Abstract
The Child Behavior Checklist Dysregulation Profile (DP) in youth has been shown to be a predictor of psychopathology later in life. We examined the activity of the Hypothalamic Pituitary Adrenal (HPA) axis in youth with remitted, new, persistent, and no DP. Data from 489 youth (47% boys) participating in a Dutch longitudinal general population study were included (Wave 1 mean age=11.5, Wave 2=14.2). Wave 2 diurnal cortisol patterns and levels in response to a laboratory stress paradigm were compared in youth with DP at Wave 1 only, Wave 2 only, both Waves, and neither Wave. Youth with the DP at Wave 2 only or at both time points showed blunted cortisol responses to stress relative to the other two groups. There were no group or sex differences in diurnal cortisol activity. More research is needed to determine how the association between DP symptoms and HPA axis functioning changes over time.
Keywords: Dysregulation profile, hypothalamic pituitary adrenal axis, cortisol, stress
Introduction
With increasing emphasis on the need for prevention of psychiatric disorders (Beardslee, Chien, & Bell, 2011; O’Connell, Boat, & Warner, 2009), there has been a call for research identifying risk in youth in order to prevent debilitating psychopathology later in life. The Dysregulation Profile (DP), assessed using a widely used parent-report questionnaire measure called the Child Behavior Checklist (CBCL) (Achenbach & Rescorla, 2001), has been shown to be a particularly strong predictor of poor mental health outcomes later in life (Althoff, Verhulst, Rettew, Hudziak, & van der Ende, 2010; Biederman et al., 2009; Holtmann et al., 2011; Meyer et al., 2009). The DP, which consists of clinically elevated scores on the Aggressive Behavior, Anxious-Depressed, and Attention Problems scales of the CBCL, was originally identified by Biederman and colleagues (Biederman et al., 1995) as a profile common among children with bipolar disorder. As research on the DP has advanced, however, it has become clear that the profile is predictive of not only bipolar disorder, but also many other serious and debilitating emotional and behavioral problems, such as substance use, major depression, personality disorders, and suicidality (Althoff, et al., 2010; Biederman, et al., 2009; Holtmann, et al., 2011; Meyer, et al., 2009). Recently, Althoff and colleagues (Althoff, et al., 2010) showed that the DP (measured in childhood) predicted anxiety disorders, mood disorders, substance use disorders, disruptive behavior disorders, and major depressive disorder in adulthood, 14 years later.
Based on the literature, the DP appears to be best described as a profile reflective of problems in regulating behavior, attention, and emotion (hence the name Dysregulation Profile). Therefore, children with this profile are likely to have difficulties in multiple areas of functioning and may be particularly at risk for co-occurring disorders later in life. Because of its clear clinical relevance and the ease with which it can be measured, the DP may be an excellent identifier of populations in need of preventive interventions to ameliorate existing psychopathology, and to prevent even more severe problems in the long-term.
To fully understand the etiology of the DP, and to ultimately design effective treatment and prevention protocols, it is necessary to study the biological processes that may differentiate youth with the DP from youth with other psychopathology and from healthy youth. However, the biological correlates of the DP have not yet been sufficiently examined. Holtmann, Zepf, and colleagues (Holtmann, Duketis, Goth, Poustka, & Boelte, 2010; Zepf, Wockel, Poustka, & Holtmann, 2008) found elevated thyroid stimulating hormone (TSH) levels and evidence of dysfunction in the serotonin system among youth with the DP. However, a more recent study failed to find any association between indicators of thyroid function and the DP in youth (Zepf et al., 2011). These studies have led the way in attempting to understand the function or dysfunction of biological systems related to the regulation of mood and stress among youth with the DP. However, more research is clearly needed to identify biomarkers and examine the etiology of this important identifier of severe psychopathology and long-term impairment.
One of the most widely studied biological systems in the development of psychiatric disorders is the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis regulates the response and adaptation to changes, including stressors, in the environment. When exposed to stress, the central nervous system is activated, and corticotropin releasing hormone (CRH), adrenal corticotrophic hormone (ACTH), and cortisol are released in the brain. The increased cortisol levels elicit the inhibition of the HPA axis, and once the stressor is gone, cortisol levels return to their baseline levels (Jacobson & Sapolsky, 1991).
