Abstract
Background:
Candidal colonization in complete denture wearers is a commonly encountered condition that worsens in the presence of untreated Diabetes Mellitus. The aim of this study was to evaluate the correlation between oral candidiasis in denture-bearing mucosa and elevated blood glucose levels in complete denture wearers and to evaluate the effect of oral hypoglycemic drug therapy in controlling oral candidal colonization in denture-bearing mucosa of complete denture wearers with Type II Diabetes Mellitus.
Materials and Methods:
This prospective observational study involved the participation of 15 complete denture wearers with Type II Diabetes Mellitus. The sample collection was made prior and after oral hypoglycaemic drug intervention, by swabbing the rugal surfaces of palatal mucosa, cultured and the density of the candidal colony formed was analyzed and interpreted as colony forming units (CFU) per mL. The candidal samples CFU and corresponding pre- and post-prandial blood glucose levels were estimated, analyzed and compared using Karl Pearson correlation analysis and paired t-test (α = 0.05).
Results:
The Karl Pearson correlation analysis showed that there was a positive correlation between the blood glucose levels (PPS and FBS) and the candidal colonization (CFU) (P < 0.05). The mean values of all the variables were analyzed using the paired t-test. There was significant reduction in the mean values of blood glucose levels (P < 0.001) and the mean values of the CFU (P < 0.001) following oral hypoglycemic drug therapy.
Conclusion:
Positive correlation was observed between oral candidiasis in complete denture-bearing mucosa and elevated blood glucose levels and oral hypoglycemic drug therapy has a positive effect in controlling oral candidal colonization in complete denture wearers with Type II Diabetes Mellitus.
Keywords: Candidal colonization, complete dentures, diabetes mellitus, oral hypoglycemic drugs
INTRODUCTION
Uncontrolled Diabetes mellitus can be a very serious metabolic disorder, which can affect every possible tissue function and cellular infrastructure in human body. Aging oral mucosa like all the other tissues in the body is not resistant to the harmful effects of Diabetes mellitus.[1,2] Diabetic microangiopathy can cause thinning of epithelial membrane thickness, decrease in salivary flow, causing xerostomia, and decreased immune response.[3] This makes the oral mucosa vulnerable to opportunistic microbial invasion.[2,4,5,6]
Furthermore, some microorganisms become more virulent in a high glucose environment. Another mechanism that can lead to the increased prevalence of infections in diabetic patients is an increased adherence of microorganisms to diabetic compared to nondiabetic cells, and this has been described for fungal infection caused by Candida albicans.[7]
The denture-bearing mucosa in diabetic patients is subject to even greater stress than normal aging mucosa. The occlusal forces, tissue trauma due to maladaptation of denture bases, surface charges of denture base materials,[8,9] dimensional changes in the denture bases due to salivary and fluid sorption affects the health of the underlying denture-bearing tissues due to decreased capillary blood flow. Several authors have stated the increased potential for mycotic invasion in denture-bearing mucosa. The effect of mycotic colonization under the vulnerable denture-bearing surface due to elevated blood glucose levels as in uncontrolled diabetes mellitus have not been studied extensively.
Hence, this study was done with the following aims and objectives:
To evaluate the correlation between oral candidiasis in denture-bearing mucosa and elevated blood glucose levels in complete denture wearers with Type II Diabetes Mellitus.
To evaluate the effect of oral hypoglycemic drug therapy and its influence in controlling oral candidal colonization in denture-bearing mucosa of complete denture wearers with Type II Diabetes Mellitus.
MATERIALS AND METHODS
Completely edentulous patients reporting to the Department of Prosthodontics for treatment were screened for sample selection. The sample selection was based upon the following inclusion and exclusion criteria.
Inclusion criteria
Complete denture wearers with undiagnosed/untreated Type II Diabetes Mellitus in Indian (Chennai) population
Age: Above 40 years
No gender discretion
Patients who are not under any antibiotic/cytotoxic/immunosuppressive drug therapy.
