Abstract
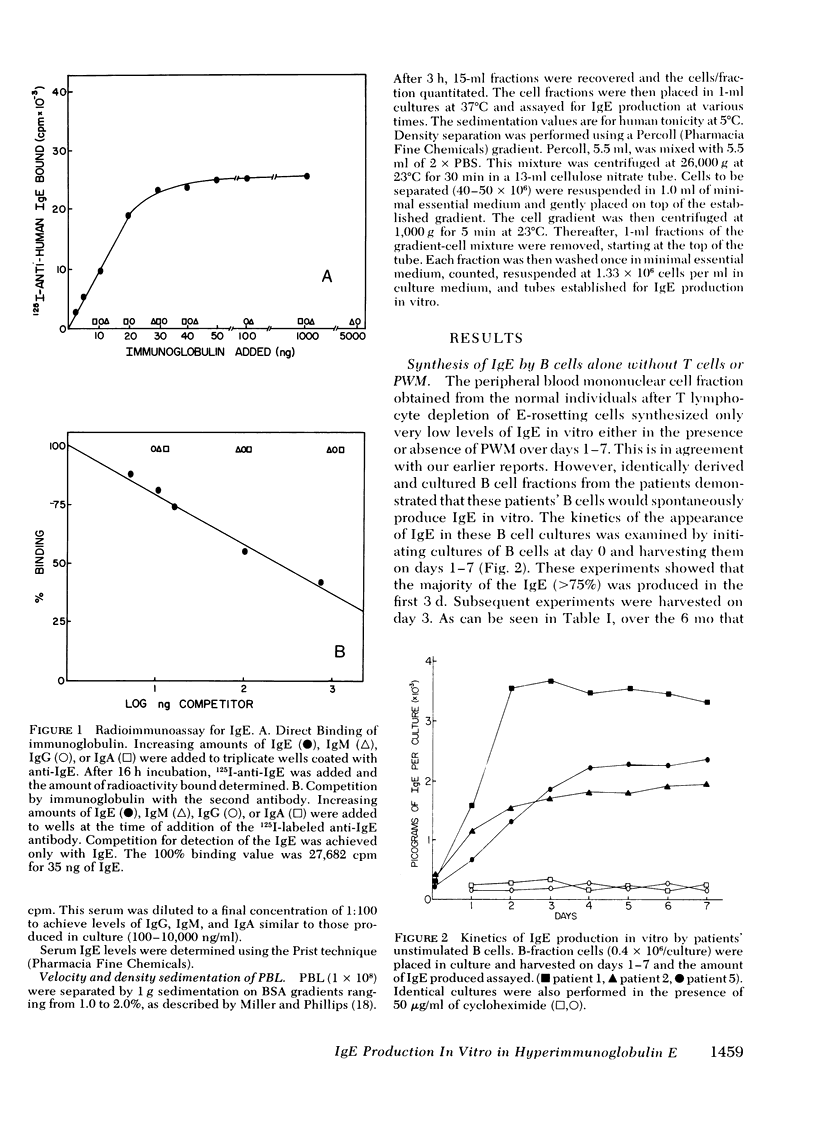
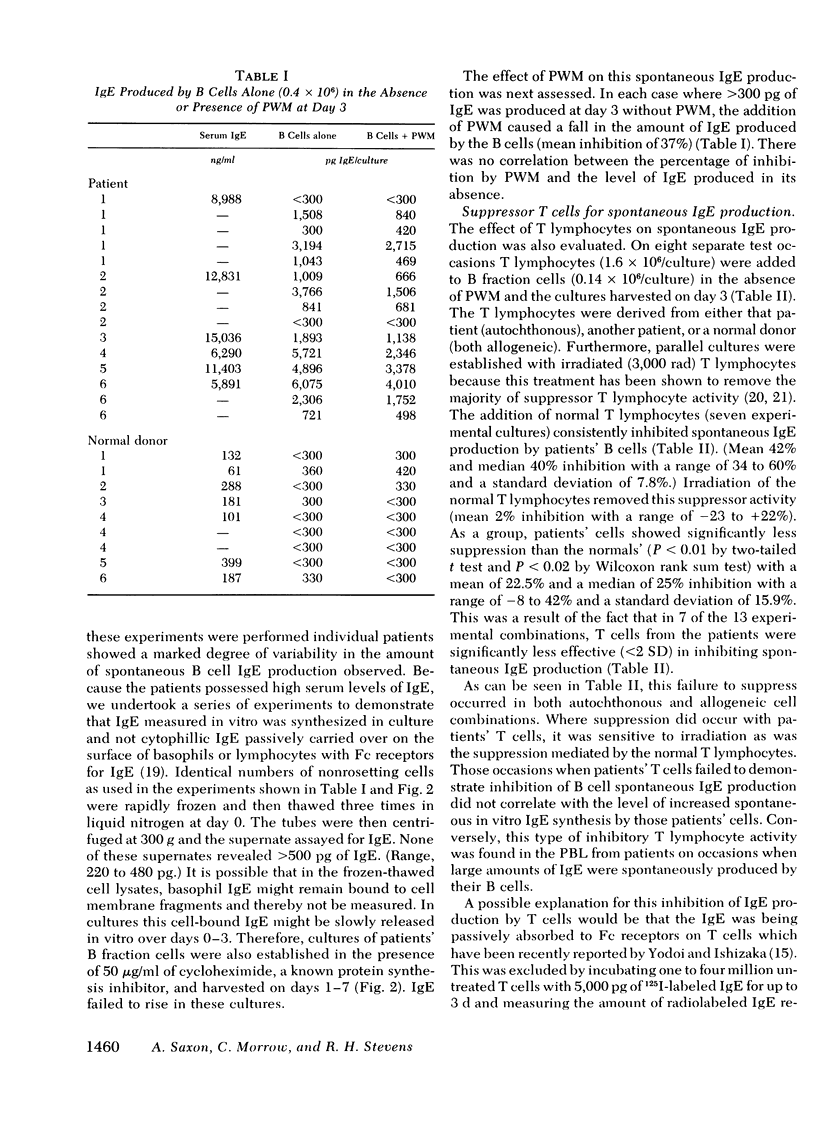
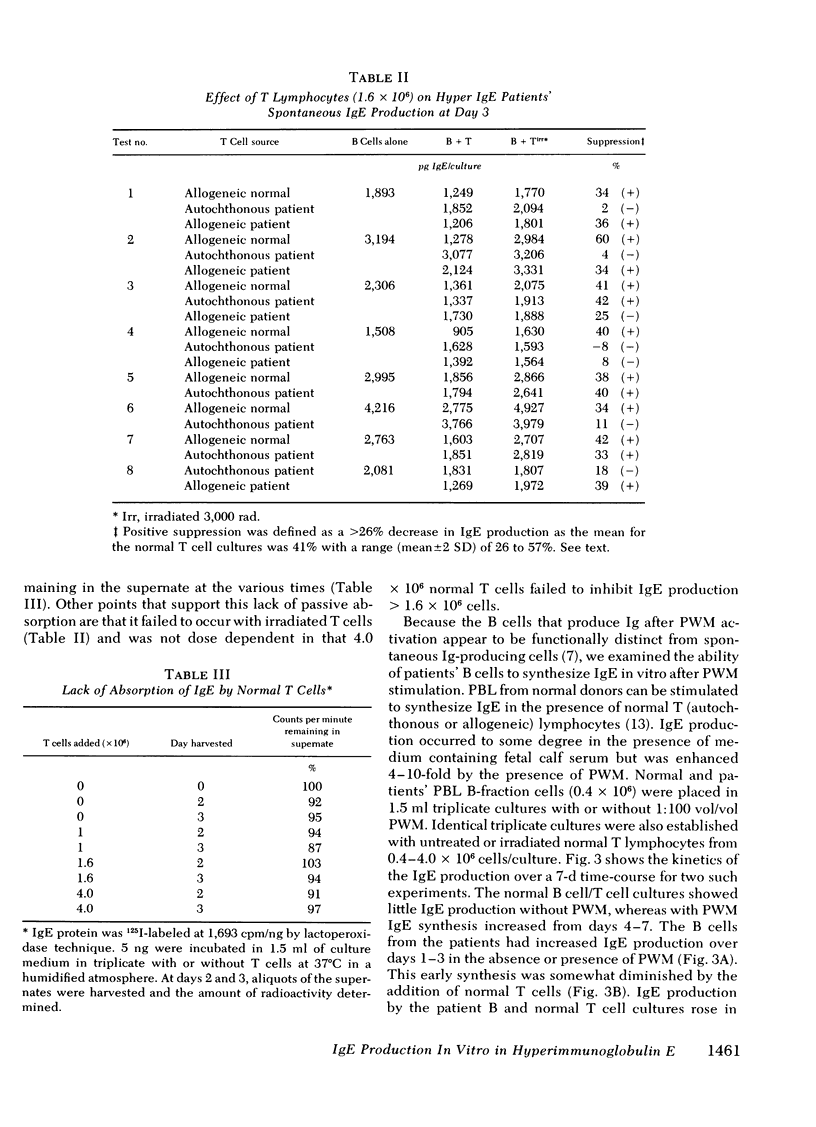
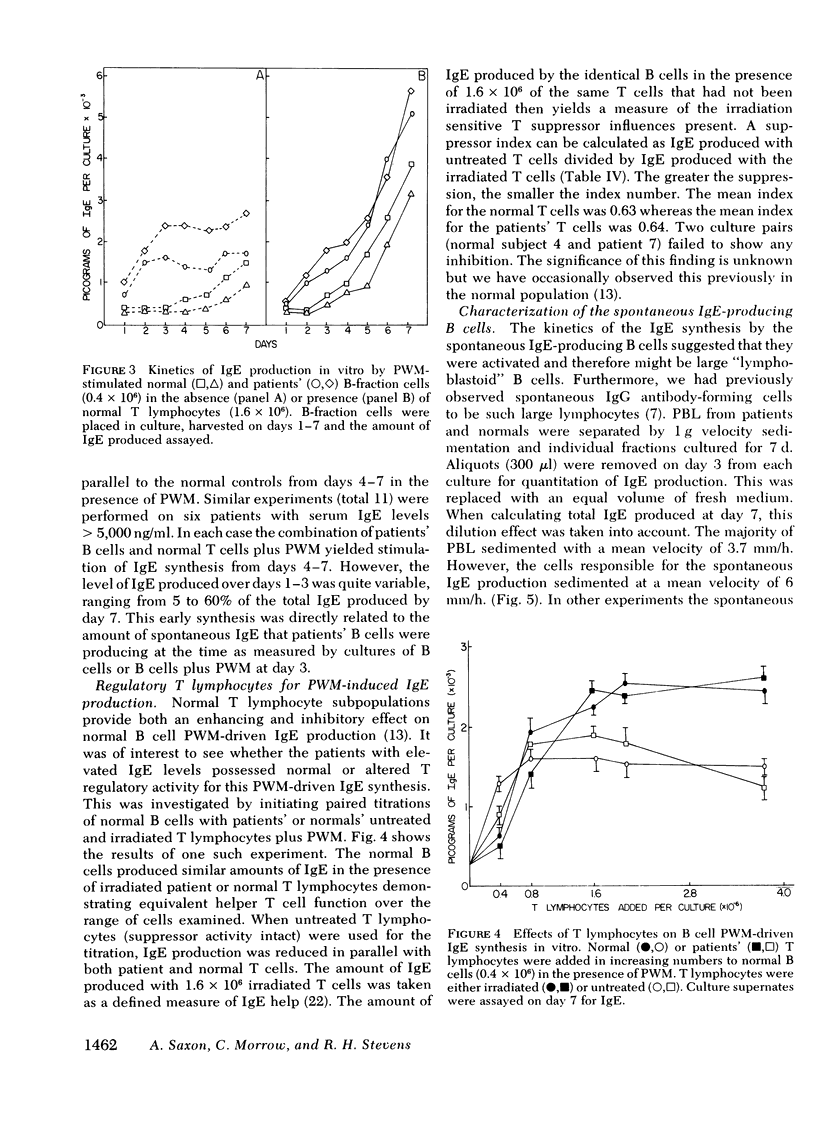
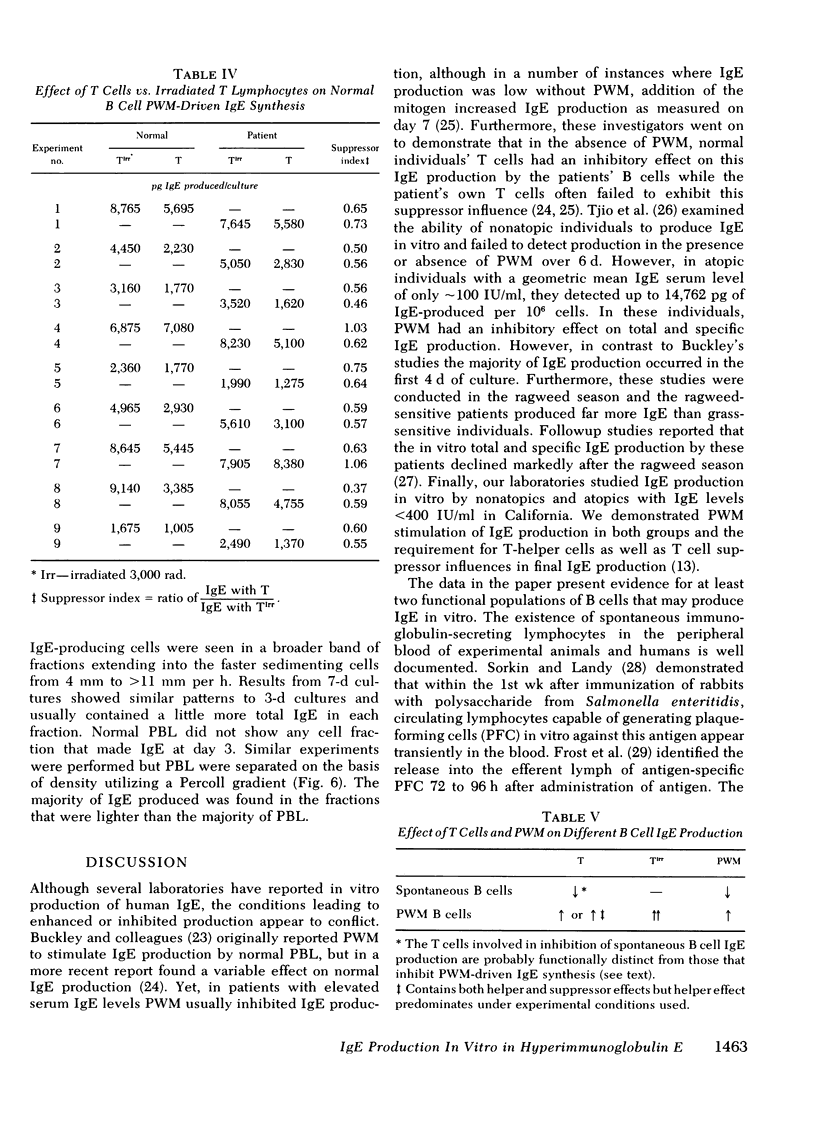
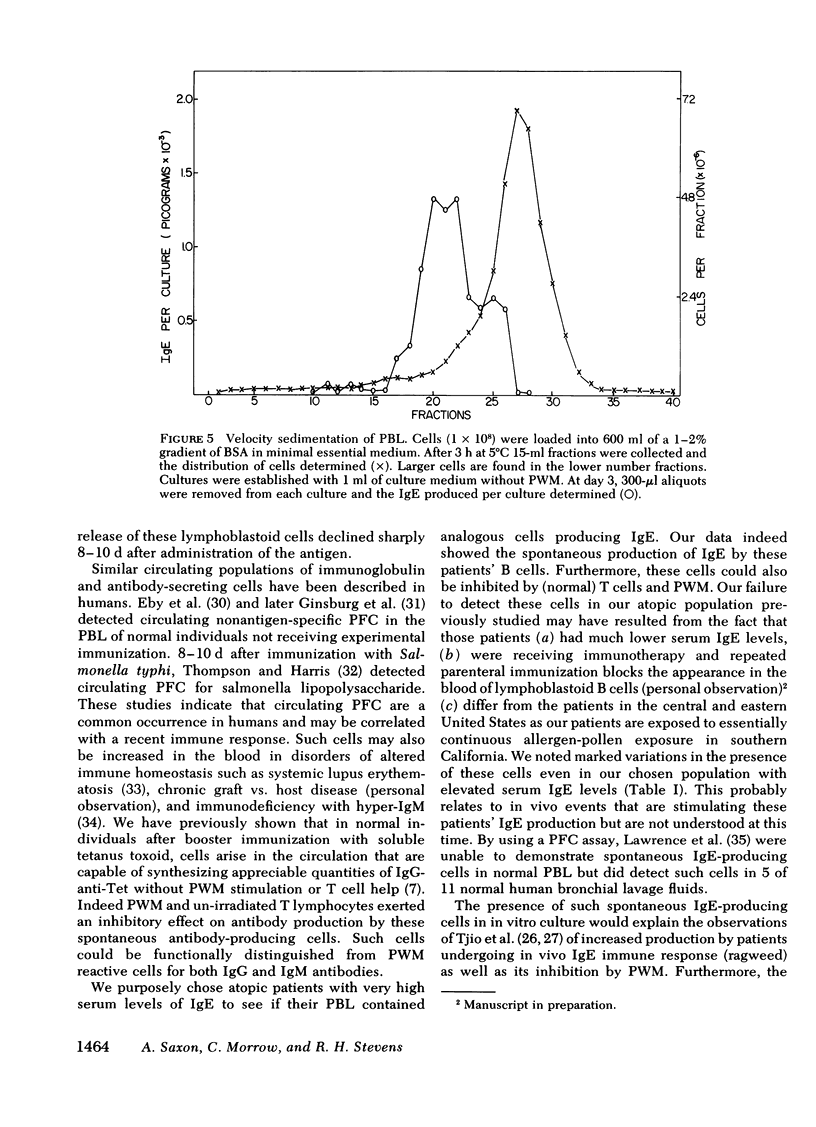
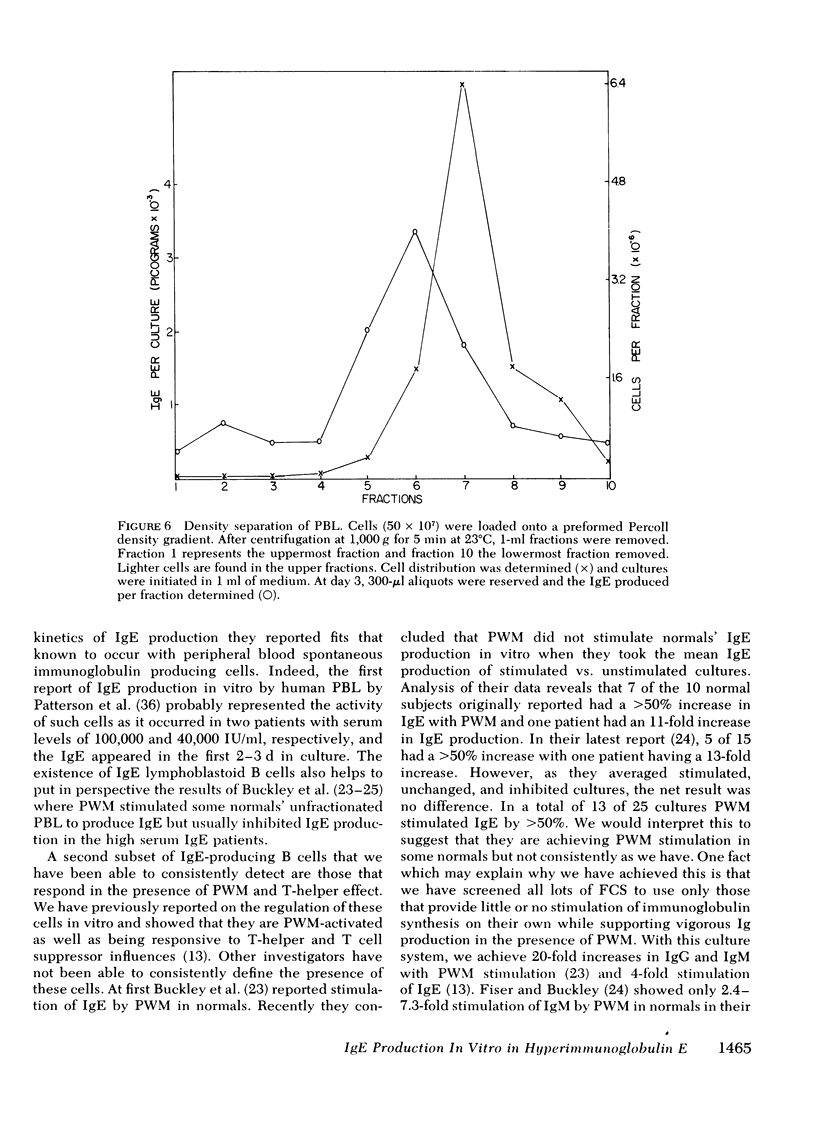
The B lymphocyte subpopulations producing immunoglobulin (Ig)E and the regulatory T cells modulating this IgE production in normals, and in atopic patients with respiratory allergy, atopic dermatitis, and markedly elevated serum IgE levels (>5,000 ng/ml), were investigated. Peripheral blood lymphocytes (PBL) were separated into T and B cell fractions and the ability of B cells to produce IgE in the presence or absence of pokeweed mitogen (PWM) and/or T cells ws determined. The patients had a circulating population of cells which spontaneously produced up to 6 ng of IgE in vitro (per 4 X 10(5) non-E-rosetting cells) in the absence of T lymphocytes and PWM. PBL from normals did not possess such cells. This IgE synthesis occurred primarily (>75%) over the first 72 h of culture. There was a wide range in their activity between patients and from the same