Variations in HPA axis activity, usually assessed using repeated measurements of salivary cortisol during a normal day or during a laboratory stress task, have been found in clinical and healthy populations (Chida & Hamer, 2008; Chida & Steptoe, 2009). Individuals with psychopathology such as depression, anxiety, aggression, substance use, and other emotional and behavioral problems often display maladaptive cortisol responses, such as cortisol levels that do not sufficiently increase in response to a stressor, or that do not recover after the removal of a stressor (Burke, Davis, Otte, & Mohr, 2005; Chida & Hamer, 2008; Greaves-Lord et al., 2007; Greaves-Lord et al., 2009). HPA-axis responsivity to stress is influenced by many different factors, including the severity of psychopathology (Burke, et al., 2005; Chida & Hamer, 2008; Kudielka, Hellhammer, & Wust, 2009). For example, in healthy subjects, there is evidence that individuals with emotion regulation deficits show increased cortisol levels in response to a stressor compared to those without such deficits (Lam, Dickerson, Zoccola, & Zaldivar, 2009; Quirin, Kuhl, & Dusing, 2011). In contrast, patients with more severe impairment of emotion regulation abilities (e.g., those with bipolar or borderline personality disorder) show blunted HPA-axis responding to stress (Nater et al., 2010; Steen et al., 2011). In other words, cortisol levels do not adequately increase following a stressor for those with severely impaired emotion regulation. This blunted cortisol response indicates a maladaptive response to stress, which can contribute to more severe levels of psychopathology over time (Burke, et al., 2005). Given that the DP appears to predict severe adult psychopathology, we were interested in determining whether youth with the DP would also display alterations in HPA-axis functioning, such as blunted cortisol responses to stress.
Although HPA-axis responses have not yet been examined in youth with the DP, we turn to existing research on some of the DP sub-components for hypothesis formation. Research on the association between some of the sub-components of the DP and HPA-axis responding have been inconsistent. For example, studies have found positive, negative, and no association between HPA-axis responses in samples of youth with aggressive behavior (Cappadocia, Desrocher, Pepler, & Schroeder, 2009) and attention problems (Hastings, Fortier, Utendale, Simard, & Robaey, 2009; Ma, Chen, Chen, Liu, & Wang, 2011; Stadler et al., 2011; van West, Claes, & Deboutte, 2009). Some have hypothesized that a reason for this inconsistency could be the frequent lack of measurement of common co-occurring problems such as anxiety, depression, and emotional dysregulation within these samples (Cappadocia, et al., 2009; Hastings, et al., 2009; Stadler, et al., 2011) or to the vulnerability of salivary cortisol measurements to be influenced by extraneous factors (Hellhammer, Wust, & Kudielka, 2009). Males and females also show differential cortisol levels even in the absence of psychopathology, with males typically displaying more pronounced responses to stress but a lower cortisol awakening response compared to females (Kudielka, et al., 2009; Marsman et al., 2008). Thus, sex differences are critical to consider when examining HPA-axis functioning in youth.
Because the DP is a precursor to severe, long-lasting psychopathology, it is likely that stable high levels of these symptoms are associated with HPA axis dysfunction, especially in response to stress. In particular, it is important to know how different levels of severity and stability of the DP relate to HPA axis activity. Furthermore, despite clear sex differences in HPA axis functioning and in the development of psychopathology (Kudielka, et al., 2009; Marsman, et al., 2008; Nolen-Hoeksema & Girgus, 1994; Zahn-Waxler, Shirtcliff, & Marceau, 2008), the role of sex in the relation between psychopathology and HPA axis functioning has been relatively understudied (Zahn-Waxler, et al., 2008).
In this investigation, we aimed to determine (1) whether differences in DP severity and stability were related to differences in HPA axis activity, both diurnally and in response to stress, and (2) whether these effects differed between boys and girls. Because the DP has been indicative of severe and pervasive psychopathology, we hypothesized that youth with stable high levels of DP symptoms rather than unstable DP symptoms would display blunted cortisol both diurnally and in response to stress. Further, we hypothesized that this effect would be moderated by the sex of subjects (Steen, et al., 2011). We expected that the cortisol response would be more blunted for girls with the DP as compared to boys, consistent with previous findings on sex differences (Kudielka, et al., 2009).
