Abstract
Ameloblastomas are regarded as a homogeneous group of neoplasms with locally invasive character. They generally do not show induction of dental hard tissue formation except in few cases. Biological behavior and histogenesis of these tumors is still unexplored as there is lack of relevant studies and long follow-up of these patients. So, we aimed to report this rare case of dentinoameloblastoma with unique presence of ghost cells in middle-aged female involving maxilla with emphasis on its biological behavior. We conclude that although histogenesis of this tumor is not clear but biological potential is similar to conventional ameloblastoma requiring wider excision.
Keywords: Ameloblastoma, biological potential, dentinoid, ghost cells
INTRODUCTION
Ameloblastomas arise from odontogenic epithelium and are locally invasive butfollow a benign course in the majority of cases.[1] Ameloblastoma generally do not show induction of dental hard tissue formation except odontoameloblastoma which is classified as a distinct entity in World Health Organization (WHO) classification of odontogenic tumors.[2] Because of the presence of odontogenic ectomesenchyme in odontoameloblastoma, inductive changes take place leading to the formation of dentin and enamel in parts of the tumor.[3] But in few instances dentinoid formation without concomitant enamel has also been reported.[2]
Large series published on ameloblastomas often make no mention of features other than those classically described and most infrequent findings are reported as case studies. This has lead to a generally accepted view that ameloblastomas are fairly homogeneous in their clinical and pathological presentation.
Till date very few cases of ameloblastoma with hard tissue formation have been reported in English literature and no author has investigated the biological behavior of this tumor. Considering its rarity and unexplored biological behavior directed us to report an unusual case of ameloblastoma with dentinoid induction-Dentinoameloblastoma along with unique presence of ghost cells. We have also assessed the biological behavior of the lesion using α-SMA as an immunohisochemical marker.
CASE REPORT
A 45-year-old female patient complained of painless swelling in upper front region of jaw and face from past 1 year. The swelling was apparently asymptomatic and static in size till the patient got her upper right tooth extracted due to tooth decay. After a week of tooth extraction, the patient noticed a swelling on buccal aspect of extracted tooth which is since then continuously increased and reached to the size of 2 × 5 cm in diameter. The swelling involved right side of the face extending from lateral to right ala of nose to infraorbital margin superiorly to inferior border of mandible inferiorly with diffuse margins. There was slight tenderness on palpation on anterior extension (near ala of nose) of the lesion. Bilateral submandibular lymph nodes were palpable.
Intraoral examination revealed firm, smooth, nontender, nonindurate painless swelling measuring 2 × 5 cm in diameter with diffuse borders extending from maxillary first premolar to first molar on right side and posteriorly to the maxillary tuberosity area along with obliteration of right buccal vestibule [Figure 1].
Figure 1.
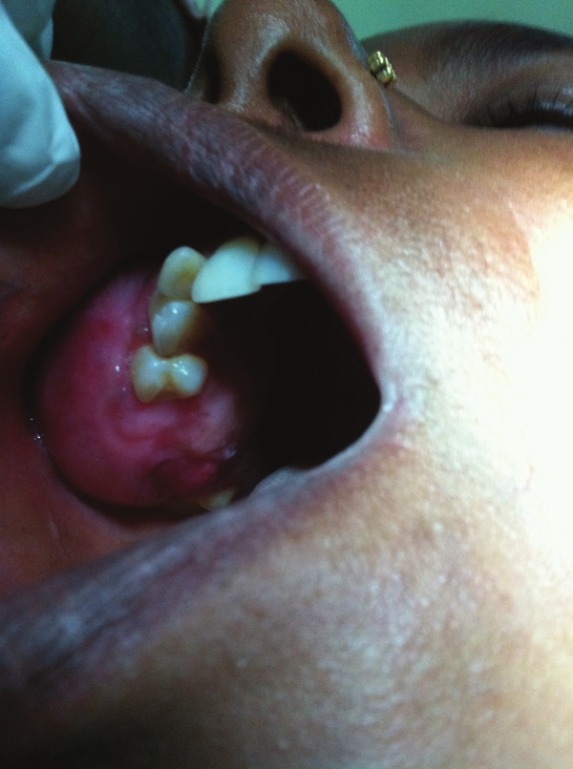
Intraoral photograph showing smooth lobulated swelling, 2 × 5 cm in diameter extending from maxillary first premolar to first molar on right side and posteriorly to the maxillary tuberosity area
Cone beam computed tomography (CBCT) evaluation demonstrated a lesion in the right maxillary alveolus extending from distal aspect of 13 and 14 posteriorly to mesial aspect of 17 and inferiorly from alveolar ridge superiorly upto the floor of maxillary sinus. There is complete loss of bony structures and perforation of buccal and palatal cortical plates with internal flecks of calcifications and resorption of root in relation to 14 [Figures 2-5].