patient studied on repeated occasions (from <300 to 6,000 pg per culture). This spontaneous IgE production was inhibited by PWM (mean inhibition, 37%) or normal T lymphocytes (mean inhibition, 42%). The patients lacked T lymphocytes capable of inhibiting this spontaneous IgE synthesis in 7 of 13 experiments. Functionally distinct B cells were identified in the patients and normals that responded to PWM with IgE production in vitro and required T-helper cell activity. Patients had normal PWM-responsive B cell IgE biosynthetic activity and T-helper function for these B cells. Suppressor T cell activity for PWM-driven IgE synthesis was also evaluated. Both the normals' and the patients' T lymphocytes provided similar levels of T cell suppressor function for PWM-driven IgE production. Patients with elevated serum IgE possessed these inhibitory T cells at times when the T lymphocytes which suppressed spontaneous igE production were absent from their PBL.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley R. H., Becker W. G. Abnormalities in the regulation of human IgE synthesis. Immunol Rev. 1978;41:288–314. doi: 10.1111/j.1600-065x.1978.tb01469.x. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Wray B. B., Belmaker E. Z. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972 Jan;49(1):59–70. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Eby W. C., Chong C. A., Dray S., Molinaro G. A. Enumerating immunoglobulin-secreting cells among peripheral human lymphocytes. A hemolytic plaque assay for a B cell function. J Immunol. 1975 Dec;115(6):1700–1703. [PubMed] [Google Scholar]
- Fiser P. M., Buckley R. H. Human IgE biosynthesis in vitro: studies with atopic and normal blood mononuclear cells and subpopulations. J Immunol. 1979 Oct;123(4):1788–1794. [PubMed] [Google Scholar]
- Frost H., Braun D. G., Poskitt D., Cahill R. N., Trnka Z. Antipolysaccharide antibodies of restricted heterogeneity secreted by a single lymph node. J Exp Med. 1976 Mar 1;143(3):707–711. doi: 10.1084/jem.143.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S., Hyslop N., Alami S., Farah F., Schneeberger E. E., Rosen F. S. Hyper immunoglobulin M immunodeficiency. (Dysgammaglobulinemia). Presence of immunoglobulin M-secreting plasmacytoid cells in peripheral blood and failure of immunoglobulin M-immunoglobulin G switch in B-cell differentiation. J Clin Invest. 