Method
Participants
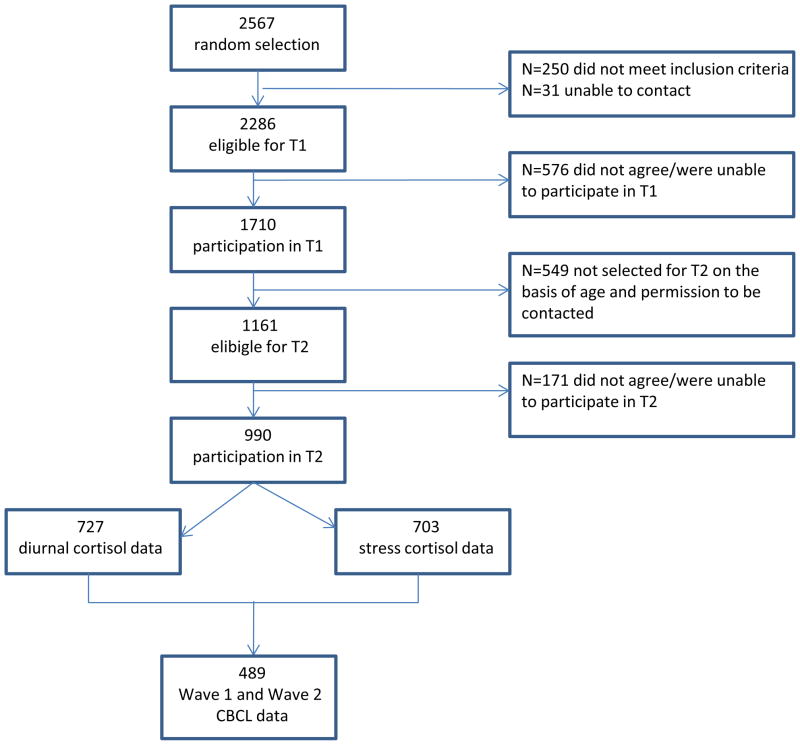
The sample for this study is part of a larger sample that participated in a longitudinal general population study (Tick, van der Ende, & Verhulst, 2007). For this larger study, 2567 children and adolescents were randomly drawn from municipal registers of 35 representative municipalities, including urban and rural areas, in the Dutch province of South Holland. At the first measurement (Wave 1), between December 2003 and April 2005, 1710 of the 2286 eligible families participated. For details on the initial data collection, see (Tick, et al., 2007; Tick, van der Ende, & Verhulst, 2008). Approximately three years later, between January 2006 and March 2009, 1161 individuals were invited to participate in Wave 2 of the study if (1) they were born between January 1st 1988 and August 31st 1997, (2) they participated at Wave 1, and (3) they gave permission to be contacted for follow-up research. One hundred seventy one individuals refused or were unable to participate, resulting in 990 children and adolescents between 8 and 20 years old who participated at Wave 2. Of these 990 children and adolescents, 489 had complete Wave 1 and Wave 2 CBCL data, participated in Wave 2 cortisol assessment procedures, and were thus included in the current study. Figure 1 describes participation and attrition for the current study. These participants did not differ from those without Wave 2 cortisol data on sex (χ2(1)=1.86, ns), but those without Wave 2 cortisol data were slightly older at Wave 1 (mean age=12.4) compared to those with Wave 2 cortisol data (mean age=11.4; F(1, 1705)=33.19, p<.001, η2=.02). Subjects who participated in the assessment of cortisol response to stress had mothers (F(1, 1681)=20.00, p<.001, η2=.01) and fathers (F(1, 1562)=6.86, p<.01, η2=.004) with higher-level jobs than those who did not participate. Written informed consent was obtained from all participating youth and their parents. The Medical Ethics Committee of the Erasmus Medical Center approved the study. For the current study, the mean age of participants at Wave 1 was 11.5 years (SD=2.7, range=6–16), and mean age at Wave 2 was 14.2 years (SD=3.6, range=8–20). Forty-seven percent of the sample was male.
Figure 1.
Flow Diagram Showing Participation and Attrition
Measures and Procedure
Diurnal and Stress-related Cortisol Levels at Wave 2
At Wave 2, salivary cortisol samples were taken at ten time points in total by passively drooling into a test tube, a reliable and stress-free approach(Kirschbaum & Hellhammer, 1994).
Diurnal Cortisol Levels
Four tubes for assessment of salivary cortisol levels on a normal day were sent to the participants by mail prior to the stress procedure. Detailed written and verbal instructions were given on the time (a normal day) and manner of sample collections, and to preserve the tubes in the freezer until the testing day. Instructions included asking participants to refrain from consuming caffeine or chocolate on the day of, ingesting dairy products one hour prior, and eating 30 minutes prior to saliva collection. Participants were instructed to provide the first sample directly upon awakening, the second 30 minutes afterwards, the third at 12 p.m. and the fourth at 8 p.m.
