Abstract
Purpose
To report the 5-year results of a prospective trial of three-dimensional conformal external beam radiotherapy (3D-CRT) to deliver accelerated partial breast irradiation in the prone position (P-APBI).
Methods
Post-menopausal patients with Stage I breast cancer with non palpable <2 cm tumors, negative margins, and negative nodes, positive hormonal receptors, and no extensive intraductal component (EIC) were eligible. The trial was offered only once eligible patients had refused to undergo standard whole-breast radiotherapy. Patients were simulated and treated on a dedicated table for prone set-up. The 3D-CRT delivered was 30 Gy in five 6 Gy/daily fractions over 10 days with port film verification at each treatment. Ipsilateral breast, ipsilateral nodal, contralateral breast, and distant failure (IBF, INF, CBF, DF) were estimated using the cumulative incidence method. Disease-free, overall, and cancer-specific survival (DFS, OS, CSS) were recorded.
Results
One hundred patients accrued to this IRB- approved prospective trial, one with bilateral breast cancer. One patient withdrew consent after simulation and another elected to interrupt radiotherapy after receiving two treatments. Ninety-eight patients are evaluable for toxicity and, in one case, both breasts were treated with PBI. Median patient age was 68 years (range 53–88 years); in 55% the tumor size was <1 cm. All patients had hormonal receptor positive cancers: 87% underwent adjuvant anti-hormonal therapy.
At a median follow-up of 64 months (range, 2–125 months), there was one local recurrence (1% IBF) and one contralateral breast cancer (1% CBF). There were no deaths due to breast cancer by 5 years. Grade 3 late toxicities occurred in 2 patients (1 breast edema, 1 transient breast pain). Cosmesis was rated good/excellent in 89% of patients with at least 36 months follow-up.
Conclusions
Five-year efficacy and toxicity of 3D-CRT to deliver prone-PBI are comparable to other experiences with similar follow-up.
Keywords: Accelerated partial breast radiotherapy, Prone set-up
INTRODUCTION
Different methods to deliver accelerated regimens of partial breast irradiation (PBI) have been developed that result in excellent 5-year outcomes with respect to local tumor control and acceptable cosmesis, as systematically reviewed by Offersen et al. (1) While the results of several prospective randomized trials that compare PBI to whole-breast radiotherapy are maturing, a consensus panel of experts has defined a set of clinical guidelines to select patients best suitable to PBI (2). There is, however, debate over the choice of the best technique for PBI. Interstitial brachytherapy has been the most widely investigated PBI technique to date and has the longest follow-up (3) (4) but recent reports suggest that mammosite or an external beam delivery technique may be as effective (5) (6). In addition, evidence is emerging with regard to the efficacy of intra-operative radiotherapy. (7)
Since the early 1990s, we have chosen the prone set-up to study external beam partial breast irradiation. Initially pilot-tested at the Anonymous University (Anon U) (8) as part of a grant from the Anon Program, these studies were continued at Anon U, supported by a grant from the Department of Defense, as Anon Trial, a prospective trial of prone accelerated partial breast irradiation (P-APBI), delivered by 3D-CRT. The choice of dose and fractionation and the feasibility and acute toxicity results of the first 47 patients were previously reported (9,10).
Details on planning and dosimetry results are reported in a companion paper that compares the Anon U technique to that applied in the NSABP/RTOG trial. (11) This report focuses on the results related to local control, cosmesis, and treatment-associated toxicities at 5 years for the 98 patients evaluable for toxicity who were treated with this technique.
METHODS AND MATERIALS
Study population
The study population consisted of 100 consecutive women who were referred for postoperative RT after lumpectomy and were offered access to the protocol, following their documented refusal to undergo standard whole-breast radiotherapy. Post-menopausal women with newly diagnosed breast cancer were eligible if tumors were originally detected by mammography (i.e. non-palpable), found to be pT1 breast cancers, excised with negative margins (defined as at least a 5 mm margin either at initial surgery or at re-excision), N0 or sentinel node negative, and hormone receptor-positive. Between June 2000 and September 2005, one hundred patients signed an informed consent to participate in this IRB-approved prospective trial: one patient withdrew consent after simulation, before planning was performed (before starting PBI) and another elected to interrupt radiotherapy after receiving two treatments. One patient had bilateral breast cancer and underwent bilateral PBI. This report focuses on the dosimetry of 101 planned cases (including a patient with bilateral breast cancer), the treatment outcomes of all 100 patients based on intent-to-treat analysis, and the treatment toxicities of the 98 patients who completed treatment and were evaluable for toxicity (including one patient with bilateral breast cancer).