1979 Aug;64(2):385–391. doi: 10.1172/JCI109473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and mitogen-induced immunoglobulin-secreting cells in human peripheral blood: evaluation by a modified reverse hemolytic plaque assay. J Immunol. 1978 Jan;120(1):33–39. [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and pokeweed mitogen-induced immunoglobulin-secreting cells in systemic lupus erythematosus. Clin Exp Immunol. 1979 Jan;35(1):76–88. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. A subpopulation of normal human peripheral B lymphcytes that bind IgE. J Clin Invest. 1977 Apr;59(4):616–624. doi: 10.1172/JCI108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. W. Distribution of plaque-forming cells in the mouse for a protein antigen. Evidence for highly active parathymic lymph nodes following intraperitoneal injection of hen lysozyme. Immunology. 1976 Jun;30(6):895–906. [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K. Cellular events in the IgE antibody response. Adv Immunol. 1976;23:1–75. doi: 10.1016/s0065-2776(08)60318-1. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. 1970 Aug;7(8):687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Biology of immunoglobulin E. Molecular basis of reaginic hypersensitivity. Prog Allergy. 1975;19:60–121. [PubMed] [Google Scholar]
- Jarrett E. E. Stimuli for the production and control of IgE in rats. Immunol Rev. 1978;41:52–76. doi: 10.1111/j.1600-065x.1978.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Lawrence E. C., Blaese R. M., Martin R. R., Stevens P. M. Immunoglobulin secreting cells in normal human bronchial lavage fluids. J Clin Invest. 1978 Oct;62(4):832–835. doi: 10.1172/JCI109195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Kochwa S., Smith C., Ishizaka K., McIntyre O. R. Clinical aspects of IgE myeloma. N Engl J Med. 1969 Nov 27;281(22):1217–1220. doi: 10.1056/NEJM196911272812204. [DOI] [PubMed] [Google Scholar]
- Patterson R., Suszko I. M., Metzger W. J., Roberts M. In vitro production of IgE by human peripheral blood lymphocytes: effect of cholera toxin and beta adrenergic stimulation. J Immunol. 1976 Jul;117(1):97–101. [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Saxon A., Portis J. Lymphoid subpopulation changes in regional lymph nodes in squamous head and neck cancer. Cancer Res. 1977 Apr;37(4):1154–1158. [PubMed] [Google Scholar]