Stress Reactivity Cortisol Levels
Stress procedure sessions took place approximately one day after saliva collection at home. The procedure began at approximately 12 p.m. or at 3 p.m. in order to minimize differences in cortisol levels due to the diurnal curve. Stress procedure sessions commenced with an explanation of the procedure by the experiment leader. After the completion of questionnaires and a ten-minute baseline period, the social stress tasks began, which were designed to elicit a stress reaction (see Dieleman, van der Ende, Verhulst, & Huizink, 2010 and Evans, Greaves-Lord, Euser, Franken, & Huizink, 2012 for full details on the procedure). These entailed a mental arithmetic task, a public speaking task, and a computer mathematics task. The task order was the same for all participants. The session ended with a five-minute recovery period and a relaxing nature documentary (25 minutes). After each period/task, during the middle of the movie and at the end of it, the participant was asked to provide saliva samples (see Figure 2; Cort5–10). These samples represent cortisol levels about 20 minutes earlier due to the delay in observable cortisol response (Sapolsky, Romero, & Munck, 2000).
Figure 2.
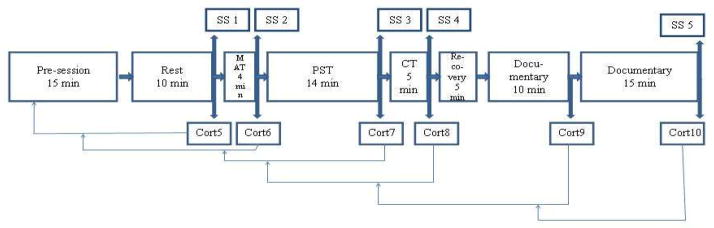
Schematic Depiction of the Stress Procedure (from (Evans, et al., 2012))
Note: SS=Subjective stress measurement; MAT=Mental arithmetic task; PST=Public speaking task; CT=Computer task; Cort5-Cort10=Cortisol tubes 5 through 10. Arrows extending from Cort5–10 point to moments during the procedure to which cortisol levels correspond, due to the delay in observable cortisol increase after the onset of the stressor (Sapolsky et al., 2000).
Saliva samples were kept in a freezer at −20 degrees Celsius (Aardal & Holm, 1995). The samples were collectively sent to either the Kirschbaum Laboratory in Dresden, Germany (371 participants’ cortisol samples) or to a laboratory at Erasmus Medical Center in Rotterdam, the Netherlands for analysis. Cortisol levels did not significantly differ between laboratories, after controlling for age. A time-resolved fluorescence immunoassay was implemented to determine the cortisol concentration in the saliva samples (details available upon request).
Subjective Stress During Baseline and Tasks
Self-reported subjective stress (SS) was assessed after the rest period and after each of the tasks, prior to collection of Cort 5–8 and Cort 10 (see Figure 2). Participants used a visual thermometer (0=not at all to 8=very much) to answer seven questions (see Figure 3). For each period/task, a total score was computed by summing the scores on these seven questions. Task SS was the average of the total scores from each of the stress tasks.
Figure 3.

Subjective Stress Questions
Dysregulation Profile at Waves 1 and 2
To assess the Dysregulation Profile at both assessment waves, the Child Behavior Checklist (CBCL) (Achenbach & Rescorla, 2001) was used. The CBCL is a widely used quantitative and empirically based measure of psychopathology in children and adolescents. The Dutch version of the CBCL has well-established reliability and validity (de Groot, Koot, & Verhulst, 1996; Tick, et al., 2007, 2008). This questionnaire contains 120 problem items on which parents rate their child’s behavior in the preceding 6 months on a three-point scale, where 0=not true, 1=somewhat or sometimes true, and 2=very true or often true. The Dysregulation Profile (DP) is defined by clinically significant levels on three CBCL subscales: Anxious/Depressed, Attention Problems, and Aggressive Behavior. As in previous studies using non-clinical population-based samples in the Netherlands (Cents et al., 2011; Velders et al., 2011) and in the United States (Spencer et al., 2011), we used the 80th percentile as our cutoff. Thus, participants were determined to have the DP if their scores were in the 80th percentile or higher on all three component scales of the DP (Anxious/Depressed, Attention Problems, and Aggressive Behavior). A 4-level categorical DP variable was then computed based on the presence or absence of DP at each wave: 0=no DP; 1=DP at Wave 1 only; 2=DP at Wave 2 only; 3=DP at both Waves (Persistent DP).
