Abstract
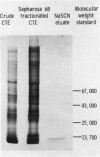
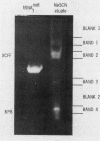
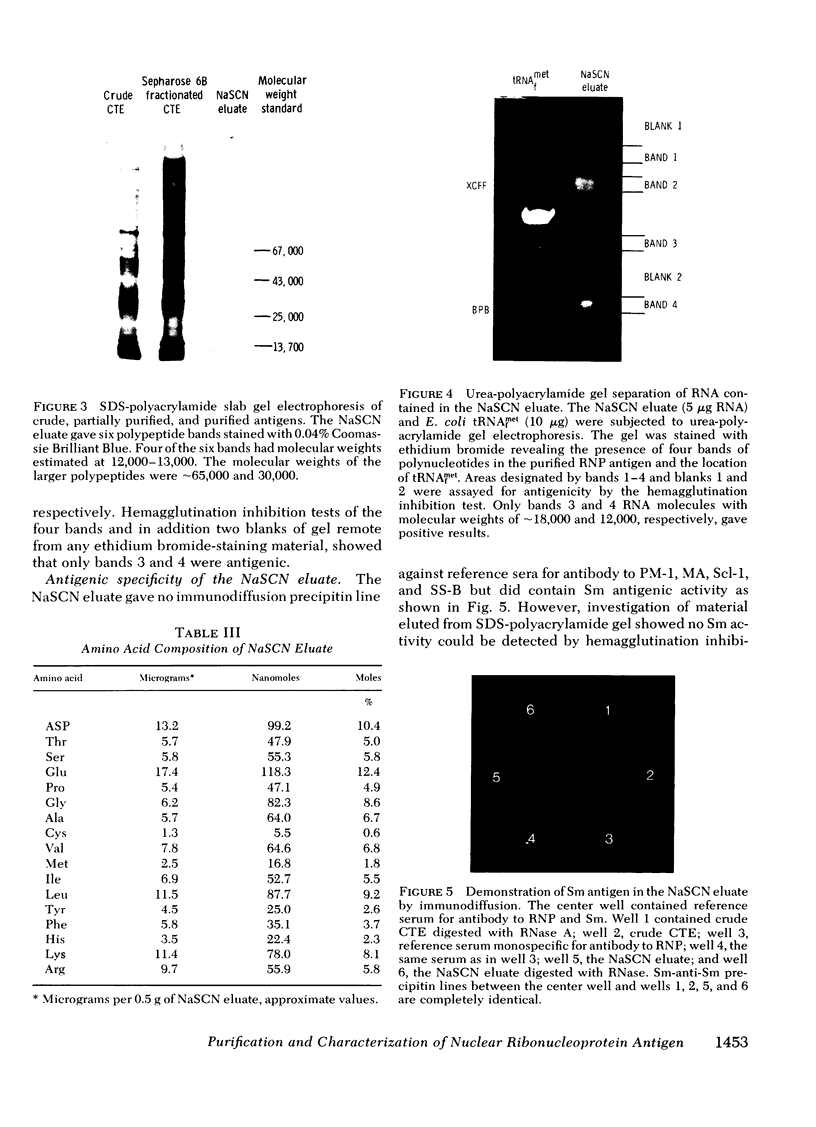
We have achieved a high degree of purification of nuclear ribonucleoprotein antigen from calf thymus nuclear extract through antibody affinity chromatography. Antibody to nuclear ribonucleoprotein was purified from the serum of a patient with mixed connective tissue disease and Sepharose 4B was covalently coupled with the purified human antibody. The sodium thiocyanate eluate from the affinity column contained active ribonucleoprotein antigen and the specific activity of the antigen in this eluate was 488 times higher than the original nuclear extract. The protein component of the eluate consisted of six polypeptides determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two of them were shown to be antigenic by a hemagglutination inhibition test. The molecular weights of these two peptides were ∼13,000 and those of the other four were 13,000, 13,000, 30,000, and 65,000. The ribonucleic acid component of the eluate was shown by urea-polyacrylamide gel electrophoresis to contain five polynucleotides. Two of the five, estimated to contain 40 and 60 nucleosides, had antigenic activity. The other three polynucleotides which had more than 77 nucleosides had no antigenic activity. No modified nucleosides were found in these ribonucleic acid molecules. Even in this highly purified ribonucleoprotein antigen, Sm antigen was detected by immunodiffusion. This evidence indicated that there are some molecular associations between ribonucleoprotein and Sm antigens as has previously been suggested.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Setzer D., Gehrke C. W. Characterization of a unique enzyme complex composed of S-adenosyl-L-methionine-tRNA-methyltransferase and aminoacyl-tRNA synthetase activities. Nucleic Acids Res. 1977 Nov;4(11):3803–3819. doi: 10.1093/nar/4.11.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon-Segovia D., Ruiz-Arguelles A., Fishbein E. Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature. 1978 Jan 5;271(5640):67–69. doi: 10.1038/271067a0. [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D., Ruiz-Arguelles A., Llorente L. Antibody penetration into living cells. II. Anti-ribonucleoprotein IgG penetrates into Tgamma lymphocytes causing their deletion and the abrogation of suppressor function. J Immunol. 1979 May;122(5):1855–1862. [PubMed] [Google Scholar]
- Davis G. E., Gehrke C. W., Kuo K. C., Agris P. F. Major and modified nucleosides in tRNA hydrolysates by high-performance liquid chromatography. J Chromatogr. 1979 May 21;173(2):281–298. doi: 10.1016/s0021-9673(00)92297-0. [DOI] [PubMed] [Google Scholar]
- Douvas A. S., Stumph W. E., Reyes P., Tan E. M. Isolation and characterization of nuclear ribonucleoprotein complexes using human anti-nuclear ribonucleoprotein antibodies. J Biol Chem. 1979 May 10;254(9):3608–3616. [PubMed] [Google Scholar]
- FAHEY J. L., HORBETT A. P. Human gamma globulin fractionation on anion exchange cellulose columns. J Biol Chem. 1959 Oct;234:2645–2651. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Physical association of two nuclear antigens and mutual occurrence of their antibodies: the relationship of the SM and RNAprotein (MO) systems in SLE sera. J Immunol. 1973 May;110(5):1318–1324. [PubMed] [Google Scholar]
- Notman D. D., Kurata N., Tan E. M. Profiles of antinuclear antibodies in systemic rheumatic diseases. Ann Intern Med. 1975 Oct;83(4):464–469. doi: 10.7326/0003-4819-83-4-464. [DOI] [PubMed] [Google Scholar]
- Parker M. D. Ribonucleoprotein antibodies: frequency and clinical significance in systemic lupus erythematosus, scleroderma, and mixed connective tissue disease. J Lab Clin Med. 1973 Nov;82(5):769–775. [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., LaRoque R. L., Velez C., Daly V., Kaiser A. D., Holman H. R. Association of autoantibodies to different nuclear antigens with clinical patterns of rheumatic disease and responsiveness to therapy. J Clin Invest. 1971 Feb;50(2):350–359. doi: 10.1172/JCI106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]