Figure 2.
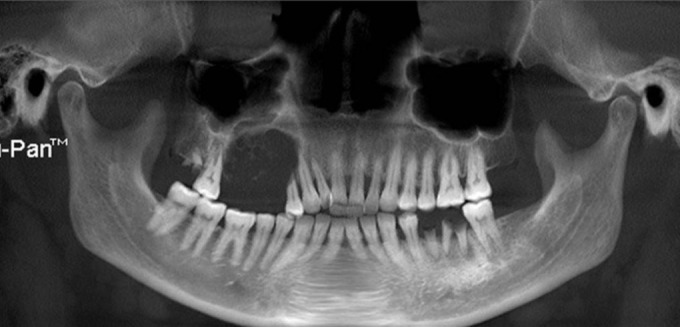
Panoramic CBCT demonstrating the lesion causing loss of bony structures with internal calcifications and resorption of root in relation to 14
Figure 5.
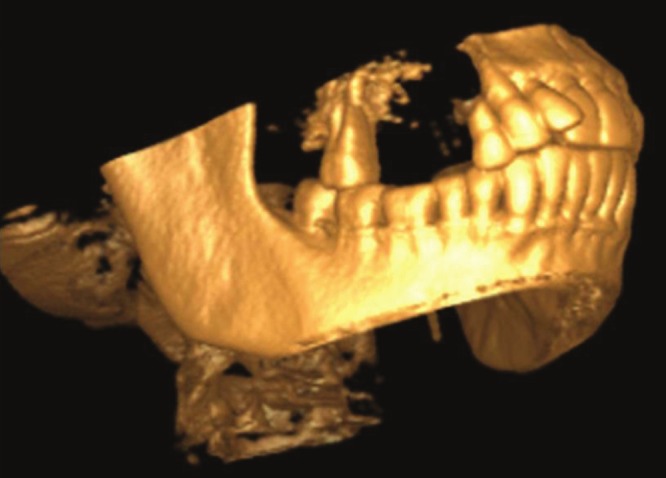
3-D CBCT volumetric reconstruction demonstrates complete perforation of buccal and lingual cortical plates with flecks of calcification
Figure 3.
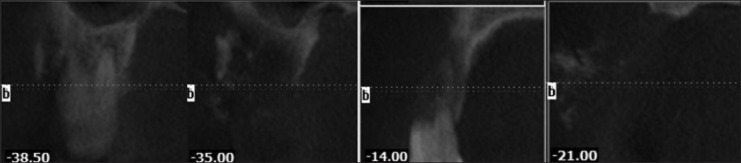
Serial cross-sectional images demonstrating the lesion
Figure 4.
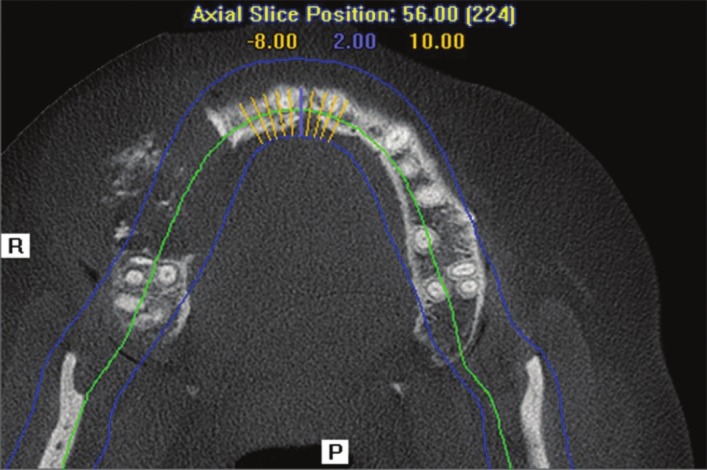
Axial CBCT section at level of maxillary alveolus demonstrate buccal and palatal cortical plates destruction with areas of calcification and soft tissue extent of the lesion
Hematological and urine examinations did not reveal any abnormal findings.
Based on clinicoradiographic findings, ossifying fibroma, calcifying epithelial odontogenic tumor (CEOT), adenomatoid odontogenic tumor (AOT) were considered in differential diagnosis and incisional biopsy was performed.