Willingness to participate in the study
Exclusion criteria
Patients who are under antibiotic drug therapy
Patients with neuromuscular/musculoskeletal disorders
Patients with psychiatric illness
Patients who are unwilling to participate in the study
Methodology
Completely edentulous patients who satisfied the inclusion criteria were included in this prospective observational study, which involved a therapeutic intervention. The selected patients were then screened for blood glucose level examination using a standard glucometer (Accu-check). Patients who had random blood glucose level more than 130 mg/dL 3 h after the last food intake (WHO guidelines) were selected for the study. The blood glucose levels were then confirmed using the Hemoglobin A1c test (HbA1C). Then, the patients were subjected to the glucose tolerance test and Type II Diabetes Mellitus was confirmed. Thirty-five (20 males and 15 females) patients presented with Diabetes Mellitus and with increased blood glucose levels. Among them, 15 patients mean age 52 with SD ± 5 were willing to participate in the study (4 females and 11 males).
The study duration and the importance of the study were explained to the patient. The patients willing to participate gave a signed informed consent to participate in the study. The study started after obtaining ethical clearance from the University Ethical Clearance Board. This study consisted of three phases.
Phases of study
Phase I
Phase II (30 days after drug intervention)
Phase III (60 days after drug intervention)
Phase I
Isolation of candidal samples
The blood glucose levels were evaluated and recorded before the candidal sample collections were made. Both the fasting blood sugar (FBS) and the postprandial blood sugar (PPS) levels were recorded. Candidal samples were obtained in morning by swabbing from the palatal and rugal denture-bearing surface of the maxilla. The swab was then shifted to a transport medium and then subjected to culture.
Culturing
The collected candidal sample was streaked on the SDA plates. The culture plates were then incubated at 37°C in an incubator and examined for the growth of yeast colonies after 48 h. Candidal growth was identified as round, smooth creamy white colonies and the number of colonies on the culture plates were counted manually and expressed as CFU/mL (colony forming units/mL)
Phase of drug intervention
In these phases (Phases II and III), the blood glucose levels and the density of the candidal colony in CFU were evaluated after drug intervention. The patients were given oral hypoglycemics as prescribed by the diabetologist. The oral hypoglycemics were administered after the first phase of sample collection. The drugs were administered to control the level of blood glucose in diabetic patients.
The blood glucose level and the CFU levels of Candida were evaluated after 30 days and 60 days following drug intervention.
Phase II
The candidal sample collections were done after 30 days of drug administration. The sample collection was done using cotton swab. The swab with the sample collected were then transferred to a transport medium and then subjected to culture. Culturing of the candidal sample was carried out, and the density of the candidal colony was assessed and expressed in CFU/mL. The colony forming units and the blood glucose level (FBS and PPS) were evaluated and recorded.
Phase III
The candidal sample collection was done after 60 days of drug intervention. The blood glucose levels, both the PPS and FBS levels, were evaluated before the sample collection. The candidal sample collection was made by swabbing from the palatal surface of the maxillary dentures and rugal folds. The swab collected was then transferred to the transport medium and cultured. The density of candidal colonization was evaluated after 48 h and expressed in CFU/mL. The blood glucose level and the CFU level of all the patients were recorded and tabulated.
RESULTS
The correlations between the variables were analyzed. The correlation analyzed was between the following.
CFU vs. PPS and CFU vs. FBS (preinterventional at day 0)
CFU vs. PPS and CFU vs. FBS (postinterventional at day 30)
CFU vs. PPS and CFU vs. FBS (postinterventional at day 60)
The P value obtained was found to be P < 0.05. Thus confirming a positive correlation between the blood glucose levels (FBS and PPS) and the candidal colony density (CFU).
Interpretation
Fasting blood sugar level
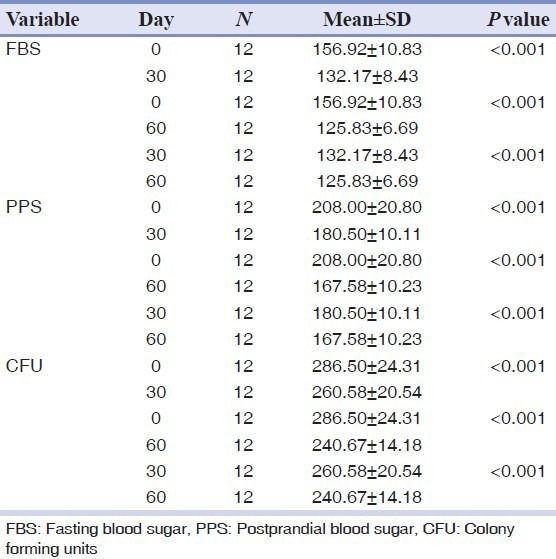
The fasting blood glucose level of the patients at the beginning of the study was 156.92 mg/dL (mean). After the drug intervention, the FBS level shows a reduction after 30 days and after 60 days. The P value obtained for all the mean values is <0.01, on comparing between day 0 and 30, day 0 and day 60 and between day 30 and day 60.
