Abstract
Flow cytometric (FCM) analysis of DNA content was performed on 82 lymph node and peripheral blood specimens from 46 patients with mycosis fungoides and the Sézary syndrome. Overall, 32 of the 46 patients (70%) had aneuploidy detected by FCM. Aneuploidy was present in 63% of the patients at the time of diagnosis before systemic therapy. In these patients, aneuploidy was frequently detected in blood and lymph node specimens scored as negative by cytology and histology, suggesting that unsuspected extracutaneous dissemination is present in many patients at the time of diagnosis.
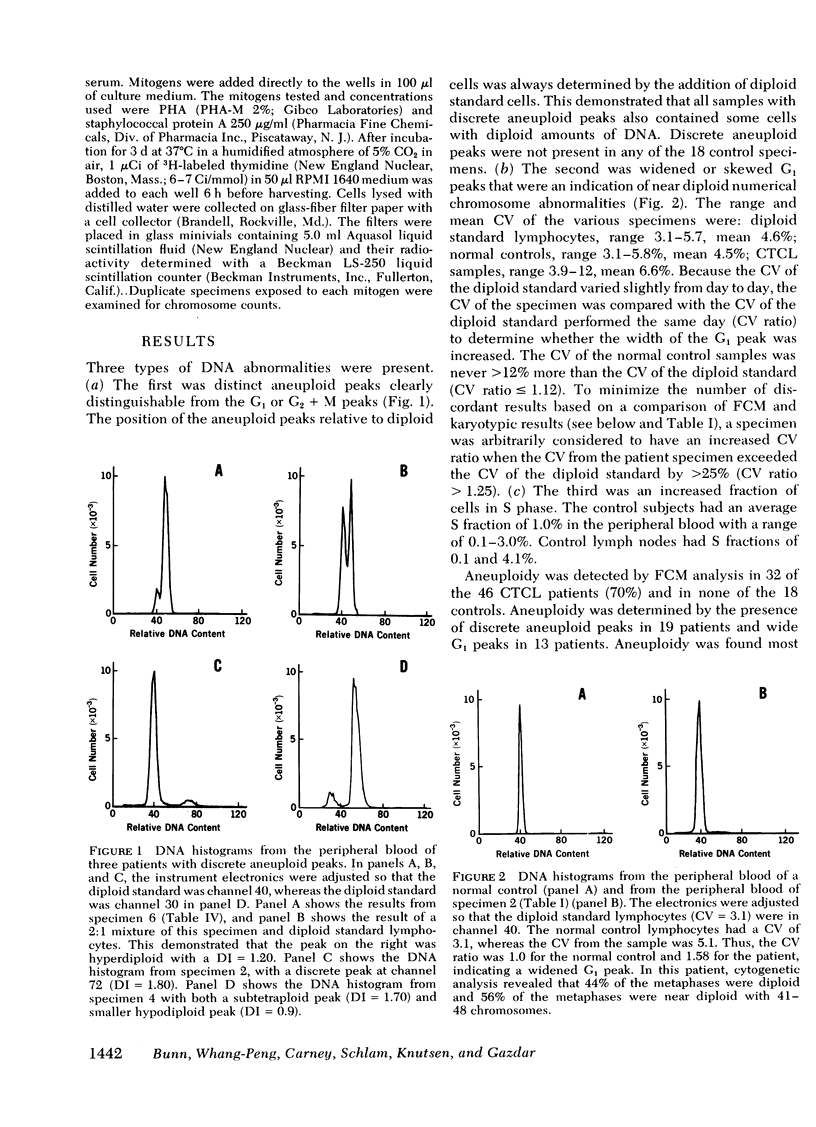
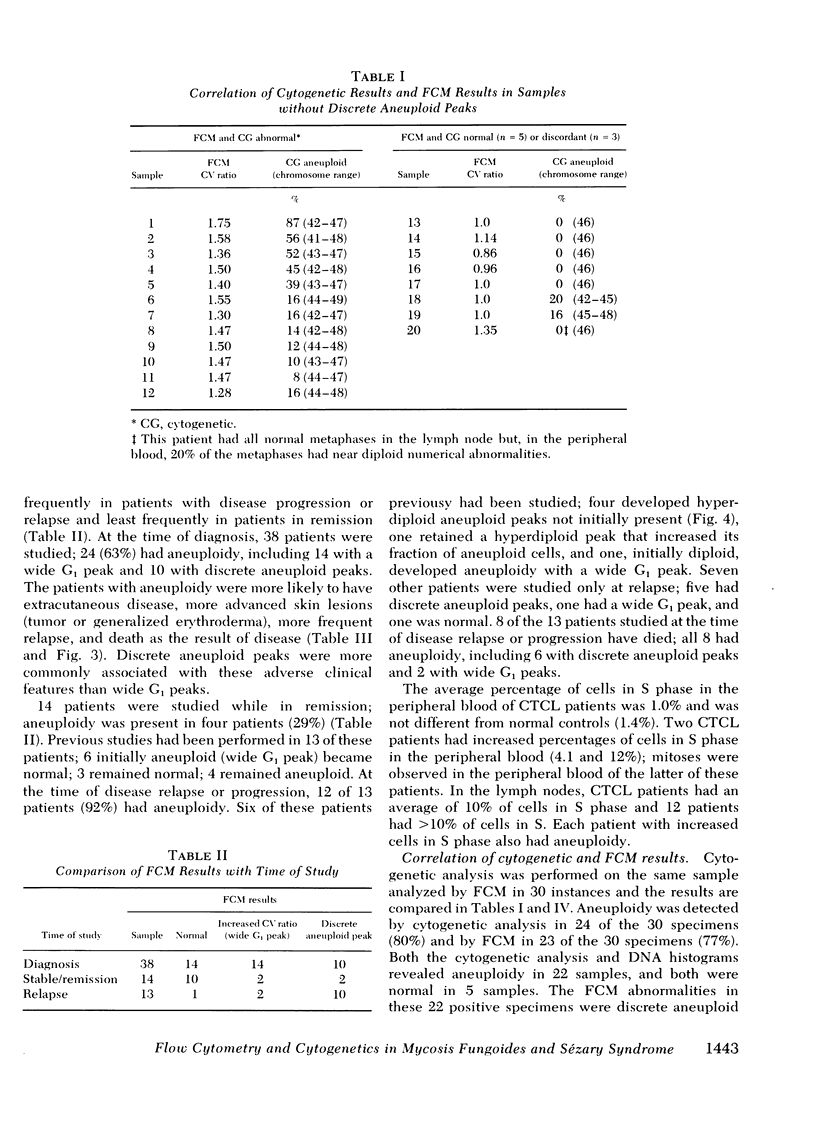
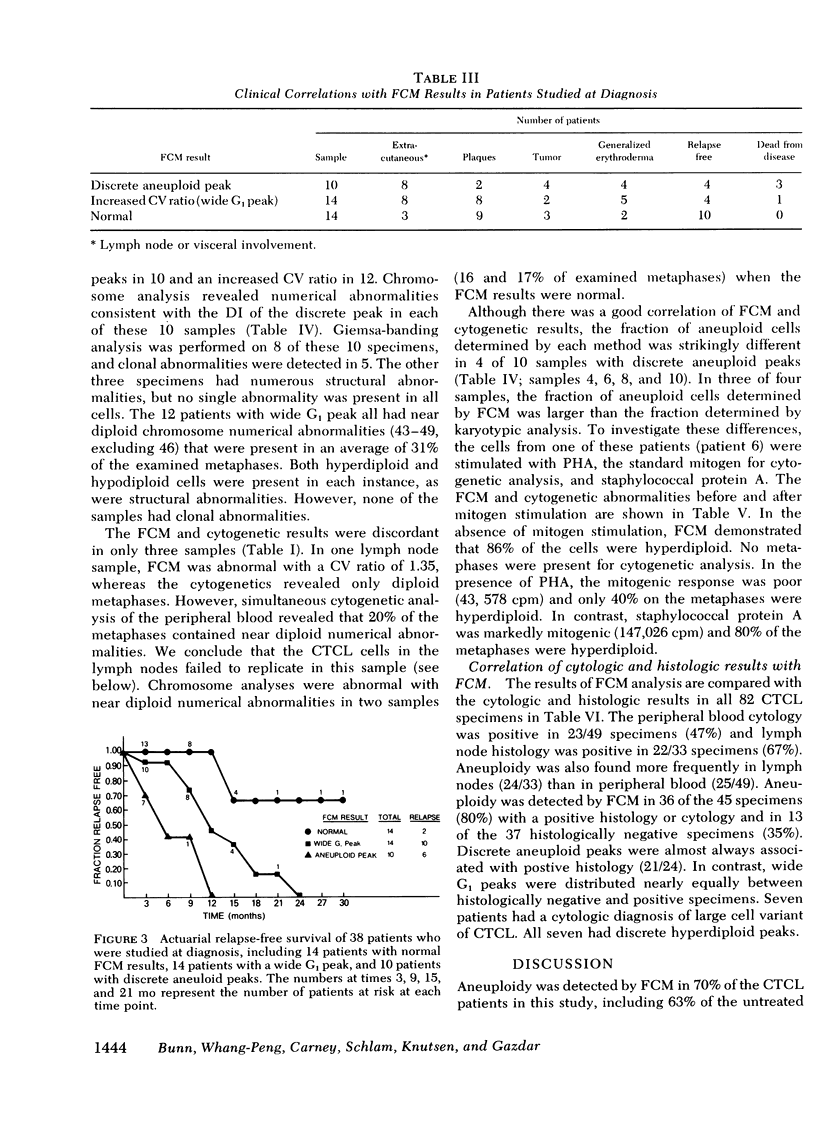
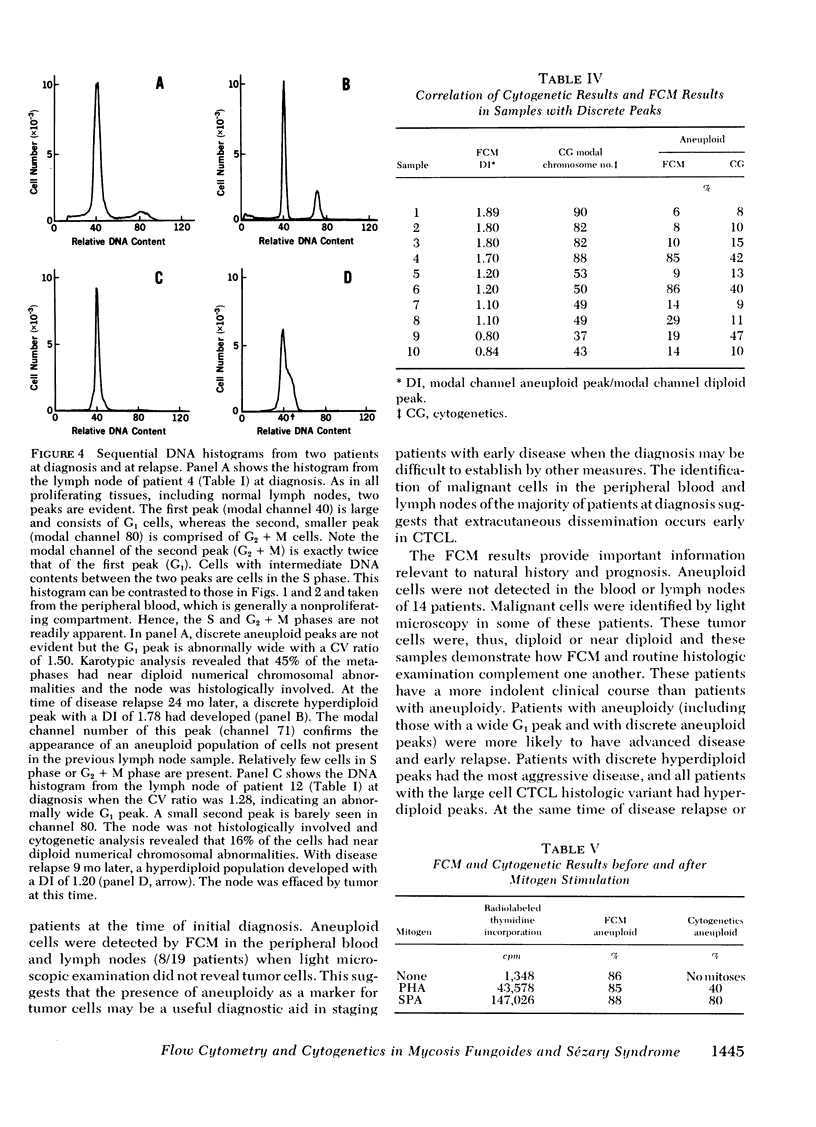
Direct comparison with Giemsa-banded cytogenetic studies showed an excellent correlation of FCM results and cytogenetic chromosome number. However, FCM frequently detected a larger fraction of aneuploid cells, and mitogen-stimulation studies suggest this is the result of preferential stimulation of normal lymphocytes by phytohemagglutinin. Thus, mitogens with a preference for malignant T cells, such as staphylococcal protein A, should be used for cytogenetic analysis of malignant T-cell disorders.