Histopathological examination of partially decalcified tissue with hematoxylin and eosin stain revealed well-encapsulated mass with peripheral areas showing interconnected odontogenic islands with tall columnar to cuboidal cells with polarized hyperchromatic nucleus at periphery with central stellate reticulum like areas, merging with sheets of closely packed ovoid to spindle cells interspersed with abundant amounts of an eosinophilic homogeneous extracellular material representing dentinoid or osteodentin is evident [Figures 6 and 7]. This dentinoid material stained positively for collagen van Gieson and Masson's trichrome. Numerous ghost cells lying in close association to epithelium were also observed. Connective tissue stroma is mature showing dense collagen bundles infiltrated with few chronic inflammatory cells seen.
Figure 6.
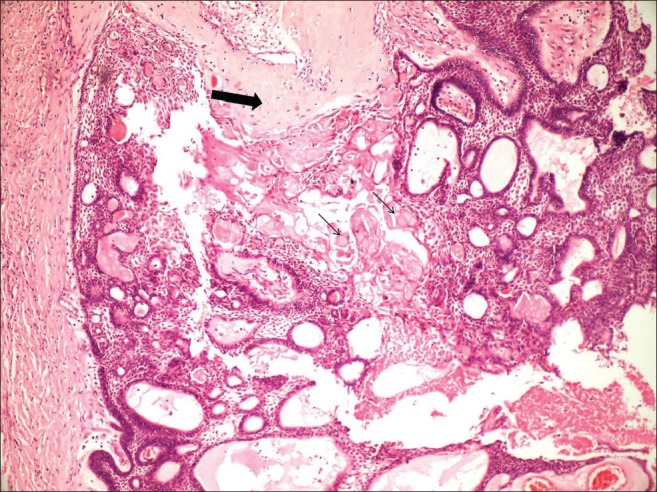
Interconnected odontogenic islands with ameloblast-like cells and central stellate reticulum-like areas in association with dentinoid-like material (large arrow) and ghost cells (small arrow) (H and E, Scanner view)
Figure 7.
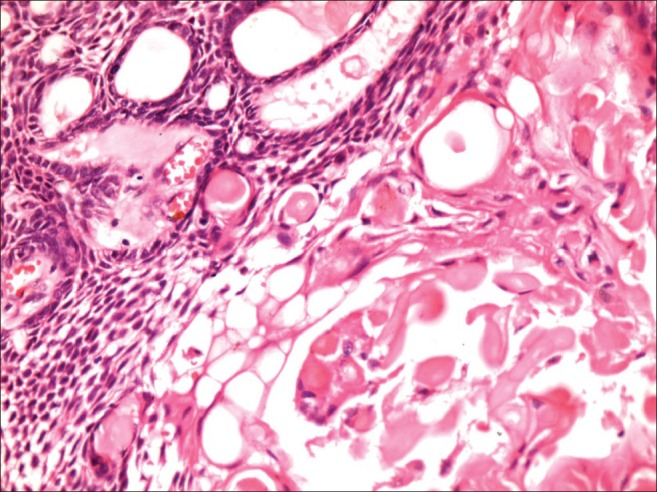
Odontogenic islands in association with numerous ghost cells (H and E, ×40)
Based on the available supporting evidences, final diagnosis of plexiform ameloblastoma with dentinoid induction along with ghost cells-dentinoameloblastoma was given. Immunohistochemical staining with α-SMA was carried out to know the biological behavior of the lesion. There was no increase in α-SMA-positive myofibroblasts in connective tissue stroma [Figure 8].
Figure 8.
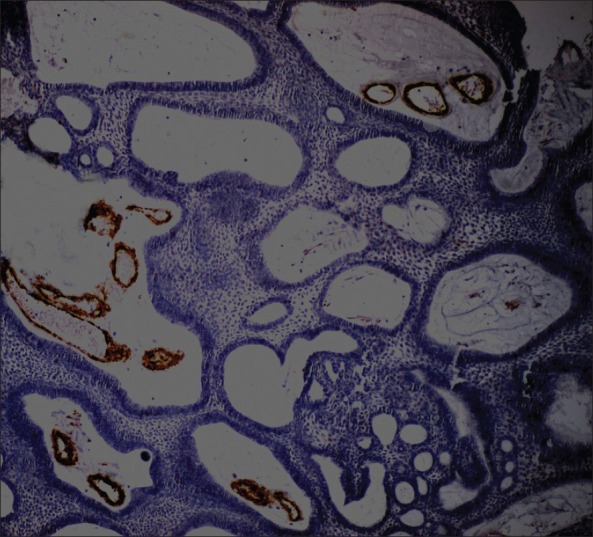
α-SMA-positive staining in vicinity of blood vessels in odontogenic islands
Current trends favor a more radical approach encompassing not only tumor itself but also a minimum border of apparently healthy bone. Hemimaxillectomy was performed involving surrounding healthy soft tissue. Histopathological examination of excised specimen reconfirmed the initial diagnosis. The postoperative recovery of patient was uneventful and 1 month later prosthetic rehabilitation was initiated. The patient was followed up for 2 years with no evidence of recurrence.
