Abstract
Background:
Addition of disinfectant to irreversible hydrocolloid impression materials can eliminate the disinfection step to avoid dimensional changes associated with it. The aim of the present study was to evaluate the effect of various disinfectant mixing liquids on the properties of commercially available irreversible hydrocolloid impression materials.
Materials and Methods:
Four commercially available irreversible hydrocolloid impression materials (Zelgan, Vignette, Tropicalgin, and Algitex) were mixed with disinfectant liquid containing chlorhexidine (0.1 and 0.2%) and sodium hypochlorite (0.1 and 0.5%). After mixing with disinfectant liquids, materials were evaluated for pH changes during gelation, gelation time, flow, gel strength, permanent deformation and detail reproduction.
Results:
Significant changes in gelation time were observed in irreversible hydrocolloid impression materials upon mixing with disinfectant liquids. In general, chlorhexidine increased the gelation time, whereas sodium hypochlorite reduced it. However, no significant changes in the flow were observed both with chlorhexidine and sodium hypochlorite. Gel strength was found to decrease when mixed with chlorhexidine, whereas an increase in gel strength was observed upon mixing with sodium hypochlorite. Permanent deformation of the most irreversible hydrocolloid impression materials was below the specification limit even after mixing with disinfectant liquids. Sodium hypochlorite significantly reduced the surface detail reproduction, whereas no change in detail reproduction was observed with chlorhexidine.
Conclusion:
Chlorhexidine solution can be used to mix irreversible hydrocolloid impression materials in regular dental practice as it did not significantly alter the properties. This may ensure effective disinfection of impressions.
Keywords: Alginate, chlorhexidine, disinfection of impressions, self-disinfectant alginate, sodium hypochlorite
INTRODUCTION
Infectious diseases from saliva or blood via contaminated impressions can spread to the clinicians, patients, or laboratory personnel.[1] It is a general practice to disinfect all dental impressions using aqueous solutions of alcohols, aldehydes, chlorine compounds, phenolics, biguanides, iodine compounds, and quaternary ammonium compounds.[2] No single disinfectant is suitable for all the materials,[3] and selection of disinfectant is important to minimize the risk of disease transmission without affecting the accuracy of details reproduced in the impression. Impressions are either sprayed with or immersed in the disinfectant liquid for a period of time. However, investigations have shown significant dimensional changes in the impressions after disinfection, especially with irreversible hydrocolloid impression materials.[3,4,5,6,7,8]
Irreversible hydrocolloids or alginates are routinely used for recording the preliminary impressions in dentistry. They are supplied as powder in pre-weighed packets or in bulk, mixed with water to form a sol which gets converted into gel due to a chemical reaction.[9,10] Because the set material consists of a gel structure, they have a tendency to absorb water, especially during the disinfection procedure making it dimensionally unstable. To address this problem, attempts have been made to incorporate disinfectants into irreversible hydrocolloid impression materials with varying success.[11] Incorporation of disinfectants makes them self-disinfectant and eliminates the need for separate disinfection procedure avoiding dimensional changes associated with the disinfection.[12] Materials such as didecyldimethyl ammonium chloride,[13] chlorhexidine,[11,14,15] quaternary ammonium compounds,[11] magnesium oxide,[16] fluoride[15,17] etc., have been used as disinfectant additives. It was observed that added disinfectant materials imparted antimicrobial activity to the impression materials with reduced overall quantity of the bacteria on the impression surface eliminating the need for conventional disinfection.[18] However, some studies have reported that even self-disinfectant irreversible hydrocolloids must be disinfected conventionally.[11] Among the disinfectants, chlorhexidine is most widely investigated at 0.1 and 0.2% concentrations.[14,15,19]
Although several research investigations have been carried out to evaluate the effect of various disinfectants, only few studies have evaluated their effect on the properties of irreversible hydrocolloid impression materials.[12,14] Existing literature indicates that most studies have investigated the flow, dimensional accuracy, and stability of self-disinfecting irreversible hydrocolloid impression materials[12,14,20] without much emphasis on the clinically important parameters such as gelation time, strength, and elastic recovery.[21] Therefore, purpose of the present study was to evaluate the properties of four commercially available irreversible hydrocolloid impression materials on mixing with different disinfectant liquids.
MATERIALS AND METHODS
Four commercially available irreversible hydrocolloid impression materials namely Zelgan [Dust-free alginate, Dentsply DeTrey GmbH Pvt. Ltd., Germany], Vignette [Chromatic alginate, Dentsply DeTrey], Tropicalgin [Chromatic alginate, Zhermack Spa, Italy], and Algitex [Conventional alginate, Dental products of India, India] were evaluated in the present study. A total of four disinfectant liquids, chlorhexidine at 0.1 and 0.2% and sodium hypochlorite at 0.1 and 0.5%, [in house preparation, Kasturba Hospital, Manipal, India] were used as mixing liquids for the selected irreversible hydrocolloid impression materials. Antimicrobial activity of these disinfectant agents was established by previous research investigations.[14,22,23] These disinfectant liquids, at the stated concentrations, were mixed with irreversible hydrocolloid impression materials and were evaluated for gelation time, flow, gel strength, permanent deformation, and surface detail reproduction.