At diagnosis, some histologically positive specimens contained only diploid cells by FCM and cytogenetic analysis. These patients had a more indolent clinical course than patients with aneuploidy. Aneuploidy was detected by FCM as either wide G1 or as discrete aneuploid peaks. The presence of aneuploidy at any time in the clinical course implied a poor prognosis. Discrete hyperdiploid peaks were associated with large cell histology, early relapse, and aggressive clinical course. The development of hyperdiploidy at relapse was documented in four patients and was associated with a transition to large cell histology and a poor prognosis. Similar studies may elucidate differences in natural history and mechanism for transition in histology in other lymphomas and solid tumors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlogie B., Göhde W., Johnston D. A., Smallwood L., Schumann J., Drewinko B., Freireich E. J. Determination of ploidy and proliferative characteristics of human solid tumors by pulse cytophotometry. Cancer Res. 1978 Oct;38(10):3333–3339. [PubMed] [Google Scholar]
- Barlogie B., Hittelman W., Spitzer G., Trujilo J. M., Hart J. S., Smallwood L., Drewinko B. Correlation of DNA distribution abnormalities with cytogenetic findings in human adult leukemia and lymphoma. Cancer Res. 1977 Dec;37(12):4400–4407. [PubMed] [Google Scholar]
- Bichel P., Frederiksen P., Kjaer T., Thommesen P., Vindelov L. L. Flow microfluorometry and transrectal fine-needle biopsy in the classification of human prostatic carcinoma. Cancer. 1977 Sep;40(3):1206–1211. doi: 10.1002/1097-0142(197709)40:3<1206::aid-cncr2820400334>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Braylan R. C., Fowlkes B. J., Jaffe E. S., Sanders S. K., Berard C. W., Herman C. J. Cell volumes and DNA distributions of normal and neoplastic human lymphoid cells. Cancer. 1978 Jan;41(1):201–209. doi: 10.1002/1097-0142(197801)41:1<201::aid-cncr2820410129>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Mullaney P. F., Steinkamp J. A. Methods and applications of flow systems for analysis and sorting of mammalian cells. Methods Cell Biol. 1975;9(0):179–246. doi: 10.1016/s0091-679x(08)60076-x. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossen P. E., Mellor J. E., Finley A. G., Ravich R. B., Vincent P. C., Gunz F. W. The Sézary syndrome: cytogenetic studies and identification of the Sézary Cell as an abnormal lymphocyte. Am J Med. 1971 Jan;50(1):24–34. doi: 10.1016/0002-9343(71)90201-4. [DOI] [PubMed] [Google Scholar]
- Cullen M. H., Lister T. A., Brearley R. I., Shand W. S., Stansfeld A. G. Histological transformation of non-Hodgkin's lymphoma: a prospective study. Cancer. 1979 Aug;44(2):645–651. doi: 10.1002/1097-0142(197908)44:2<645::aid-cncr2820440234>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Edelson R. L., Kirkpatrick C. H., Shevach E. M., Schein P. S., Smith R. W., Green I., Lutzner M. Preferential cutaneous infiltration by neoplastic thymus-derived lymphocytes. Morphologic and functional studies. Ann Intern Med. 1974 Jun;80(6):685–692. doi: 10.7326/0003-4819-80-6-685. [DOI] [PubMed] [Google Scholar]
- Gahrton G., Zech L., Robèrt K. H., Bird A. G. Mitogenic stimulation of leukemia cells by Epstein-Barr virus. N Engl J Med. 1979 Aug 23;301(8):438–439. doi: 10.1056/nejm197908233010823. [DOI] [PubMed] [Google Scholar]
- Golomb H. M. Clinical implications of chromosome abnormalities in acute non-lymphocytic leukemia: current status. Virchows Arch B Cell Pathol. 1978 Nov 17;29(1-2):73–79. doi: 10.1007/BF02899339. [DOI] [PubMed] [Google Scholar]