DISCUSSION
Dentinoameloblastoma has been originally described by Slabbert et al.,[2] who observed intimate association of dentinoid like material with odontogenic epithelium in unicystic neoplasm containing typical follicles of ameloblastoma along with psammomatous-type dystrophic calcifications in male patient of Asian ethnic origin. However, the present case showed plexiform ameloblastoma along with large amount of dentinoid/osteodentin-like material with an additional unique presence of ghost cells. Moridani et al.,[4] reported a similar case of plexiform ameloblastoma and adenomatoid odontogenic tumor along with hard tissue formation. Presence of ghost cells in ameloblastoma is not a usual feature but ameloblastic fibrodontoma, odontoma has shown convincing evidences of presence of ghost cells. The ghost cells are anucleate and retain the outline of the cell membrane. These cells undergo dystrophic mineralization characterized by fine basophilic granularity, which may eventually result in large sheets of calcified material. On occasion, ghost cells may become displaced in the connective tissue wall, eliciting a foreign-body giant cell response.[5] Recently Sonone et al.,[6] also reported a case of adenoid ameloblastoma with dentinoid induction with presence of ghost cells.
In the cases reported by Evans, et al.,[1] Slabbert, et al.,[2] Orlowski, et al.,[7] Matsumoto, et al.,[8] Tajima, et al.,[9] and in the present report, hard tissue formation was found with tumor diagnosed as ameloblastoma, while in six other reports the tumor was AOT. Bone formation was observed in one neoplasm but in other reports the hard tissue had been interpreted as dentin or dentinoid. Interstitial ossification has been reported in two cases of polycystic ameloblastoma[10] and enamel matrix formation was described in two reports. Our present case revealed presence of dentinoid-like material which showed positive staining for massons trichome and van Gieson staining.
Odontoameloblastoma has been categorized as odontogenic tumor with/without hard tissue formation in WHO 2005 classification.[11] WHO and Philipsen and Reichart[12] have defined it as a neoplasm that includes odontogenic ectomesenchyme in addition to odontogenic epithelium that resembles an ameloblastoma (SMA) in both structure and behavior. Because of the presence of odontogenic ectomesenchyme, inductive changes take place leading to the formation of dentin and enamel in parts of the tumor.
Orlowski et al.,[7] in 1991 described an odontogenic tumor that exhibited features of unicystic plexiform ameloblastoma with dentinogenesis. They chose not to use the name “odontoameloblastoma” or “dentinoameloblastoma” although dental hard tissue formation occurred. The emphasis was placed on the neoplastic component, rather than a product.
Dentinoameloblastoma shows dentinoid induction without concomitant enamel formation in structural and functional ameloblastoma where as odontoameloblastoma shows enamel and dentinoid formation in ameloblatoma. So, authors feel that dentinoameloblastoma forms an undifferentiated stage of odontoameloblastoma as currently accepted thoughts on tooth embryogenesis suggest that enamel formation can only occur subsequent to induction of ameloblasts by dentin. Hard tissue formation observed in dentinoameloblastoma is hamartomatous or because of inductive stimuli produced by the proliferating epithelium over the mesenchymal tissue is still unresolved. Papagerakis, et al.,[13] demonstrated that ameloblastic epithelial cells in mixed odontogenic tumors expressed gene products normally present in ectomesenchymal cells and resulted in conversion and coexpression of mesenchymal phenotype. Thus, it is probable that neoplastic epithelial cells committed to ameloblastic differentiation could produce the dentinoid which exists in some tumors. It has been seen that induction in ameloblastomas is extremely rare which suggest that attempts to classify odontogenic tumors on the basis of induction will be unsound.
Myofibroblasts are a unique group of cells phenotypically intermediate between smooth muscle cells and fibroblast which have the potential to facilitate progression of neoplastic epithelial lesions[14] and are identified by immiunohistochemical marker α-SMA. Vered, et al.,[15] suggested that when more myofibroblats are present in the stroma, a more aggressive behavior of the odontogenic cyst/tumor can be anticipated. However, our present case did not show increase in myofibroblasts. So, we suggested that the biological behavior of dentinoameloblastoma would be expected to be the same as that of conventional ameloblastomas and therapy would consist of a wide excision or jaw resection in large tumors.