Preparation of the specimens
Control samples were prepared by mixing selected materials powder with deionized water. All experimental procedures were carried out at a temperature of 22 ± 1°C and 50 ± 10% relative humidity. The temperature of mixing liquid was maintained at 20 ± 1°C. The powder was dispensed by weight and water or disinfectant liquid was dispensed by volume. All the materials were mixed for 45 seconds using rubber bowl and alginate mixing spatula by a single operator to standardize the manipulative variables. However, mixing time was reduced to 30 seconds with 0.5% sodium hypochlorite mixing solution as it accelerated the setting of irreversible hydrocolloid impression materials leading to rapid gelation. At the end of mixing time, a uniform creamy consistency was formed, which was filled into a polyvinyl chloride mould of 30 mm internal diameter and 16 mm height on a flat glass slab. Immediately after filling the mould, a second mould of 15mm internal diameter and 19 mm height was forced into the irreversible hydrocolloid mix in the first mould until it extruded onto the top. This was done to ensure proper filling of the mold with irreversible hydrocolloid mix without entrapping the air bubbles. Subsequently, a flat glass plate was pressed on top of the second mould to remove excess material and the material was left in the mould for 5 minutes to set after which the specimens were retrieved and subjected to testing [Figure 1].
Figure 1.
Mold used for the preparation of irreversible hydrocolloid specimens
Measurement of pH changes during setting
Measurement of pH changes in the irreversible hydrocolloid impression materials during gelation was carried out as described by Anastassiadou, et al., 1995.[24] Irreversible hydrocolloid mix was placed in a plastic cylinder of 15mm internal diameter and 19 mm height. Immediately after loading the material, a spherical indent was made on the irreversible hydrocolloid surface using a round glass rod so as to provide room for the glass-combined electrode of pH meter (CyberScan 510, Eutech Instruments Pte Ltd., Ayer Rajah Crescent Singapore) to be in contact with the surface of the impression material. After indent was made, 0.2 ml of deionized water was placed into the indent. The pH meter electrode was kept in the indent covered with water to contact the aqueous phase of gel and pH changes were recorded 60 seconds from the start of mixing until 5 minutes at every 30 seconds interval (n = 3).
Measurement of gelation time
Gelation time was measured as described by Lemon, et al., 2003.[25] Sixty seconds after the start of mixing, flat end of a polished polymethyl methacrylate rod of 6 mm diameter and 10 cm length was placed in contact with the surface of the irreversible hydrocolloid mix and withdrawn immediately. This procedure was repeated at 5 seconds intervals till the impression material no longer adhered to the rod. Gelation time was established from the beginning of mixing until the material no longer adhered to the rod (n = 5).
Measurement of flow
Flow was determined as described by Wang, et al., 2007.[14] A standard 0.5 ml of irreversible hydrocolloid mix (n = 3) was injected onto a glass plate (15 × 15 × 2 mm) using a disposable syringe. Another glass plate was placed on top of this mix and 1.5 kg load was placed on the upper plate for 5 seconds. Diameter of the impression disk was measured at three different places and average diameter, in mm, was considered as flow.
Measurement of gel strength
Gel strength was measured by a method as described by MacPherson et al., 1967 with slight modifications.[26] At the end of 6 minutes from the start of mixing, specimens were retrieved from the mould (n = 10) and placed on the bottom plate of universal testing machine (Instron, Model 3366, Instron Corp, High Wycombe, United Kingdom). Specimens were stressed at a rate of 10 mm/minute. Maximum compressive load at which the material failed as indicated by a significant reduction in the load during testing was considered for the measurement of strength.
Measurement of permanent deformation
Permanent deformation was measured according to a previously reported method with few modifications.[26] Six minutes from the start of mixing, specimens (n = 5) were placed in the universal testing machine (Instron). Specimens were loaded until the specimen length was compressed to 10% of the original length (10% deformation) and the same was maintained for 15 seconds. After 15 seconds, the load was removed and the specimens were allowed to recover for 30 seconds. Length of the specimen was measured at the end of recovery time and change in length was considered for calculating the permanent deformation using the following formula:
![]()
Measurement of surface detail reproduction
Measurement of surface detail reproduction was carried out as described by Taylor, et al., 2007.[21] A stainless steel die was fabricated with three lines of 25, 50, and 75 μm widths according to ANSI/ADA Specification No. 18.[27] Stainless steel die was covered with a PVC tube of 10 mm height to contain the mixed material. Die was cleaned with alcohol and allowed to dry prior to each impression recording. The irreversible hydrocolloid impression material was mixed as described above and loaded into PVC tube. It was covered with a glass slab, and a weight of 1 kg was applied for 5 seconds. The mix was allowed to set for 5 minutes before being removed from the die. Immediately after the impression was removed, dental stone mix was poured into the impression and allowed to set for 90 minutes. After retrieving the cast, its surface was observed under the stereo microscope for surface detail reproduction. Depending on the reproduction of the three lines on the cast, samples were graded as 1 = sharp detail, continuous line; 2 = continuous line, but with some loss of sharpness; 3 = deterioration of line detail; and 4 = rough appearance with loss of continuity of the line (n = 3).