Postprandial blood sugar
These results show that there is a significant reduction in the postprandial glucose level after drug administration. The mean value at day 0 is 208 mg/dL, at the end of day 30 after drug intervention the mean value is 180 mg/dL, and at day 60 is 167.58 mg/dL.
This reduction continues but the margin of reduction is lesser when compared to that between day 0 and day 30.
CFU
The mean CFU value at day 0 before the drug intervention is 286.50, after drug intervention the mean value shows a decrease in the colony forming units, the reduction of the CFU level is by about 20 CFU at day 30 and by 40 CFU at day 60.
The results confirms that as the blood glucose level decreases there is also a decrease in the candidal density as expressed by colony forming units.
From these changes, we can confirm the effect of the oral hypoglycemic drug therapy in reducing the blood glucose level in type II diabetic patients and also the density of candidal colonization with reference to the mean values seen after phase of drug intervention.
DISCUSSION
The null hypothesis formulated for this study was that there is no significant correlation between the blood glucose levels and oral candida colonization in complete denture wearers with Type II Diabetes Mellitus and oral hypoglycemic drug therapy has no influence in controlling oral candidal colonization in denture-bearing mucosa of complete denture wearers with Type II Diabetes Mellitus.
The results of this study negated the null hypothesis.
Statistical analysis was done to evaluate the relationship between the variables. The factors analyzed were between:
PPS and CFU (preinterventional and postinterventional values)
FBS and CFU (preinterventional and postinterventional values)
The results from this study [Table 1] demonstrate that there is a statistically significant correlation between the blood glucose level and candidal colonization.
Table 1.
Karl Pearson correlation analysis
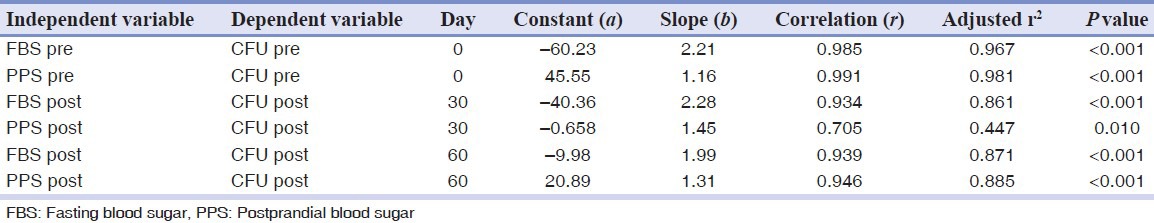
There was a reduction in the candidal colony forming units from day 0 and day 60. Similarly, there was a reduction in the blood glucose levels (FBS and PPS) from day 0 to day 60. The candidal isolates were higher at day 0 from the diabetic denture wearers when compared to that at day 60. The reduction in blood glucose levels was seen after drug intervention. Both the PPS and FBS levels were reduced [Table 2].
Table 2.