Simulation and treatment planning
The details of the prone PBI treatment technique have been previously reported. (8)(10) Specifically, each patient underwent CT simulation for treatment planning positioned prone on a specially designed breast table that allowed the indexed breast tissue to fall freely below the table with no obstruction to the radiation beams. An orthogonal laser alignment was used to identify a plane likely to intersect the tumor cavity, based on mammography and/or surgical description of the location (reference plane). On the reference plane, five radio-opaque markers were placed on the skin, the back of the patient, bilaterally on mid-axillary lines, and midline to the sternum and lateral breast. After CT verification of the plane, the five markers were replaced with permanent tattoos, as landmarks for daily treatment positioning. CT imaging started at the level of the mandible and extended below the infra-mammary fold, to include the entire lungs, with 2.5 or 3.75 mm CT slice thickness, using a GE Light speed helical CT scanner.
CT images were transferred to a Varian Eclipse™ treatment planning system (Varian Medical Systems, Palo Alto, CA). The surgical cavity, identified as the area of architectural distortion in the breast tissue, defined the clinical target volume (CTV). When necessary, information obtained from the surgical report, mammography, and other available imaging test results were incorporated. Although not intentionally included by the CTV, the surgical incision was outlined by a wire placed over the incision before CT scanning. Adding a 2 cm margin to the CTV created the planning target volume (PTV). After uniform expansion, the PTV was limited anterior by the skin and posterior by the chest wall. An additional 7 mm margin was added to the PTV to the field edge to account for beam penumbra, for a total margin of approximately 2.7 cm. For dosimetric reporting, the PTV_EVAL was generated from the PTV by cropping 0.5 cm from the skin edge and excluding the chest wall. The ipsilateral lung and heart were outlined. The normal ipsilateral breast tissue volume was defined by applying radio-opaque wires in the supine position at the site of the medial, lateral, inferior, and superior borders of the classic opposite tangent breast fields to define the volume that would have been treated by classic whole-breast tangents in the supine position.
Dose–volume constraint guidelines
Treatment planning was performed using the CT-defined volumes, most often through an opposed pair of mini-tangents. When required to increase dose distribution homogeneity, wedges were used. The isocenter was located approximately 5–7 cm from the midline along an axis passing through the center of the PTV. The dose was normalized to 100% at the isocenter before choosing an isodose volume that encompassed the PTV, typically 95%. Dose inhomogeneity was maintained at <110%.
Additional normal tissue dose guidelines included limiting 50% of the ipsilateral breast volume to receive <50% of the prescribed dose. In addition, the volume of heart and lung included in the treatment fields was expected to be <10%. Field arrangements were designed to avoid the contralateral breast and ipsilateral lung and heart tissue completely. No specific guidelines with regards to the dose allowed to the contralateral breast were defined in the protocol. The dose fractionation schedule was 30 Gy delivered in five fractions of 6 Gy to the 95% isodose surface, given within 10 days (Monday, Wednesday, Friday, Monday, Wednesday).
Treatment room lasers were used to verify consistent positioning of the patient on the table. Daily set-up reproducibility was ensured by leveling marks on the torso and triangulation marks placed on the back, ipsilateral side, and breast tissue. The set-up was designed to identify a plane orthogonal to the table that also crossed the tumor cavity. Before each fraction, portal films of each field verified treatment positioning. Accepted variance was limited to 5 mm from the isocenter position indicated on the digitally reconstructed radiographs.