Statistical analysis
Data were analyzed using Graphpad Prism software version 5. Results were subjected to one-way ANOVA test to compare the effect of selected disinfectants on the properties of irreversible hydrocolloid impression materials. Differences were subjected to Tukey's multiple comparison test and a ‘p’ value less than 0.05 is considered significant.
RESULTS
Measurement of pH changes during gelation
Changes in pH observed during the gelation of selected irreversible hydrocolloid impression materials on mixing with disinfectant liquids are presented in Figure 2. Figure 2a shows pH changes observed with Zelgan material. Control specimens of Zelgan have shown pH of 9.17 ± 0.76 at 1 minute which decreased to 8.91 ± 0.61 at 5 minutes. Zelgan mixed with 0.5% sodium hypochlorite has shown pH of 12.41 ± 0.66 at 1 minute which reduced to 9.16 ± 0.19 at 5 minutes. However, when mixed with 0.1 and 0.2% chlorhexidine, it has shown pH of 9.33 ± 0.12 and 9.35 ± 0.42 at 1 minute, respectively, and at 5 minutes pH was found to be 9.27 ± 0.12 and 9.30 ± 0.37, respectively. Control specimens of Vignett [Figure 2b] have shown pH of 10.16 ± 1.06 at 1 minute which steadily decreased to 8.89 ± 0.18 at 5 minutes. On mixing Vignette with 0.1% chlorhexidine, pH was found to be less than control specimens (9.91 ± 0.63) at 1 minute but at 5 minutes, it was found to be 9.44 ± 0.12 which was higher than control specimens. Similar trend was observed with 0.2% chlorhexidine. On the other hand, Vignette mixed with 0.1% sodium hypochlorite has shown higher pH of 10.67 ± 0.20 at 1 minute and its pH was found to be close to control pH. Control specimens of Tropicalgin [Figure 2c] have shown pH of 8.07 ± 0.56 at 1 minute which was found to decrease during gelation and its pH was found to be 6.81 ± 0.20 at 5 minutes. On mixing Tropicalgin with disinfectant liquid, pH increased especially with 0.1% sodium hypochlorite which showed pH of 10.53 ± 0.25 at 1 minute. However, at 5 minutes, pH of all Tropicalgin specimens mixed with disinfectant liquids was found to be close to 7. Control specimens of Algitex [Figure 2d] have shown pH of 9.44 ± 1.03 at 1 minute which reduced to 7.92 ± 0.27 at 5 minutes. Algitex mixed with 0.5% sodium hypochlorite has shown pH of 10.04 ± 0.35 at 1 minute which reduced to 7.52 ± 0.17 at 5 minutes. Algitex mixed with 0.2% chlorhexidine showed pH of 7.16 ± 0.05 which marginally increased to 7.36 ± 0.05 at 5 minutes.
Figure 2.
