studies are critical to informing the development of safe and effective oral and vaginal forms of HIV pre-exposure prophylaxis (PrEP) for adolescent and pregnant women, who are typically excluded from PrEP clinical trials. Defining the biological mechanisms that place specific populations at increased risk of HIV acquisition, including those that are more likely to be victims of sexual violence, such as adolescent and pregnant women, will accelerate the identification of novel prevention strategies.
Footnotes
Disclosures
The authors have no potential conflicts of interest to declare.
References
- 1.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lingappa JT, Hughes K, Baeten J, Fife K, De Bruyn G, Farquhar C, Kapiga S, Makhema J, Celum C. Partners in Prevention HSV/HIV Transmission Study Team: Infected Partner’s Plasma HIV-1 RNA Level and the HIV-1 Set Point of Their Heterosexual Seroconverting Partners. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2011. [Google Scholar]
- 3.Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 4.Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, Gray GE, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS ONE. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenny C, Hooton TM, Bowers A, Copass MK, Krieger JN, Hillier SL, et al. Sexually transmitted diseases in victims of rape. New Eng J Med. 1990;322:713–716. doi: 10.1056/NEJM199003153221101. [DOI] [PubMed] [Google Scholar]
- 7.Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis. 2005;32:351–357. doi: 10.1097/01.olq.0000154504.54686.d1. [DOI] [PubMed] [Google Scholar]
- 8.Barnabas RV, Webb EL, Weiss HA, Wasserheit JN. The role of coinfections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. AIDS. 2011;25:1559–1573. doi: 10.1097/QAD.0b013e3283491e3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slaughter L, Brown CR, Crowley S, Peck R. Patterns of genital injury in female sexual assault victims. Am J Obstet Gynecol. 1997;176:609–616. doi: 10.1016/s0002-9378(97)70556-8. [DOI] [PubMed] [Google Scholar]
- 10.Adams JA, Girardin B, Faugno D. Signs of genital trauma in adolescent rape victims examined acutely. J Pediatr Adolesc Gynecol. 2000;13:88. doi: 10.1016/s1083-3188(00)00015-2. [DOI] [PubMed] [Google Scholar]
- 11.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 12.Hiscott J, Kwon H, Genin P. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J Clin Investig. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvet JP, Gresenguet G, Belec L. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin Microbiol Infect. 1997;3:19–23. doi: 10.1111/j.1469-0691.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 14.Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Roan NR, Muller JA, Liu H, Chu S, Arnold F, Sturzel CM, et al. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe. 2011;10:541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 17.Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berlier W, Cremel M, Hamzeh H, Levy R, Lucht F, Bourlet T, et al. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum Reprod. 2006;21:1135–1142. doi: 10.1093/humrep/dei496. [DOI] [PubMed] [Google Scholar]
- 19.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 20.Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol. 2012;189:1024–1035. doi: 10.4049/jimmunol.1200005. [DOI] [PubMed] [Google Scholar]
- 21.Prakash M, Patterson S, Gotch F, Kapembwa MS. Recruitment of CD4 T lymphocytes and macrophages into the cervical epithelium of women after coitus. Am J Obstet Gynecol. 2003;188:376–381. doi: 10.1067/mob.2003.16. [DOI] [PubMed] [Google Scholar]
- 22.Mavedzenge SN, Weiss HA, Montgomery ET, Blanchard K, De Bruyn G, Ramjee G, et al. Determinants of differential HIV incidence among women in three southern African locations. J Acquir Immune Defic Syndr. 2011;58:89–99. doi: 10.1097/QAI.0b013e3182254038. [DOI] [PubMed] [Google Scholar]
- 23.Finkelhor D, Turner H, Ormrod R, Hamby SL. Violence, abuse, and crime exposure in a national sample of children and youth. Pediatrics. 2009;124:1411–1423. doi: 10.1542/peds.2009-0467. [DOI] [PubMed] [Google Scholar]
- 24.Swartzendruber A, Brown JL, Sales JM, Murray CC, DiClemente RJ. Sexually transmitted infections, sexual risk behavior, and intimate partner violence among African American adolescent females with a male sex partner recently released from incarceration. J Adolesc Health. 2012;51:156–163. doi: 10.1016/j.jadohealth.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diclemente RJ, Wingood GM, Crosby RA, Salazar LF, Head S, Rose E, et al. Anal sex is a behavioural marker for laboratory-confirmed vaginal sexually transmissible infections and HIV-associated risk among African-American female adolescents. Sex Health. 2009;6:111–116. doi: 10.1071/SH08062. [DOI] [PubMed] [Google Scholar]
- 26.Geisler WM. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53(Suppl 3):S92–S98. doi: 10.1093/cid/cir698. [DOI] [PubMed] [Google Scholar]
- 27.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–110. [PubMed] [Google Scholar]
- 28.Prevention CfDCa. 2010 Sexually Transmitted Disease Surveillance. [accessed September 4 2010];2010 Available from: http://www.cdc.gov/std/stats10/adol.htm.