Paired t-test to compare the mean between different time periods
Samaranayake, et al. observed the adhesion of candidal species was seen in serum-coated acrylic strips.[9] The candidal colonization was observed to be greater in diabetic complete denture wearers and also the isolation of candidal organisms from them was more when compared to nondiabetic denture wearers.[10,11] Webb, et al. observed the virulence factor of candida when increased can predispose to oral candidiasis, and it increases in magnitude when the immune status is compromised as seen in diabetic patients.[12]
Dorocka-Bobkowska, et al. has observed the risk of denture stomatitis increased in diabetic patients, and the frequency of candidal isolation was more when compared to nondiabetics.[13] Darwazeh, et al. observed that candidal organisms were more frequently isolated from the fingertips and oral cavity of denture wearers proving that denture wearers with candida had a higher prevalence for candidal contamination[14] and Daniluk, et al. stated that the inflammation caused due to denture stomatitis had a very marked presence of candidal species.[15]
Increased colonization of opportunistic mycopathogens was observed by Lockhart, et al. in the elderly.[16] Khosravi, et al. observed that there was an increased prevalence of candida species with higher densities in the oral cavity of subjects with Diabetes Mellitus.[17]
The candidal colonization in a controlled diabetic was of less density as observed in this study. In this study, no variation in the pattern of candidal colonization between the genders was present. Oral hypoglycemic drugs significantly reduced the elevated blood glucose level. Controlling Diabetes Mellitus can improve cellular health by minimizing rapid cellular exfoliation associated with diabetes mellitus[18,19,20] and limits intracellular water loss, improves xerostomic changes, enhances the effectiveness of immune mechanism by improved neutrophil function, reversing microangiopathy at various stages.
This improves the desuppression of the immune system effectively by enhancing the function of secretory immunoglobulins and lysozymes that can provide effective protection against fungal infection. Deceleration of microangiopathy maintains the integrity of the basement membrane thus controlling the rate of epithelial turn over and maintenance of optimal thickness of the epithelial membrane, and hence regulation of the blood glucose level facilitates improved protection against the fungal mycelium.
Controlling elevated blood glucose levels to the optimum level following antidiabetic drug therapy facilitates regulation of adipokines and cytokines which are inflammatory mediators released from adipose tissues, thus controlling excessive inflammatory response present in uncontrolled diabetic patients. The denture-bearing tissues in diabetic patients are subjected to varying levels of stress and trauma due to maldirected occlusal forces, progressive and uneven wear of resin teeth, decreased vertical dimension, and trauma from thermal injury caused by poor conduction of heat by the denture bases. Thus, controlling diabetes mellitus greatly minimizes inflammatory response in the denture-bearing area.
Inflammatory changes can cause alteration in the volume of the tissues present in the denture-bearing area. Edema and compression added with angiopathy results in faster degeneration of the epithelium present in the denture-bearing area making it highly susceptible to fungal infections. Thus antidiabetic drug therapy by controlling blood glucose levels minimizes the chance of inflammation and subsequent fungal invasion in the denture-bearing mucosa.
This study offers further scope of research as the patients can be followed for increased periods of time, the efficacy and effectiveness of group specific oral hypoglycemic drugs can be studied, and the study can also be extended to Type II Diabetes Mellitus patients undergoing insulin therapy.
CONCLUSION
Within the limitations of the study, it could be concluded:
There is a positive correlation between oral candidiasis in denture-bearing mucosa and elevated blood glucose levels in complete denture wearers with Type II Diabetes Mellitus.
The oral hypoglycemic drug therapy has a positive effect in controlling oral candidal colonization in complete denture wearers and by controlling the blood glucose level in uncontrolled Type II diabetic denture wearers with oral hypoglycemic drugs, it is possible to reduce increased colonization of candida in denture-bearing mucosa and thus enhance improved health.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Soell M, Hassan M, Miliauskaite A, Haikel Y, Selimovic D. The oral cavity of elderly patients in diabetes. Diabetes Metab. 2007;33(Suppl 1):S10–8. doi: 10.1016/s1262-3636(07)80053-x. [DOI] [PubMed] [Google Scholar]