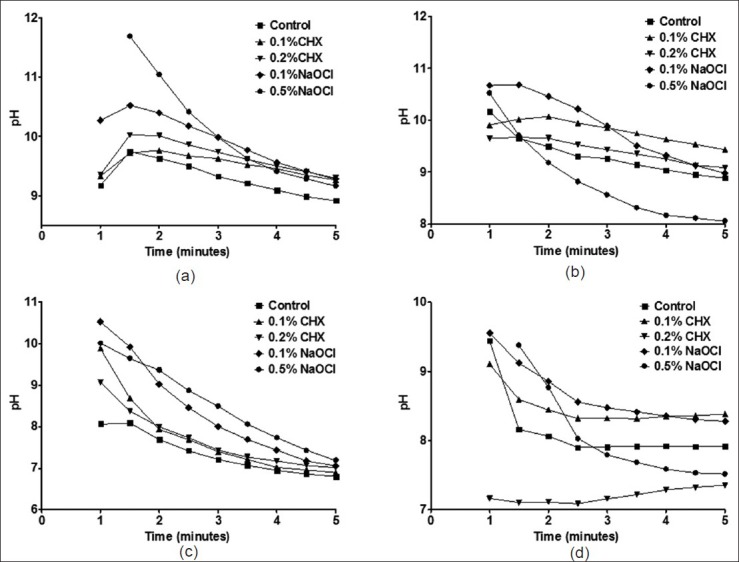
pH changes during gelation of Irreversible hydrocolloids (a) Zelgan, (b) Vignett, (c) Tropicalgin, (d) Algitex
Gelation time
Gelation time of control and test groups are presented in Figure 3. Control specimens of Zelgan showed gelation time of 109.61 ± 5.68 seconds, whereas Zelgan mixed with 0.1 and 0.5% chlorhexidine has shown longer gelation time of 130.80 ± 5.54 and 181.58 ± 4.15 seconds, respectively (P < 0.05). Similar increase in gelation time was observed with both Tropicalgin and Algitex when mixed with chlorhexidine. However, mixing Vignette with chlorhexidine was found to reduce the gelation time (P < 0.05). Control specimens of Vignette showed gelation time of 86.20 ± 4.92 seconds, whereas Vignette mixed with 0.1 and 0.2% chlorhexidine showed gelation time of 79.52 ± 2.11 and 68.01 ± 2.64 seconds, respectively. Sodium hypochlorite reduced gelation time of all the materials used in the study except Algitex. Zelgan mixed with 0.1 and 0.5% sodium hypochlorite showed gelation time of 70.00 ± 6.73 and 53.50 ± 1.00 seconds, respectively, which was less than the gelation time observed with control specimens (P < 0.05). However, Tropicalgin on mixing with 0.5% sodium hypochlorite showed prolonged gelation time beyond 30 minutes (not shown in Figure 3). Vignette mixed with both chlorhexidine and sodium hypochlorite showed reduced gelation time. Although reduced gelation time was not statistically significant for Vignett mixed with 0.1% chlorhexidine such a reduction may make the clinical manipulation of the material difficult. Algitex when mixed with 0.1 and 0.5% sodium hypochlorite showed gelation time of 174.80 ± 6.76 and 172.00 ± 9.38 seconds respectively, which was higher than that observed with control specimens (P < 0.05)
Figure 3.
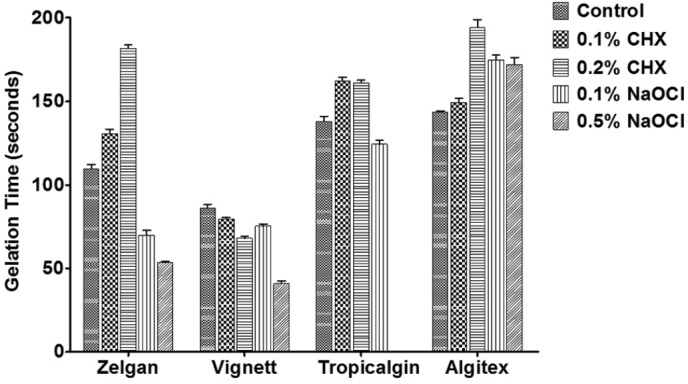
Gelation time of irreversible hydrocolloids mixed with disinfectant liquids
Flow
Flow of selected materials mixed with disinfectant liquids is presented in Figure 4. Flow of the selected impression materials could not be measured when they were mixed with 0.5% sodium hypochlorite as these materials either set rapidly or fragmented without flow. Control specimens of Zelgan showed flow of 40.22 ± 2.82 mm. Zelgan mixed with 0.1 and 0.2% chlorhexidine showed flow of 42.77 ± 0.62 mm and 42.05 ± 0.39 mm, respectively, which was statistically not significant compared to control group. However, when Zelgan was mixed with 0.1% sodium hypochlorite, it reduced the flow to 34.38 ± 1.27 mm which was significantly less than control group (P < 0.05). Vignette control group showed flow of 36.94 ± 0.68 mm, whereas when 0.1 and 0.2% chlorhexidine were used for mixing, it showed flow of 39.38 ± 0.93 mm and 39.05 ± 0.85 mm, respectively. Vignette mixed with 0.1% sodium hypochlorite showed reduced flow of 34.22 ± 0.56 mm (P < 0.05). No significant difference in the flow of Tropicalgin was observed when mixed with the disinfectant liquids. Algitex on mixing with 0.1% chlorhexidine showed a significant decrease in the flow, whereas 0.1% sodium hypochlorite significantly increased the flow (P < 0.05).
Figure 4.