Potential Covariates
In previous studies examining cortisol levels, age (Gunnar, Talge, & Herrera, 2009), body mass index (BMI) (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999), pubertal status (Hankin, Badanes, Abela, & Watamura, 2010), socioeconomic status (SES) (Miller et al., 2009), and physical and sexual abuse (Cicchetti, Rogosch, Gunnar, & Toth, 2010) have been previously used as covariates. In the current study, SES variables were the Wave 1 occupational levels of both mother and father, coded into no work, elementary, lower, middle, higher, and academic. Because there were few fathers in the no work category (N=8), it was combined with the “elementary” category for father job level. Age at cortisol assessment (Wave 2) was used as a potential covariate. Wave 2 height and weight were measured prior to the test session and used to compute BMI. Pubertal status was measured using self-reported Tanner stages (Marshall & Tanner, 1970). Physical and sexual abuse was assessed within retrospective parent report and self-report life events questionnaires collected at Wave 2. If either informant reported that the child had ever experienced physical or sexual abuse, abuse was coded as 1. If neither informant reported abuse, the abuse variable was coded 0. Contraceptive use may also influence HPA axis functioning. However, a recent study using the current sample found no association between contraceptive use and HPA axis functioning in females (Evans, et al., 2012), therefore we did not include this variable as a covariate in the current examinations.
Statistical Analyses
Data preparation
The four-level DP variable was dummy coded prior to entry into regression analyses. Specifically, three dummy-coded variables were computed, where the “no DP at either wave” group was the reference category.
In order to determine an index of diurnal cortisol levels, the area under the curve with respect to ground (AUCg diurnal) was calculated according to Preussner et al. (2003) (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). AUCg indexes the total amount of cortisol secreted during a period of time, as it indicates the total area between zero and the curve that is constituted by the levels of cortisol at each measurement period. The four cortisol levels assessed during a normal day were used to calculate AUCg diurnal. AUCg diurnal was square-root transformed to approximate a normal distribution.
An AUC with respect to increase (AUCi) is the most preferred indicator of the cortisol response to stress (Pruessner, et al., 2003). However, our sample showed a general lack of increase in cortisol levels in response to the stressful tasks, and cortisol levels at the onset of the procedure were already elevated compared to all of the proceeding cortisol measurements. Therefore, AUCi was less preferable in this case and we instead divided the stress procedure into three parts and tested the linear association between cortisol and DP group at each part (Evans, et al., 2012). This provided an estimation of cortisol levels at the beginning (CortAtOnset; average of Cort5–6), during the stressful tasks (CortDuringTasks; average of Cort7–9), and after the tasks (CortRecovery; Cort10) by DP group. CortAtOnset, CortDuringTasks, and CortRecovery were log-transformed to approximate a normal distribution.
Analytic Plan
Descriptive analyses were conducted in SPSS version 19.0. Analyses of Variance (ANOVAs) and correlations were conducted to explore the relations of age, mother and father job level, BMI, and abuse with AUCg diurnal, CortAtOnset, CortDuringTasks, and CortRecovery. The difference between Task and Baseline SS was computed to check that the stress tasks induced stress.
Multiple regression analyses were conducted in Mplus version 6.12 (Muthen & Muthen, 1998–2010). Mplus handles missing cases under the Missing at Random (MAR) assumption using Full-Information Maximum Likelihood (FIML). Multivariate regressions were run in Mplus to determine whether DP and its interaction with sex were related to HPA axis activity at Wave 2. AUCg diurnal was entered as the dependent variable in the first analyses. CortAtOnset, CortDuringTasks, and CortRecovery were the dependent variables in the next regression analyses. These regression analyses tested DP (three dummy-coded variables) and sex as independent variables without the interaction term. Then, another model was run for each dependent variable to determine whether the interaction between sex and DP was significantly related to diurnal cortisol levels (AUCg diurnal) and cortisol responses to stress (CortAtOnset, CortDuringTasks, CortRecovery). For each model, covariates were included if they were significantly related to the dependent variable. Because we had specific, directional predictions, and all DP dummy variables were entered into a single regression model, alpha level adjustment was not necessary. With a sample of 489 and alpha=.05, the power to detect a small interaction effect (d=.2) after controlling for two independent variables is .88. Power to detect a medium or large effect would be 1.00. Therefore, there should be sufficient power to identify interactions in the current study.