- Golomb H. M., Vardiman J. W., Rowley J. D., Testa J. R., Mintz U. Correlation of clinical findings with quinacrine-banded chromosomes in 90 adults with acute nonlymphocytic leukemia: an eight-year study (1970-1977). N Engl J Med. 1978 Sep 21;299(12):613–619. doi: 10.1056/NEJM197809212991201. [DOI] [PubMed] [Google Scholar]
- Hart J. S., Trujillo J. M., Freireich E. J., George S. L., Frei E., 3rd Cytogenetic studies and their clinical correlates in adults with acute leukemia. Ann Intern Med. 1971 Sep;75(3):353–360. doi: 10.7326/0003-4819-75-3-353. [DOI] [PubMed] [Google Scholar]
- Kakati S., Sandberg A. A. Chromosomes in solid tumors. Virchows Arch B Cell Pathol. 1978 Nov 17;29(1-2):129–137. doi: 10.1007/BF02899346. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzner M., Edelson R., Schein P., Green I., Kirkpatrick C., Ahmed A. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975 Oct;83(4):534–552. doi: 10.7326/0003-4819-83-4-534. [DOI] [PubMed] [Google Scholar]
- MOORHEAD P. S., NOWELL P. C., MELLMAN W. J., BATTIPS D. M., HUNGERFORD D. A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960 Sep;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- Nowell P. C., Rowlands D. T., Jr, Daniele R. P., Berger B. M., Guerry D. Changes in membrane markers and chromosome patterns in chronic T-cell leukemia. Clin Immunol Immunopathol. 1979 Mar;12(3):323–330. doi: 10.1016/0090-1229(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Pierre R. V. Cytogenetics in malignant lymphoma. Virchows Arch B Cell Pathol. 1978 Nov 17;29(1-2):107–112. doi: 10.1007/BF02899343. [DOI] [PubMed] [Google Scholar]
- Risdall R., Hoppe R. T., Warnke R. Non-Hodgkin's lymphoma: a study of the evolution of the disease based upon 92 autopsied cases. Cancer. 1979 Aug;44(2):529–542. doi: 10.1002/1097-0142(197908)44:2<529::aid-cncr2820440222>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Schechter G. P., Bunn P. A., Fischmann A. B., Matthews M. J., Guccion J., Soehnlen F., Munson D., Minna J. D. Blood and lymph node T lymphocytes in cutaneous T cell lymphoma: evaluation by light microscopy. Cancer Treat Rep. 1979 Apr;63(4):571–574. [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Shackney S. E., Edelson R., Bunn P. A., Jr The kinetics of Sézary cell production. Cancer Treat Rep. 1979 Apr;63(4):659–661. [PubMed] [Google Scholar]
- TJIO J. H., WHANG J. Chromosome preparations of bone marrow cells without prior in vitro culture or in vivo colchicine administration. Stain Technol. 1962 Jan;37:17–20. doi: 10.3109/10520296209114563. [DOI] [PubMed] [Google Scholar]
- Testa J. R., Mintz U., Rowley J. D., Vardiman J. W., Golomb H. M. Evolution of karyotypes in acute nonlymphocytic leukemia. Cancer Res. 1979 Sep;39(9):3619–3627. [PubMed] [Google Scholar]
- Tribukait B., Gustafson H., Esposti P. Ploidy and proliferation in human bladder tumors as measured by flow-cytofluorometric DNA-analysis and its relations to histopathology and cytology. Cancer. 1979 May;43(5):1742–1751. doi: 10.1002/1097-0142(197905)43:5<1742::aid-cncr2820430525>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Bunn P., Knutsen T., Schechter G. P., Gazdar A. F., Matthews M. J., Minna J. D. Cytogenetic abnormalities in patients with cutaneous T-cell lymphomas. Cancer Treat Rep. 1979 Apr;63(4):575–580. [PubMed] [Google Scholar]
- van Vloten W. A., van Duijn P., Schaberg A. Cytodiagnostic use of Feulgen-DNA measurements in cell imprints from the skin of patients with mycosis fungoides. Br J Dermatol. 1974 Oct;91(4):365–371. doi: 10.1111/j.1365-2133.1974.tb13073.x. [DOI] [PubMed] [Google Scholar]