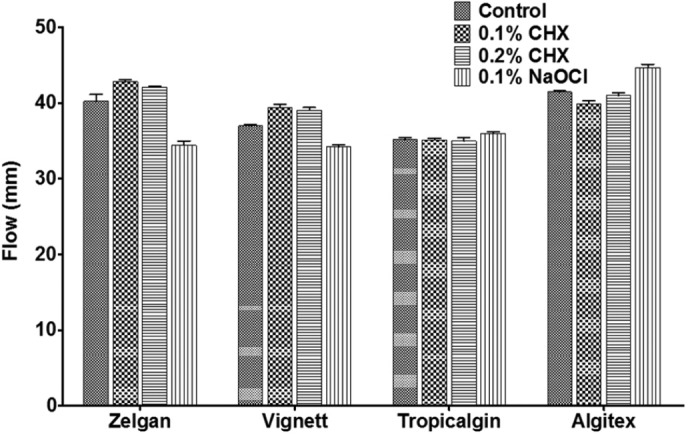
Flow of irreversible hydrocolloids mixed with disinfectant liquids
Gel strength
Gel strength of selected irreversible hydrocolloid impression materials mixed with disinfectant materials is presented in Figure 5. All the selected materials have shown a significant reduction in gel strength when mixed with chlorhexidine and the reduction in strength was more at higher concentration. Similar reduction in gel strength was also observed with sodium hypochlorite except for Zelgan. Control specimens of Zelgan showed gel strength of 0.79 ± 0.03 MPa, whereas Zelgan mixed with 0.1 and 0.5% sodium hypochlorite has shown increased gel strength of 1.25 ± 0.06 and 1.35 ± 0.04 MPa, respectively. Tropicalgin mixed with 0.5% sodium hypochlorite did not set even after 30 minutes, and hence its strength could not be measured.
Figure 5.
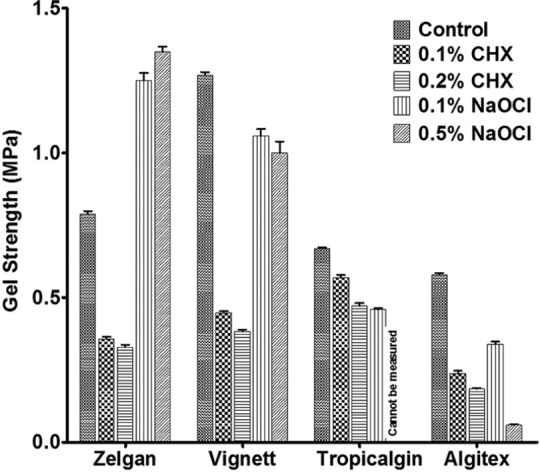
Gel strength of irreversible hydrocolloids mixed with disinfectant liquids
Permanent deformation
Permanent deformation of selected irreversible hydrocolloid impression materials mixed with disinfectant materials is shown in Figure 6. Results indicated that permanent deformation was not significantly affected by mixing with disinfectant liquids except 0.5% sodium hypochlorite. According to American Dental Association specification number 18, the maximum clinically acceptable permanent deformation is 3%.[27] Among the selected materials, Algitex showed permanent deformation which was above the clinically acceptable limit and its permanent deformation was found to increase significantly when mixed with 0.5% sodium hypochlorite (P < 0.05). In addition, Zelgan mixed with 0.2% chlorhexidine and Vignette mixed with 0.5% sodium hypochlorite showed permanent deformation that was slightly higher than the acceptable limit.
Figure 6.
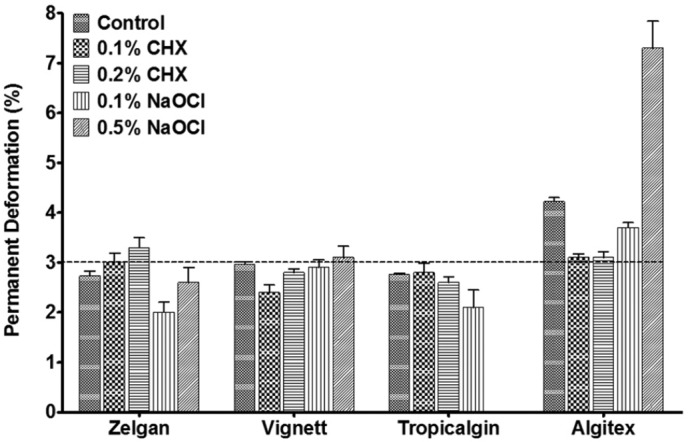
Permanent deformation of irreversible hydrocolloids mixed with disinfectant liquids
Surface detail reproduction
Mean surface detail reproduction observed with selected irreversible hydrocolloid impression materials is presented in Figure 7. Control group of all the irreversible hydrocolloid impression materials reproduced excellent surface details of the metal die. However, detail reproduction was found to be affected severely when they were mixed with 0.5% sodium hypochlorite. In contrast, both concentrations of chlorhexidine did not affect the detail reproduction significantly.
Figure 7.