- Saxon A., Stevens R. H. Stimulation and regulation of human IgE production in vitro using peripheral blood lymphocytes. Clin Immunol Immunopathol. 1979 Dec;14(4):474–488. doi: 10.1016/0090-1229(79)90100-4. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H. Suppression of immunoglobulin production in normal human blood: characterization of the cells responsible and mediation by a soluble T lymphocyte-derived factor. Clin Immunol Immunopathol. 1978 Aug;10(4):427–437. doi: 10.1016/0090-1229(78)90155-1. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Sorkin E., Landy M. Antibody production by blood leucocytes. Experientia. 1965 Nov 15;21(11):677–680. doi: 10.1007/BF02144077. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Differential synthesis of IgM and IgA anti-tetanus toxoid antibody in vitro following in vivo booster immunization of humans. Cell Immunol. 1979 Jun;45(1):142–150. doi: 10.1016/0008-8749(79)90369-1. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Immunoregulation in humans: control of antitetanus toxoid antibody production after booster immunization. J Clin Invest. 1978 Dec;62(6):1154–1160. doi: 10.1172/JCI109234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T. Regulation of reaginic antibody formation in animals. Prog Allergy. 1975;19:122–194. [PubMed] [Google Scholar]
- Thomson P. D., Harris N. S. Detection of plaque-forming cells in the peripheral blood of actively immunized humans. J Immunol. 1977 Apr;118(4):1480–1482. [PubMed] [Google Scholar]
- Tjio A. H., Hull W. M., Gleich G. J. Production of human immunoglobulin E antibody in vitro. J Immunol. 1979 May;122(5):2131–2133. [PubMed] [Google Scholar]
- Waldmann T. A., Blaese R. M., Broder S., Krakauer R. S. Disorders of suppressor immunoregulatory cells in the pathogenesis of immunodeficiency and autoimmunity. Ann Intern Med. 1978 Feb;88(2):226–238. doi: 10.7326/0003-4819-88-2-226. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Ishizaka K. Lymphocytes bearing Fc receptors for IgE. I. Presence of human and rat T lymphocytes with Fc epsilon receptors. J Immunol. 1979 Jun;122(6):2577–2583. [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]