Cosmesis and toxicities
Cosmetic results were evaluated by the patient who was asked to classify her global cosmetic result after breast conservation treatment as excellent, good, or poor. Toxicity was reported based on LENT/SOMA classification of late effects. (12)
Statistical methods
An optimal two-stage Simon design was used to conduct this Phase II study. The study was designed to test the null hypothesis that the 3-year local recurrence rate is ≥ 9% vs. the alternative that the 3-year local recurrence rate is ≤ 3% (a 0.05; power of 0.80). Thirty-one patients were to be enrolled in the first stage and up to 99 patients if the trial continued through the second stage. If two or fewer local recurrences had developed in the first 31 patients who completed at least 1 year of follow-up, accrual would continue up to completion of the second stage. If five or more local recurrences were observed at any point, the trial would have been stopped. The primary study endpoint was defined to be any ipsilateral breast local recurrence (whether a true local recurrence within the radiation field or breast local recurrence outside the field) including both isolated recurrence and concomitant with distant disease.
RESULTS
Study accrual and follow-up
One hundred patients enrolled in the trial: one withdrew consent before treatment (she changed her mind and underwent whole-breast radiotherapy), one elected to stop treatment after two fractions, and one had both breasts treated by PBI. One hundred patients were evaluable for treatment outcome with an intent-to-treat analysis. Observations from 98 patients who completed treatment were evaluable for late toxicity and cosmetic assessment. The median follow-up was 64 months (range, 2–125).
Patient and treatment-related characteristics
Patient and treatment-related characteristics for all eligible patients are shown in Tables 1 (a and b). The median patient age was 68 years, and the median tumor size was 0.9 cm (range 0.1mm–19mm). Tumor size was <10 mm in 56 patients (one patient with bilateral breast cancer had a 2 mm tumor in one breast and a 15 mm tumor in the other) and >10 mm < 20 mm in 45. All patients had negative margins pathologically (in 20 patients after re-excision). All patients were hormonal receptor positive (HR+) and were prescribed anti-hormonal therapy: 87% received anti-hormonal therapy. In 78 cases a Ki67 assessment of proliferation was available, and resulted to be <14 in 69/78.
Table 1a.
Patients characteristics (N=100)
| Age (years) | |
| Median | 68 |
| Range | 53–88 |
| Post Menopausal | 100 |
| Tumor Side | |
| Right Breast | 48 (48%) |
| Left Breast | 51 (51%) |
| Bilateral | 1 (1%) |
| Hormonal Therapy* | |
| None | 13 (13%) |
| Yes | 85 (87%) |
Information available for 98 cases
Table 1b.
Tumor characteristics (N=101, 1 patient with bilateral breast cancer)
| Tumor dimension (cm) | |
| Median | 9 mm |
| Range | 1.2mm –19mm |
| <1 cm | 56 (55%) |
| >1cm and <2 cm | 45 (45%) |
| Histology | |
| Invasive ductal | 74 (75%) |
| Invasive lobular | 16 (16%) |
| Invasive tubular | 6 (6%) |
| Invasive Ductal+Lobular | 1 (1%) |
| Invasive colloid | 2 (2%) |
| N/A* | 1 |
| Nodal Status | |
| N0 | 101** (100%) |
| pN0 at sentinel node or axillary dissection | 96 (96%) |
| No nodal assessment because of <5mm primary | 5 (5%) |
| ER+ | 99 (99%) |
| ER− | 1 (1%) |
| PR+ | 65 (65%) |
| PR− | 35 (35%) |
| HER2 (+) | |
| Yes | 5** (5%) |
| No | 93 (95%) |
| N/A | 2 |
| Ki67 (available in 78/100) | |
| Proliferation <14% | 68 (87%) |
| Proliferation >14% | 9 (11%) |
insufficient tissue to perform the test
one patient with bilateral breast cancer
1pt received trastuzumab
One hundred and one treatment plans from 100 patients were evaluable for dosimetric information. Median volume of PTV_EVAL was 161.7cc with a range of 46.8cc–900.3cc. Median PTV/total breast volume was 17% with a range of 6%–46%. Median coverage by the 95% isodose line of the CTV and PTV were 99% and 97%, respectively. The 25% and 75% isodose lines covered a median of 48% and 38% of the whole breast volume, respectively. Median maximum dose to the breast was 3210cGy with a range of 3000cGy–3960cGy. Table 2 provides a comparison of these results with those of conformal PBI in the supine position published by Chen et al. (13) (with the permission of the corresponding author).
Table 2.