Results
Descriptive Results
Forty-seven percent of the sample was boys (n=230). Most (88%) of the sample did not have the DP at either time point (n=430). Approximately 4% of participants had the DP at Wave 1 only (n=20), 5% had the DP at Wave 2 only (n=23), and 3% had the DP at both waves (n=16). This group was named the “Persistent DP” group. The prevalence of persistent DP (3%) was smaller than at Wave 1 (5%) and Wave 2 (4%). All three of these percentages, however, fall within the range of previously reported rates of DP in the general population and twin samples (Althoff, Rettew, Faraone, Boomsma, & Hudziak, 2006; Althoff, et al., 2010). Mean DP score was significantly higher at Wave 1 (Mean=11.85, SD=8.54) compared to Wave 2 (Mean=10.26, SD=8.10; t(480)=30.43, p<.001)). Mean Wave 1 and Wave 2 DP scores and cortisol levels by DP group are shown in Table 1.
Table 1.
Means and standard deviations of Dysregulation Profile (DP) raw scores and cortisol variables by DP group
| W1 DP raw score | W2 DP raw score | W2 AUCg Diurnal | W2 CortAt Onset | W2 Cort During Tasks | W2 Cort Recovery | |
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| No DP | 10.0 (6.7) | 8.3 (6.1) | 89.6 (15.2) | 2.0 (0.5) | 2.0 (0.5) | 1.8 (0.5) |
| W1 DP only | 30.2 (6.5) | 17.5 (7.6) | 91.9 (13.0) | 1.9 (0.8) | 1.5 (0.6) | 1.4 (0.6) |
| W2 DP only | 18.1 (4.7) | 26.9 (6.0) | 85.0 (13.0) | 1.7 (0.8) | 1.7 (0.5) | 1.7 (0.5) |
| Persistent DP | 30.5 (4.5) | 31.5 (8.4) | 86.6 (15.8) | 1.5 (0.8) | 1.5 (0.9) | 1.2 (0.9) |
Note: AUCgDiurnal has been square root transformed; CortAtOnset, CortDuringTasks, and CortRecovery were natural log transformed. DP: Dysregulation Profile; W1: Wave 1; W2: Wave 2; M: Mean; SD: Standard Deviation.
The DP groups did not significantly differ in age (F(3, 320)=.39, ns). The mean ages were: No DP=15.19 (SD=3.50), Wave 1 DP Only=15.63 (SD=3.22), Wave 2 DP Only=14.84 (SD=3.64), Persistent DP=14.23 (SD=3.52). The DP groups also did not significantly differ regarding sex (χ2(3)=1.21, ns). The percentage boys within each group were: No DP=47%, Wave 1 DP Only=60%, Wave 2 DP Only=42%, Persistent DP=47%.
When examining potential covariates, abuse, mother job level, father job level, and pubertal status were not significantly related to AUCg diurnal, CortAtOnset, CortDuringTasks, or CortRecovery (see Table 2). Correlations between continuous covariates and predictors with AUCg diurnal, CortAtOnset, CortDuringTasks, and CortRecovery are shown in Table 3. Age was significantly related to CortAtOnset and BMI was significantly related to CortRecovery. Older youth had higher cortisol levels at the onset of the stress task, and those with higher BMIs had lower cortisol levels at recovery. Neither age nor BMI were associated with CortDuringTasks or to AUCg diurnal. Task SS significantly increased from Baseline SS (β=0.83, p<.001), suggesting that the tasks did increase subjective stress levels as intended. Mean subjective stress levels at each of the five assessments (see Figure 2) were: SS1 Mean=7.39, SD=6.01; SS2 Mean=10.21, SD=7.88; SS3 Mean=12.27, SD=9.17; SS4 Mean=8.14, SD=7.50; SS5 Mean=4.90, SD=6.27. Regression analyses revealed no differences in Task SS by DP group.
Table 2.