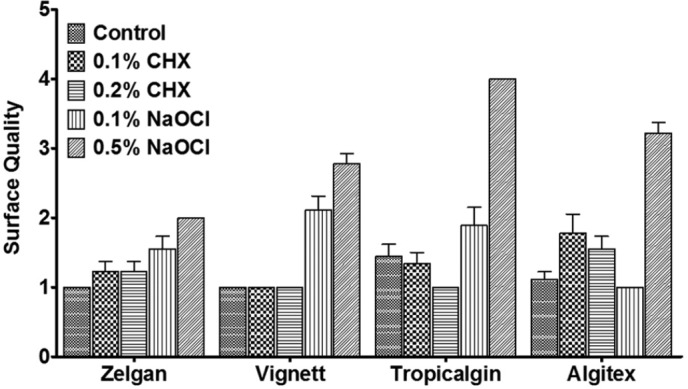
Surface detail reproduction of irreversible hydrocolloids mixed with disinfectant liquids
DISCUSSION
In the present study, commercially available irreversible hydrocolloid impression materials were mixed with varying concentrations of different disinfectant liquids, and their properties were evaluated. Results indicate that the properties of irreversible hydrocolloids are affected by mixing them with disinfectant liquids depending on the type and concentration of the disinfectant. It was also observed that the extent to which the properties are altered was dependent on the type of irreversible hydrocolloid impression material. During the gelation, a continuous alteration of hydrogen ion concentration occurs in the system.[28] In the present study, all control specimens showed initial alkaline pH between 8 and 10 and the pH was found to decrease as the gelation progressed. However, use of disinfectant liquids resulted in a more alkaline pH even after gelation. These changes in pH may affect dissociation and cross-linking of reactive ingredients, and thus may influence gelation time, strength, and permanent deformation.[29] It may also affect the color changes during the manipulation of chroma alginates. Furthermore, pH of the set irreversible hydrocolloid may significantly affect setting process of gypsum materials while making the cast.[30]
Gelation of irreversible hydrocolloid impression materials occur clinically when approximately 10% of alginate carboxyl groups are cross-linked with calcium ions.[25,28] Gelation time of irreversible hydrocolloids was found to increase on mixing them with chlorhexidine which may be due to the delay in supply of calcium ions required for the gelation. However, Vignette mixed with chlorhexidine showed reduced gelation time which may be due to the compositional differences. Sodium hypochlorite, on the other hand, reduced the gelation time which may react with sodium phosphate and minimize its availability to counteract the calcium ions. Such a process ensures that the calcium ions in the sol form are readily available to react with the soluble alginate molecules to form a gel.[28] This results in reduced gelation time as the retarding effect of trisodium phosphate is nullified by the presence of sodium hypochlorite. This observation was not consistent with Algitex which is a conventional irreversible hydrocolloid impression material.
Flow of irreversible hydrocolloid impression material allows it to record all the finer details. Upon mixing the irreversible hydrocolloid with mixing liquid, it forms a sol which is fluid and as the gelation occurs its fluidity gradually decreases. Irreversible hydrocolloids mixed with chlorhexidine have shown better flow which is attributed to their longer gelation time. Sodium hypochlorite, on the other hand, was found to accelerate the gelation of irreversible hydrocolloids and hence they showed less flow. Algitex showed longer gelation time, and hence showed higher flow when mixed with sodium hypochlorite. This may be due to the difference in the composition of Algitex compared to other irreversible hydrocolloids used in the study. Algitex is a conventional irreversible hydrocolloid, whereas all other materials used in the present study are dust-free irreversible hydrocolloids. However, both chlorhexidine and sodium hypochlorite were found to reduce the gel strength indicating that these materials altered cross-linking density in the set material. Gel strength of all irreversible hydrocolloids on mixing with disinfectants was found to be less compared to control group. Zelgan has shown higher gel strength on mixing with sodium hypochlorite. Decrease in strength was more with chlorhexidine than with sodium hypochlorite. It was also observed that materials with longer gelation time have shown reduced gel strength which can be attributed to the low cross-linking density in slow setting materials.[25,31]
Permanent deformation in irreversible hydrocolloid impression materials is related to the cross-linking density.[32] Higher cross-linking density upon gelation imparts superior strength and elasticity to the gel. Both chlorhexidine and sodium hypochlorite may have altered the cross-linking density which may result in significant distortion of the impression on removal from undercuts. However, the extent of increase in the permanent deformation was less with chlorhexidine compared to sodium hypochlorite. Permanent deformation of all the irreversible hydrocolloids used in the present study was found to be satisfying the requirement or slightly above the recommended <3% permanent deformation when mixed with chlorhexidine.