Dosimetric results of Anon Study of prone PBI (100 patients, 101 plans) compared with those from William Beaumont supine PBI (94 plans) [8]
| Dosimetric characteristics of the study population | ||||||
|---|---|---|---|---|---|---|
| Dosimetric characteristics | Anon Study Mean value | Beaumont Mean value | Anon Study Median value | Beaumont Median value | Anon Study Range | Beaumont Range |
| PTV_EVAL (cc) | 200.8 | 268.1 | 161.7 | 265.6 | 46.8–900.3 | 61.8–623.0 |
| Whole breast volume (cc) | 1135 | 1584 | 1012 | 1499 | 262–3493 | 481–3417 |
| V5/WBV | 58% | 77% | 58% | 77% | 29%–99% | 46%–94% |
| V10/WBV | 54% | 72% | 53% | 73% | 27%–96% | 43%–91% |
| V25/WBV | 50% | 62% | 48% | 63% | 23%–93% | 37%–92% |
| V50/WBV | 45% | 49% | 43% | 50% | 18%–80% | 31%–61% |
| V75/WBV | 39% | 39% | 38% | 40% | 14%–76% | 26%–53% |
| V100/WBV | 24% | 24% | 24% | 24% | 5%–49% | 2%–39% |
| Maximum dose (Ratio of PD) | 1.08 | 1.10 | 1.07 | 1.10 | 1–1.32 | 1.05–1.27 |
| CTV coverage | ||||||
| 100% IDL | 98% | 95% | 98% | 99% | 55.4%–100% | 54%–100% |
| 95% IDL | 99% | 99% | 99% | 100% | 84.5%–100% | 97.5%–100% |
| PTV coverage | ||||||
| 95% IDL | 97% | 98% | 97% | 100% | 84.5%–100% | 92.5%–100% |
| Ipsilateral breast coverage (cc) | ||||||
| 100% IDL | 244.6 | 381.8 | 205.9 | 360.1 | 9.3–1146.8 | 31.4–885.0 |
| 75% IDL | 432.3 | 627.2 | 365.9 | 596 | 122.5–1563.3 | 152.0–1621.0 |
| 50% IDL | 494.2 | 783.1 | 422.8 | 754.7 | 146.1–1987.5 | 221.7–1881.8 |
| 25% IDL | 556.5 | 977.5 | 467.5 | 951.8 | 166.9–2306.3 | 263.0–2176.7 |
| PTV/total breast volume % | 18% | 17% | 17% | 17% | 6%–46% | 8%–27% |
| Dmax (cGy)* | 3250 | 4208 | 3210 | 4232 | 3000–3960 | 3668–4880 |
Anon U prescribed dose: 6Gy x 5 daily fractions, total dose 30Gy in 10 days
Beaumont prescribed dose: 3.85Gy x 10 BID fractions, total dose 38.5Gy in 5 days
Treatment efficacy
Five–year results of treatment are presented in Table 3. One patient had a local recurrence (in breast failure, IBF) resulting in a 5-year cumulative incidence rate of isolated IBF (without concurrent distant failure) of 1%. The in breast failure was detected at routine mammography and occurred in another quadrant of the breast, 33 months from accrual to the study: this new cancer was invasive lobular, while the first cancer was invasive ductal. One patient developed contralateral breast cancer 47 months from accrual to the trial. No cases of ipsilateral nodal failure (INF) were observed. No distant failure had occurred at the 5-year mark. There were two deaths (one from non-small cell lung cancer and one from Alzheimer’s disease). The overall DFS is 95% (95% CI: 87%–98%) at 5 years.
Table 3.