- 2.Barbeau J, Seguin J, Goulet JP, de Koninck L, Avon SL, Lalonde B, et al. Reassessing the presence of Candida albicans in denture-related stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:51–9. doi: 10.1067/moe.2003.44. [DOI] [PubMed] [Google Scholar]
- 3.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 4.Oksala E. Factors predisposing to oral yeast infections. Acta Odontol Scand. 1990;48:71–4. doi: 10.3109/00016359009012736. [DOI] [PubMed] [Google Scholar]
- 5.Chomicz L, Szubinska D, Piekarczyk J, Wojtowicz A, Piekarczyk B, Starosciak B, et al. Occurrence of oral subclinical infections in insulin treated diabetics. Wiad Parazytol. 2004;50:177–80. [PubMed] [Google Scholar]
- 6.Kumar BV, Padshetty NS, Bai KY, Rao MS. Preva-lence of Candida in the oral cavity of diabetic sub-jects. J Assoc Physicians India. 2005;53:599–602. [PubMed] [Google Scholar]
- 7.Samaranayake LP, MacFarlane TW. The effect of dietary carbohydrates on the in-vitro adhesion of Candida albicans to epithelial cells. J Med Microbiol. 1982;15:511–7. doi: 10.1099/00222615-15-4-511. [DOI] [PubMed] [Google Scholar]
- 8.Radford DR, Challacombe SJ, Walter JD. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med. 1999;10:99–116. doi: 10.1177/10454411990100010501. [DOI] [PubMed] [Google Scholar]
- 9.Samaranayake LP, McCourtie J, MacFarlane TW. Factors affecting the in-vitro adherence of Candida albicans to acrylic surfaces. Arch Oral Biol. 1980;25:611–5. doi: 10.1016/0003-9969(80)90076-x. [DOI] [PubMed] [Google Scholar]
- 10.Budtz-Jörgensen E, Stenderup A, Grabowski M. An epidemiologic study of yeasts in elderly denture wearers. Community Dent Oral Epidemiol. 1975;3:115–9. doi: 10.1111/j.1600-0528.1975.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 11.Lotfi-Kamran MH, Jafari AA, Falah-Tafti A, Tavakoli E, Falahzadeh MH. Candida Colonization on the Denture of Diabetic and Non-diabetic Patients”. Dent Res J. 2009;6:23–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: A review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust Dent J. 1998;43:45–50. doi: 10.1111/j.1834-7819.1998.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 13.Dorocka-Bobkowska B, Budtz-Jörgensen E, Włoch S. Non-insulin-dependent diabetes mellitus as a risk factor for denture stomatitis. J Oral Pathol Med. 1996;25:411–5. doi: 10.1111/j.1600-0714.1996.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 14.Darwazeh AM, Al-Refai S, Al-Mojaiwel S. Isolation of Candida species from the oral cavity and fingertips of complete denture wearers. J Prosthet Dent. 2001;86:420–3. doi: 10.1067/mpr.2001.118020. [DOI] [PubMed] [Google Scholar]
- 15.Daniluk T, Tokajuk G, Stokowska W, Fiedoruk K, Sciepuk M, Zaremba ML, et al. Occurrence rate of oral Candida albicans in denture wearer patients. Adv Med Sci. 2006;51:77–80. [PubMed] [Google Scholar]
- 16.Lockhart SR, Joly S, Vargas K, Swails-Wenger J, Enger L, Soll DR. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J Dent Res. 1999;78:857–68. doi: 10.1177/00220345990780040601. [DOI] [PubMed] [Google Scholar]
- 17.Khosravi AR, Bandghorai AN, Moazzeni M, Shokri H, Mansouri P, Mahmoudi M. Evaluation of Candida albicans allergens reactive with specific IgE in asthma and atopic eczema patients. Mycoses. 2009;52:326–33. doi: 10.1111/j.1439-0507.2008.01599.x. [DOI] [PubMed] [Google Scholar]
- 18.Darwazeh AM, Lamey PJ, Samaranayake LP, MacFarlane TW, Fisher BM, Macrury SM. The relationship between colonisation, secretor status and in-vitro adhesion of Candida albicans to buccal epithelial cells from diabetics. J Med Microbiol. 1990;33:43–9. doi: 10.1099/00222615-33-1-43. [DOI] [PubMed] [Google Scholar]
- 19.Darwazeh AM, MacFarlane TW, Lamey PJ. The in vitro adhesion of Candida albicans to buccal epithelial cells (BEC) from diabetic and non-diabetic individuals after in vivo and in vitro application of nystatin. J Oral Pathol Med. 1997;26:233–6. doi: 10.1111/j.1600-0714.1997.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 20.Manfredi M, McCullough MJ, Al-Karaawi ZM, Vescovi P, Porter SR. In vitro evaluation of virulence attributes of Candida spp. isolated from patients affected by diabetes mellitus”. Oral Microbiol Immunol. 2006;21:183–9. doi: 10.1111/j.1399-302X.2006.00275.x. [DOI] [PubMed] [Google Scholar]