- 29.Hwang LY, Ma Y, Benningfield SM, Clayton L, Hanson EN, Jay J, et al. Factors that influence the rate of epithelial maturation in the cervix in healthy young women. J Adolesc Health. 2009;44:103–110. doi: 10.1016/j.jadohealth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss GB, Clemetson D, D’Costa L, Plummer FA, Ndinya-Achola JO, Reilly M, et al. Association of cervical ectopy with heterosexual transmission of human immunodeficiency virus: results of a study of couples in Nairobi, Kenya. J Infect Dis. 1991;164:588–591. doi: 10.1093/infdis/164.3.588. [DOI] [PubMed] [Google Scholar]
- 31.Mansfield MJ, Emans SJ. Adolescent menstrual irregularity. J Reprod Med. 1984;29:399–410. [PubMed] [Google Scholar]
- 32.World Health Organization. World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. I. A multicenter cross-sectional study of menarche. World Health Organization Task Force on Adolescent Reproductive Health. J Adolesc Health. 1986;7:229–235. [PubMed] [Google Scholar]
- 33.Hwang LY, Scott ME, Ma Y, Moscicki AB. Higher levels of cervicovaginal inflammatory and regulatory cytokines and chemokines in healthy young women with immature cervical epithelium. J Reprod Immunol. 2011;88:66–71. doi: 10.1016/j.jri.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madan RP, Carpenter C, Fiedler T, Kalyoussef S, McAndrew TC, Viswanathan S, et al. Altered biomarkers of mucosal immunity and reduced vaginal lactobacillus concentrations in sexually active female adolescents. PLoS ONE. 2012;7:e40415. doi: 10.1371/journal.pone.0040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrier LA, Bowman FP, Lin M, Crowley-Nowick PA. Mucosal immunity of the adolescent female genital tract. J Adolesc Health. 2003;32:183–186. doi: 10.1016/s1054-139x(02)00536-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Owen SM, Rudolph DL, Cole AM, Hong T, Waring AJ, et al. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J Immunol. 2004;173:515–520. doi: 10.4049/jimmunol.173.1.515. [DOI] [PubMed] [Google Scholar]
- 37.Hazrati E, Galen B, Lu W, Wang W, Ouyang Y, Keller MJ, et al. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J Immunol. 2006;177:8658–8666. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- 38.Saidi H, Eslahpazir J, Carbonneil C, Carthagena L, Requena M, Nassreddine N, et al. Differential modulation of human lactoferrin activity against both R5 and X4-HIV-1 adsorption on epithelial cells and dendritic cells by natural antibodies. J Immunol. 2006;177:5540–5549. doi: 10.4049/jimmunol.177.8.5540. [DOI] [PubMed] [Google Scholar]
- 39.Valimaa H, Tenovuo J, Waris M, Hukkanen V. Human lactoferrin but not lysozyme neutralizes HSV-1 and inhibits HSV-1 replication and cell-to-cell spread. Virol J. 2009;6:53. doi: 10.1186/1743-422X-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee-Huang S, Huang PL, Sun Y, Huang PL, Kung HF, Blithe DL, et al. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc Natl Acad Sci USA. 1999;96:2678–2681. doi: 10.1073/pnas.96.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee-Huang S, Maiorov V, Huang PL, Ng A, Lee HC, Chang YT, et al. Structural and functional modeling of human lysozyme reveals a unique nonapeptide, HL9, with anti-HIV activity. Biochemistry. 2005;44:4648–4655. doi: 10.1021/bi0477081. [DOI] [PubMed] [Google Scholar]
- 42.John M, Keller MJ, Fam EH, Cheshenko N, Hogarty K, Kasowitz A, et al. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis. 2005;192:1731–1740. doi: 10.1086/497168. [DOI] [PubMed] [Google Scholar]