Detail reproduction is mainly influenced by flow of the unset irreversible hydrocolloid into the details and its compatibility with the gypsum products. Sodium hypochlorite reduced the detail reproduction in irreversible hydrocolloids which could be attributed to the accelerated setting preventing it from flowing into the details. Further, it may also be related to the compatibility between gypsum product and set irreversible hydrocolloid impression material. As gypsum products generally show a pH range of 5-7 during setting, they are compatible with irreversible hydrocolloid impression materials having pH in this range. However, sodium hypochlorite increased alkalinity of the irreversible hydrocolloid impression materials, which might have altered its compatibility with dental stone affecting the detail reproduction.[30] Although similar change in pH of the irreversible hydrocolloid impression materials was observed on mixing them with chlorhexidine, the change in pH was not as much as observed with sodium hypochlorite. This could be the reason for better surface detail reproduction with the irreversible hydrocolloids mixed with chlorhexidine.
Another clinically important factor that affects the selection of disinfectant for mixing with irreversible hydrocolloid is the biological compatibility which is not evaluated in the present study. Especially, sodium hypochlorite may cause irritation or burning sensation of the oral soft tissues.
From the results, it can be observed that both chlorhexidine and sodium hypochlorite have altered the properties of irreversible hydrocolloid impression materials, and their effect on the properties is concentration dependent. Hence, lower concentrations of disinfectant may be conveniently used for mixing of irreversible hydrocolloid impression materials. However, such dilution may reduce the efficacy of disinfectant.
CONCLUSION
From the results, it can be concluded that mixing of irreversible hydrocolloid impression materials with disinfectant liquids may alter their properties depending on the type and concentration of the disinfectant. Among the disinfectant solutions that are used in the present study, chlorhexidine can be considered as a suitable disinfectant liquid for mixing with irreversible hydrocolloid impression materials as it did not affect the properties of selected irreversible hydrocolloid impression materials significantly.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Samaranayake LP, Hunjan M, Jennings KJ. Carriage of oral flora on irreversible hydrocolloid and elastomeric impression materials. J Prosthet Dent. 1991;65:244–49. doi: 10.1016/0022-3913(91)90169-w. [DOI] [PubMed] [Google Scholar]
- 2.Kwok WM, Ralph WJ. The use of chemical disinfectants in dental prosthetics. Aust Dent J. 1984;29:180–83. doi: 10.1111/j.1834-7819.1984.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson GH, Chellis KD, Gordon GE, Lepe X. Dimensional stability and detail reproduction of irreversible hydrocolloid and elastomeric impressions disinfected by immersion. J Prosthet Dent. 1998;79:446–53. doi: 10.1016/s0022-3913(98)70160-x. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad S, Tredwin CJ, Nesbit M, Moles DR. Effect of immersion disinfection with Perform-ID on alginate, an alginate alternative, an addition-cured silicone and resultant type III gypsum casts. Br Dent J. 2007;202:E1. doi: 10.1038/bdj.2006.120. discussion 36-7. [DOI] [PubMed] [Google Scholar]
- 5.Tan HK, Hooper PM, Buttar IA, Wolfaardt JF. Effects of disinfecting irreversible hydrocolloid impressions on the resultant gypsum casts: Part III-dimensional changes. J Prosthet Dent. 1993;70:532–37. doi: 10.1016/0022-3913(93)90267-r. [DOI] [PubMed] [Google Scholar]
- 6.Jones ML, Newcombe RG, Bellis H, Bottomley J. The dimensional stability of self-disinfecting alginate impression compared to various impression regimes. Angle Orthod. 1990;60:123–28. doi: 10.1043/0003-3219(1990)060<0123:TDSOSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Wilson HJ. Impression materials. Br Dent J. 1988;164:221–25. doi: 10.1038/sj.bdj.4806406. [DOI] [PubMed] [Google Scholar]
- 8.Tobias RS, Browne RM, Wilson CA. An in vitro study of the antibacterial and antifungal properties of an irreversible hydrocolloid impression material impregnated with disinfectant. J Prosthet Dent. 1989;62:601–5. doi: 10.1016/0022-3913(89)90087-5. [DOI] [PubMed] [Google Scholar]