Treatment Outcomes @ 5 years (100 patients, intent-to-treat)
| Endpoint | No. Failures | |
|---|---|---|
| All Follow-up | At 5 Years | |
| All IBF | 1 | 1 |
| IBF w/o concurrent distant failure | 1 | 1 |
| Ipsilateral Nodal failure | 0 | 0 |
| Contralateral Breast failure | 1 | 1 |
| Distant failure | 1 | 0 |
| Disease-free survival | 9 | 4 |
| Overall survival | 6 | 2 |
| Cause-specific survival | 0 | 0 |
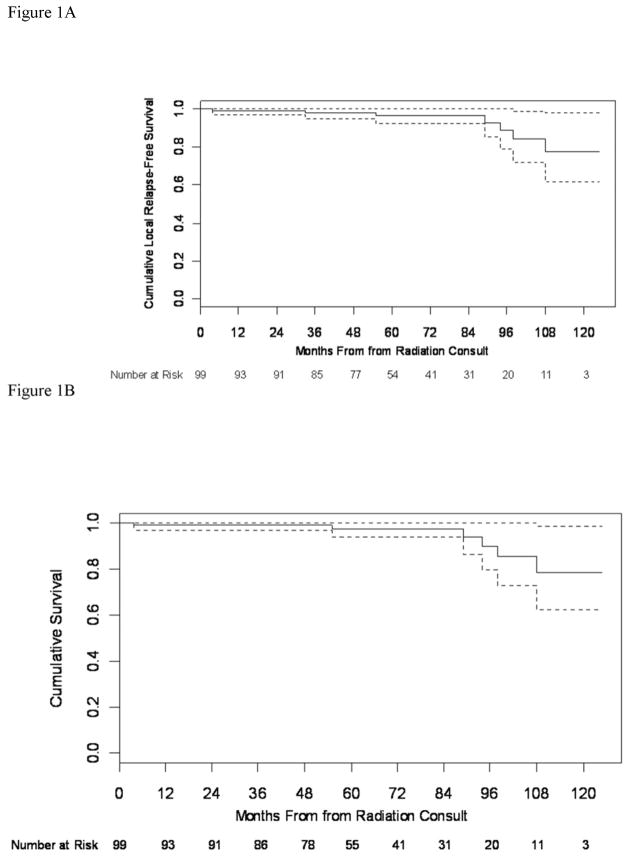
A total 6 deaths and 3 failures occurred during the entire study period. The three failures consist of the already mentioned isolated breast cancer failure and the contralateral breast cancer and of a systemic failure that occurred at 67 months. In addition to the 2 deaths during the first five years of follow-up, four additional patients have died after 5 years follow-up (1 of heart disease, 1 of multiple sclerosis, and 2 of unknown causes, at 89, 94, 98 and 108 months, respectively). Figures 1 displays the cumulative overall survival for this series.
Figure 1.
Cumulative overall survival
Treatment-related toxicities
A detailed analysis of treatment-related toxicities is presented in Table 4. The most frequent toxicities observed (Grade 1 or higher) were hyper-pigmentation (29%), fibrosis (8%), telangiectasia (3%) breast pain (13%), and breast edema (9%). Grade 3 toxicities were observed in two patients during follow up (1 transient breast pain and 1 breast edema).
Table 4.
Treatment late toxicities per LENT/SOMA classification(98 patients)
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
| Breast, Subjective: | ||||
| Pain | 85 (87%) | 11 (11%) | 1 (1%) | 1 (1%) |
| Breast Objective: | ||||
| Skin, pigmentation change | 69 (70%) | 29 (29%) | 0 (0%) | 0 (0%) |
| Edema | 89 (91%) | 7 (7%) | 1 (1%) | 1 (1%) |
| Telangiectasia | 95 (97%) | 3 (3%) | 0 (0%) | 0 (0%) |
| Fibrosis | 90 (92%) | 8 (8%) | 0 (0%) | 0 (0%) |
| Fat necrosis* | 79 (81%) | 19*(19%) | 0 (0%) | 0 (0%) |
Asymptomatic, incidental finding at mam
Cosmetic results
Table 5 presents the cosmetic results of the study population over time. Seventy-four patients provided an assessment of their cosmetic result at least 36 months after treatment: in 89% of these patients, the cosmetic result was rated as excellent (47%) or good (42%).
Table 5.