- 43.Shust GF, Cho S, Kim M, Madan RP, Guzman EM, Pollack M, et al. Female genital tract secretions inhibit herpes simplex virus infection: correlation with soluble mucosal immune mediators and impact of hormonal contraception. Am J Reprod Immunol. 2010;63:110–119. doi: 10.1111/j.1600-0897.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keller MJ, Madan RP, Torres NM, Fazzari MJ, Cho S, Kalyoussef S, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS ONE. 2011;6:e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghartey JP, Carpenter C, Gialanella P, Rising C, McAndrew TC, Mhatre M, Tugetman J, Einstein MH, Chazotte C, Herold BC. Association of bactericidal activity of genital tract secretions with Escherichia coli colonization in pregnancy. Am J Obstet Gynecol. 2012;207:297. doi: 10.1016/j.ajog.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levinson P, Kaul R, Kimani J, Ngugi E, Moses S, MacDonald KS, et al. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS. 2009;23:309–317. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 47.Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199–230. [PubMed] [Google Scholar]
- 48.Draper DL, Landers DV, Krohn MA, Hillier SL, Wiesenfeld HC, Heine RP. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am J Obstet Gynecol. 2000;183:1243–1248. doi: 10.1067/mob.2000.107383. [DOI] [PubMed] [Google Scholar]
- 49.McComb DE, Nichols RL, Semine DZ, Evrard JR, Alpert S, Crockett VA, et al. Chlamydia trachomatis in women: antibody in cervical secretions as a possible indicator of genital infection. J Infect Dis. 1979;139:628–633. doi: 10.1093/infdis/139.6.628. [DOI] [PubMed] [Google Scholar]
- 50.Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol (Oxford) 1990;71:403–410. [PMC free article] [PubMed] [Google Scholar]
- 51.Wolner-Hanssen P, Patton DL, Holmes KK. Protective immunity in pig-tailed macaques after cervical infection with Chlamydia trachomatis. Sex Transm Dis. 1991;18:21–25. doi: 10.1097/00007435-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Rank RG, Whittum-Hudson JA. Protective immunity to chlamydial genital infection: evidence from animal studies. J Infect Dis. 2010;201(Suppl 2):S168–S177. doi: 10.1086/652399. [DOI] [PubMed] [Google Scholar]
- 53.Batteiger BE, Tu W, Ofner S, Van Der Pol B, Stothard DR, Orr DP, et al. Repeated Chlamydia trachomatis genital infections in adolescent women. J Infect Dis. 2010;201:42–51. doi: 10.1086/648734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rebbapragada A, Howe K, Wachihi C, Pettengell C, Sunderji S, Huibner S, et al. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J Acquir Immune Defic Syndr. 2008;49:520–522. doi: 10.1097/QAI.0b013e318189a7ca. [DOI] [PubMed] [Google Scholar]
- 56.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 57.Anderson BL, Ghosh M, Raker C, Fahey J, Song Y, Rouse DJ, et al. In vitro anti-HIV-1 activity in cervicovaginal secretions from pregnant and nonpregnant women. Am J Obstet Gynecol. 2012;207:65, e61. doi: 10.1016/j.ajog.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valore EV, Wiley DJ, Ganz T. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun. 2006;74:5693–5702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalyoussef S, Nieves E, Dinerman E, Carpenter C, Viswanathan S, Oh J, Burd B, Angeletti RH, Buckheit KN, Fredricks DN, Madan RP, Keller MJ, Herold BC. Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions. PLoS ONE. doi: 10.1371/journal.pone.0049506. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dezzutti CS, Richardson BA, Marrazzo JM, Tugetman J, Ramjee G, Taha T, Chirenje ZM, Abdool Karim SS, Hillier SL, Herold BC on behalf of the MTN Biomedical Sciences Working Group and the HPTN 035 Protocol Team. Mucosal E. coli bactericidal activity and immune mediators are associated with HIV-1 seroconversion in women participating in the HPTN 035 trial. J Infect Dis. 2012 Oct 8; doi: 10.1093/infdis/jis555. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]