- 9.Rubel BS. Impression Materials: A comparative review of impression materials most commonly used in restorative dentistry. Dent Clin North Am. 2007;51:629–42. doi: 10.1016/j.cden.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Nandini VV, Venkatesh KV, Nair KC. Alginate impressions: A practical perspective. J Conserv Dent. 2008;11:37–41. doi: 10.4103/0972-0707.43416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan DA, Palenik CJ, Setcos JC, Miller CH. Antimicrobial activities of dental impression materials. Dent Mater. 1998;14:399–404. doi: 10.1016/s0300-5712(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 12.Ramer MS, Gerhardt DE, McNally K. Accuracy of irreversible hydrocolloid impression materials mixed with disinfectant solutions. J Prosthodont. 1993;2:156–8. doi: 10.1111/j.1532-849x.1993.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 13.Tyler R, Tobias RS, Ayliffe GA, Browne RM. An in vitro study of the antiviral properties of an alginate impression material impregnated with disinfectant. J Dent. 1989;17:137–9. doi: 10.1016/0300-5712(89)90108-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Wan Q, Chao Y, Chen Y. A Self-disinfecting irreversible hydrocolloid impression material mixed with chlorhexidine solution. Angle Orthod. 2007;77:894–900. doi: 10.2319/070606-277. [DOI] [PubMed] [Google Scholar]
- 15.Casemiro LC, Pires-de-Souza Fde C, Panzeri H, Martins CH, Ito IY. In vitro antimicrobial activity of irreversible hydrocolloid impressions against 12 oral microorganisms. Braz Oral Res. 2007;21:323–9. doi: 10.1590/s1806-83242007000400008. [DOI] [PubMed] [Google Scholar]
- 16.Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods. 2003;54:177–82. doi: 10.1016/s0167-7012(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton IR. Biochemical effects of fluoride on oral bacteria. J Dent Res. 1990;69:660–67. doi: 10.1177/00220345900690S128. [DOI] [PubMed] [Google Scholar]
- 18.Cserna A, Crist RL, Adams AB, Dunning DG. Irreversible hydrocolloids: A comparison of antimicrobial efficacy. J Posth Dent. 1994;71:387–9. doi: 10.1016/0022-3913(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 19.Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodont Res. 2002;37:389–98. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones ML, Newcombe RG, Bellis H, Bottomley J. The dimensional stability of self-disinfecting alginate impressions compared to various immersion regimes. Angle Orthod. 1990;60:123–8. doi: 10.1043/0003-3219(1990)060<0123:TDSOSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RL, Wright PS, Maryan C. Disinfection procedures: Their effect on the dimensional accuracy and surface quality of irreversible hydrocolloid impression materials and gypsum casts. Dent Mater. 2002;18:103–10. doi: 10.1016/s0109-5641(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 22.Rueggeberg FA, Beall FE, Kelly MT, Schuster GS. Sodium hypochlorite disinfection of irreversible hydrocolloid impression material. J Prosthet Dent. 1992;67:628–31. doi: 10.1016/0022-3913(92)90160-c. [DOI] [PubMed] [Google Scholar]
- 23.Samadi N, Zaree R, Bakhtiar H, Salehnia A, Azimi S. Comparative antibacterial efficacy of endemic satureja khuzistanica jamzad essential oil, sodium hypochlorite and chlorhexidine gluconate solutions as root canal irrigations. Dent Res J. 2011;8:28–32. [PMC free article] [PubMed] [Google Scholar]
- 24.Anastassiadou V, Dolopoulou V, Kaloyannides A. The relation between thermal and pH changes in alginate impression materials. Dent Mater. 1995;11:182–5. doi: 10.1016/0109-5641(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 25.Lemon JC, Okay DJ, Powers JM, Martin JW, Chambers MS. Facial moulage: The effect of a retarder on compressive strength and working and setting times of irreversible hydrocolloid impression material. J Prosthet Dent. 2003;90:276–81. doi: 10.1016/s0022-3913(03)00366-4. [DOI] [PubMed] [Google Scholar]
- 26.MacPherson GW, Craig RG, Peyton FA. Mechanical properties of hydrocolloid and rubber impression materials. J Dent Res. 1967;46:714–21. doi: 10.1177/00220345670460041401. [DOI] [PubMed] [Google Scholar]
- 27.Council adopts American Dental Association specification No.18 (alginate impression materials). Council on Dental Materials and Devices. J Am Dent Assoc. 1968;77:1354–8. doi: 10.14219/jada.archive.1968.0369. [DOI] [PubMed] [Google Scholar]
- 28.Buchan S, Peggie RW. Role of ingredients in alginate impression compounds. J Dent Res. 1966;45:1120–9. doi: 10.1177/00220345660450041701. [DOI] [PubMed] [Google Scholar]
- 29.Anastassiadou V, Dolopoulou V, Kaloyannides A. Relationship between pH changes and dimensional stability in irreversible hydrocolloid impression material during setting. Int J Prosthodont. 1995;8:535–40. [PubMed] [Google Scholar]
- 30.Otani H, Adachi M, Kanematsu Y. Surface reproduction of pH-adjusted alginate impression materials. Dent Mater. 1985;1:58–60. doi: 10.1016/s0109-5641(85)80026-9. [DOI] [PubMed] [Google Scholar]
- 31.Cook W. Alginate dental impression materials: Chemistry, structure and properties. J Biomed Mater Res. 1986;20:1–24. doi: 10.1002/jbm.820200103. [DOI] [PubMed] [Google Scholar]
- 32.Aoyama N, Hayakawa I, Akiba N, Minakuchi S. Effect of high-molecular-weight sodium alginate on the viscosity and characteristics of alginate impression materials. Prosthodont Res Pract. 2007;6:239–45. [Google Scholar]