Cosmetic results over time
| Cosmetic results | Cosmesis at last follow-up (n=87) | >12 months follow-up (n= 90) | >24 months follow-up (n= 86) | >36 months follow-up (n= 74) |
|---|---|---|---|---|
| Excellent | 45 (52%) | 45 (52%) | 42 (51%) | 35 (47%) |
| Good | 33 (38%) | 33 (38%) | 33 (40%) | 31 (42%) |
| Total Excellent/Good | 78 (90%) | 78 (90%) | 75 (90%) | 66 (89%) |
| Fair | 8 (9%) | 8 (9%) | 7 (8%) | 7 (9%) |
| Poor | 1 (1%) | 1 (1%) | 1 (1%) | 1 (1%) |
DISCUSSION
This trial was specifically directed to a clinical subset of breast cancer patients at a very low risk for local recurrence as the best group to safely test the role of partial breast radiotherapy. This selection demonstrated to be successful, as confirmed by the observed 1% local recurrence rate at five-year follow-up. Since the pattern of local recurrence elsewhere in the breast tends to be later than that at the original site, a longer follow-up remains necessary to prove the long-term validity of these findings.
The prone approach of partial breast radiotherapy confirmed to be readily feasible and to achieve excellent dose-sparing of adjacent normal tissues, with results comparable to those reported for supine set-up by the investigators at Beaumont (Table 2). In a separate publication we have reported the reproducibility results of the prone technique as part of our current trial of prone PBI that includes cone beam CT for daily position verification. (14) In that report we confirm the adequacy of a 1.5 cm margin from the CTV (the contoured surgical cavity) to the PTV, an expansion that is approximately 1 cm inferior to that required by RTOG 0413. This issue is relevant, since approximately a third of patients who had consented to participate in RTOG 0413 could not be randomized because the PTV was greater than about 25% of the total breast volume, resulting in an inability to achieve the dose-volume constraints of the trial. (15) A more widespread adoption of a prone set-up may enhance compliance to the constraints of PBI trials.
The choice of dose/fractionation of the original trial was designed based on BED calculations (9)(10) that predicted a possible increase in incidence of late toxicity, like fibrosis and telangiectasia. Consequently, accrued patients underwent blood sampling for genome-wide analysis of genes potentially associated with this risk to identify specific patients’ profiles for future exclusion from hypo-fractionated, accelerated radiotherapy protocols. The observed limited clinical incidence of late effects has led us to measure the tissue changes after prone PBI with a more sensitive, objective, and quantitative approach, by using the MoistureMeter D, a device to measure subtle skin and subcutaneous tissue changes after radiotherapy.(16) This approach will enable us to develop a more reliable phenotype of late radiation effects in the breast to correlate with the genomic studies.
The favorable characteristics of the patients in this series, in terms of their age and tumor characteristics likely explain the excellent outcome. Similar to the patients in the CALGB/ECOG/RTOG Elderly Trial, local recurrence after radiotherapy and tamoxifen was 1%. This trial prospectively randomized patients older than 70 to tamoxifen alone versus tamoxifen and radiotherapy. In this study omission of radiotherapy, that consisted of 30–32 fractions in that study, resulted in a relatively low but still significantly higher local recurrence rate (4%). (17) Moreover, modern breast cancer management is based on molecular as well as stage-related characteristics of the primary tumor. (18) For instance, the risk of local and regional relapse associated with each breast cancer molecular subtype was determined in a study of 2,985 patients with early invasive breast cancer. (19) Previous work demonstrated that immuno-histochemical analysis of a limited panel of six over-expressed protein biomarkers can adequately classify breast cancer into the major subtypes, and predict recurrence similar to genetic signatures: these markers are ER/PR receptors, Ki-67, HER2, EGFR, and cytokeratin 5/6. Based on their pattern of expression, patients were classified into the following categories: luminal A, luminal B, luminal-HER2, HER2 enriched, basal-like, or triple-negative phenotype–non basal. Multivariable analysis was used to determine the risk of local or regional relapse associated with the intrinsic subtypes, after adjusting for standard clinico-pathologic factors. At a median follow-up time of 10 years, carriers of Luminal A tumors (ER or PR positive, HER2 negative, Ki-67 <14%) treated by breast conservation and radiation had the best prognosis and the lowest rate of local and regional relapse, 8% and 3%, respectively (19).
Some investigators have interpreted the results of this study and those from the Elderly Trial as sufficient evidence to justify avoidance of adjuvant radiotherapy in carriers of luminal A tumors, particularly if elderly. We believe a more cautious approach could consist of offering less treatment, like the five- fractions schedule of PBI utilized in this trial. While Ki67 proliferation was available only in 78% of the patients in this trial, most of them were likely to carry luminal A tumors. In this cohort, with the exception of two patients, the remaining 98 demonstrated optimal compliance to the prescribed radiotherapy regimen and tolerated well a prone set-up. Similarly, the majority of the patients reported to be very satisfied with the cosmetic result obtained by the prone PBI approach.