CONCLUSION
Although ameloblastomas are generally regarded as a homogeneous group of neoplasms, detailed investigations prove clinicopathological diversity in a significant number of tumors. Many of these changes emphasize the differentiation potential of neoplastic odontogenic epithelium and add interesting parameters to the study of tissue reactions associated with this common odontogenic tumor. Further case reports on dentinoameloblastomas are expected to shed a light on the biological behavior and nature of this unique tumor.
Footnotes
Source of Support: Borne by the Authors
Conflict of Interest: None declared
REFERENCES
- 1.Evans BL, Carr RF, Phillipe LJ. Adenoid ameloblastoma with dentinoid: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:583–8. doi: 10.1016/j.tripleo.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 2.Slabbert H, Altini M, Crooks J, Uys P. Ameloblastoma with dentinoid induction: Dentinoameloblastoma. J Oral Pathol Med. 1992;21:46–8. doi: 10.1111/j.1600-0714.1992.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 3.Dive A, Khandekar S, Bodhade A, Dhobley A. Odontoameloblastoma. J Oral Maxillofac Pathol. 2011;15:60–4. doi: 10.4103/0973-029X.80028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghasemi-Moridani S, Yazdi I. Adenoid Ameloblastoma with Dentinoid: A Case Report. Arch Iran Med. 2008;11:110–2. [PubMed] [Google Scholar]
- 5.Tomich CE. Calcifying odontogenic cyst and dentinogenic ghost cell tumor. Oral Maxillofac Surg Clin North Am. 2004;16:391–7. doi: 10.1016/j.coms.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Sonone A, Hande A, Chaudhary M, Bonde R, Sheorain A, Agni N. Adenoid ameloblastoma with dentinoid and ghost cells. A composite odontogenic tumour: A rare case report and review of the literature. Oral Surg. 2011;4:77–81. [Google Scholar]
- 7.Orlowski WA, Doyle JL, Salb R. Unique odontogenic tumor with dentinogenesis and features of unicystic plexiform ameloblastoma. Oral Surg Oral Med Oral Pathol. 1991;72:91–4. doi: 10.1016/0030-4220(91)90196-j. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Mizoue K, Seto K. Atypical plexiform ameloblastoma with dentinoid: Adenoid ameloblastoma with dentinoid. J Oral Pathol Med. 2001;30:251–4. doi: 10.1034/j.1600-0714.2001.300410.x. [DOI] [PubMed] [Google Scholar]
- 9.Tajima Y, Sakamoto E, Yamamoto Y. Odontogenic cyst giving rise to an adenomatoid odontogenic tumor: Report of a case with peculiar features. J Oral Maxillofac Surg. 1992;50:190–3. doi: 10.1016/0278-2391(92)90370-f. [DOI] [PubMed] [Google Scholar]
- 10.Raubenheimer EJ, van Heerden WF, Noffke CE. Infrequent clinicopathological findings in 108 ameloblastomas. J Oral Pathol Med. 1995;24:227–32. doi: 10.1111/j.1600-0714.1995.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 11.Philipsen HP. Keratocystic odontogenic tumour. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Head and neck tumours. Pathology and Genetics. WHO Classification of tumours. IARC Press: Lyon; 2005. pp. 306–7. [Google Scholar]
- 12.Reichart PA, Philipsen HP. Chicago: Quintessence Publication; 2004. Odontogenic Tumors and Allied Lesions; pp. 171–3. [Google Scholar]
- 13.Papagerakis P, Peuchmaur M, Hotton D, Ferkdadji L, Delmas P, Sasaki S, et al. Aberrant gene expression in epithelial cells of mixed odontogenic tumors. J Dent Res. 1999;78:20–30. doi: 10.1177/00220345990780010201. [DOI] [PubMed] [Google Scholar]
- 14.Mashhadiabbas F, Moghadam SA, Moshref M, Elahi M. Immunohistochemical Detection and Ultrastructure of Myofibroblasts in the Stroma of Odontogenic Cysts and Ameloblastoma. Iran Red Crescent Med J. 2010;12:453–7. [Google Scholar]
- 15.Vered M, Shohat I, Buchner A, Dayan D. Myofibroblasts in stroma of odontogenic cysts and tumors can contribute to variations in the biological behavior of lesions. Oral Oncol. 2005;41:1028–33. doi: 10.1016/j.oraloncology.2005.06.011. [DOI] [PubMed] [Google Scholar]