In conclusion, in view of the recent evidence of the general benefit patients derive from breast radiotherapy (20), the five-fractions course tested in this study is a valid option for women at low risk of recurrence, particularly in post-menopausal women whose tumors satisfy the immuno-histochemical criteria for being carriers of luminal A cancers.
Summary.
A prospective trial of prone PBI delivered in five 6 Gy daily fractions of 3D conformal radiotherapy to the cavity with 2 cm margins is reported. Postmenopausal women with T1 breast cancer, HR positive, were eligible. The 5-year results demonstrate a 1% local recurrence rate and good-to-excellent cosmetic results in 89%, confirming the successful selection of patients and dose/fractionation for PBI.
Acknowledgments
Presented at ASTRO 52nd Annual Meeting, held in San Diego, CA, November 1-5, 2010 Supported by Department of Defense Grant DAMD17-01-1- 0345, Silvia C. Formenti, P.I.; NCI Cancer Center Support Grant P30 CA16087, JDG.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Offersen BV, Overgaard M, Kroman N, et al. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiotherapy and Oncology. 2009;90:1–13. doi: 10.1016/j.radonc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Onc Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Ott OJ, Hildebrandt G, Potter R, et al. Accelerated partial breast irradiation with multi-catheter brachytherapy: Local control, side effects and cosmetic outcome for 274 patients. Results of the German-Austrian multi-centre trial. Radiotherapy and Oncology. 2007;82:281–286. doi: 10.1016/j.radonc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Polgar C, Major T, Fodor J, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiotherapy and Oncology. 2010;94:274–279. doi: 10.1016/j.radonc.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Vicini F, Beitsch P, Quiet C, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808–817. doi: 10.1016/j.ijrobp.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 6.Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/ II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120–1127. doi: 10.1016/j.ijrobp.2009.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous, et al. T1 stage breast cancer: adjuvant hypofractionated conformal radiation therapy to tumor bed in selected postmenopausal breast cancer patients--pilot feasibility study. Radiology. 2002;222:171–178. doi: 10.1148/radiol.2221010769. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous, et al. Biologic comparison of partial breast irradiation protocols. Int J Radiat Oncol Biol Phys. 2004;60:1393–1404. doi: 10.1016/j.ijrobp.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous, et al. Prone accelerated partial breast irradiation after breast-conserving surgery: preliminary clinical results and dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;60:493–504. doi: 10.1016/j.ijrobp.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Anonymous, et al. Anon U prone accelerated partial breast irradiation: Compliance to the dosimetry requirements of RTOG-0413. Int J Radiat Oncol Biol Phys. 2010;78:S256. doi: 10.1016/j.ijrobp.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 12.Hoeller U, Tribius S, Kuhlmey A, et al. Increasing the rate of late toxicity by changing the score? A comparison of RTOG/EORTC and LENT/SOMA scores. Int J Radiat Oncol Biol Phys. 2003;55:1013–1018. doi: 10.1016/s0360-3016(02)04202-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen PY, Wallace M, Mitchell C, et al. Four-year efficacy, cosmesis, and toxicity using three-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:991–997. doi: 10.1016/j.ijrobp.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Anonymous, et al. Prospective study of cone-beam computed tomography image-guided radiotherapy for prone accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;81:568–574. doi: 10.1016/j.ijrobp.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Powell S. Radiotherapy for breast cancer in the 21st Century. The Breast Journal. 2010;16 (Suppl 1):S34–38. doi: 10.1111/j.1524-4741.2010.01001.x. [DOI] [PubMed] [Google Scholar]
- 16.Nuutinen J, Lahtinen T, Turunen M, et al. A dielectric method for measuring early and late reactions in irradiated human skin. Radiotherapy and Oncology. 1998;47:249–254. doi: 10.1016/s0167-8140(97)00234-x. [DOI] [PubMed] [Google Scholar]
- 17.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. NEJM. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 18.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. Journal of Clinical Oncology. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 20.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]