Frequencies of categorical covariates, and associations with diurnal cortisol and cortisol response to stress at Wave 2.
| Covariate | Categories | Frequencies | AUCg Diurnal* | CortAtOnset | CortDuringTasks | CortRecovery |
|---|---|---|---|---|---|---|
| Abuse (assessed at Wave 2) | Not abused | 71% | F(1, 254)=0.01, partial η2=.000 | F(1, 282)=1.43, partial η2=.005 | F(1, 282)=0.83, partial η2=.003 | F(1, 282)=1.86, partial η2=.007 |
| Abused | 29% | |||||
| Mother job level | no work | 11% | F(5, 422)=0.52, partial η2=.006 | F(5, 480)=0.91, partial η2=.009 | F(5, 480)=0.76, partial η2=.008 | F(5, 480)=0.33, partial η2=.003 |
| elementary | 4% | |||||
| lower level | 21% | |||||
| mid level | 37% | |||||
| higher level | 21% | |||||
| academic | 7% | |||||
| Father job level | no work/elementary | 5% | F(4, 401)=0.92, partial η2=.009 | F(4, 456)=0.52, partial η2=.005 | F(4, 456)=0.98, partial η2=.008 | F(4, 456)=0.43, partial η2=.004 |
| lower level | 19% | |||||
| mid level | 36% | |||||
| higher level | 27% | |||||
| academic | 13% | |||||
| Wave 2 Pubertal status (Tanner stage)a | II | 2% | F(3, 207)=2.17, partial η2=.030 | F(3, 241)=0.28, partial η2=.003 | F(3, 241)=0.36, partial η2=.004 | F(3, 241)=0.42, partial η2=.005 |
| III | 13% | |||||
| IV | 46% | |||||
| V | 39% |
Note. AUCg=Area under the curve with respect to ground;
Results of one-way analyses of variance (ANOVAs), where degrees of freedom are in parentheses; None of the ANOVAs were statistically significant at the p<.05 level (two-tailed);
There were zero participants in Tanner stage I.
Table 3.
Bivariate correlations between continuous variables, means, and standard deviations (N=489)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean (SD) | Range | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. W2 Age | 1 | 15.16 (3.49) | 8.00–20.84 | |||||||
| 2. W2 BMI | .46** | 1 | 20.10 (3.58) | 13.55–36.75 | ||||||
| 3. W2 AUCg diurnal | .03 | .09 | 1 | 7672.82 (2909.55) | 889.20 – 24027.60 | |||||
| 4. W2 CortAtOnset | .13* | .01 | .18** | 1 | 8.74 (5.77) | 1.71–52.50 | ||||
| 5. W2 CortDuringTasks | .03 | −.06 | .17** | .82** | 1 | 8.40 (5.36) | 1.12–46.82 | |||
| 6. W2 CortRecovery | −.09 | .20** | −.13** | .67** | .86** | 1 | 6.91 (3.96) | 1.05–31.31 | ||
| 7. W1 DP score | −.12* | −.01 | −.05 | −.11* | −.12* | −.11* | 1 | 11.84 (8.51) | 0.00–47.00 | |
| 8. W2 DP score | −.17** | −.05 | −.04 | −.12* | −.12* | −.08 | .69** | 1 | 10.34 (8.33) | 0.00–51.00 |
Note. W1: Wave 1; W2: Wave 2; BMI: Body Mass Index; AUCg: Area Under the Curve with respect to ground; DP: Dysregulation profile; SD: Standard Deviation;
Significant at the 0.05 level (2-tailed);
Significant at the 0.01 level (2-tailed).
Diurnal cortisol responding
No covariates were included in these models as none were significantly associated with AUCg diurnal in the descriptive analyses. In the first model testing the main effects of sex and DP in predicting AUCg diurnal at Wave 2, sex significantly predicted Wave 2 AUCg diurnal, but DP did not (see Table 4). Girls generally had higher AUCg diurnal than boys. The interaction between sex and DP was not significantly associated with Wave 2 AUCg diurnal.
Table 4.
Regression analysis of CBCL Dysregulation Profile (DP) predicting diurnal cortisol at Wave 2
| Dependent Variable: AUCg diurnal at Wave 2 | |||
|---|---|---|---|
| Independent Variable | b (SE) | β | p |
| W1 DP Onlya | −1.85 (3.99) | −.02 | ns |
| W2 DP Onlya | −3.74 (3.56) | −.05 | ns |
| Persistent DPa | −3.66 (3.99) | −.04 | ns |
| Sexb | 6.61 (1.47) | .21 | <.001 |
Note.
No DP is the reference category;
Male is the reference category; CBCL: Child Behavior Checklist; DP: Dysregulation Profile; AUCg: Area under the curve with respect to ground; b: unstandardized regression coefficient; SE: standard error; β: standardized regression coefficient(beta); ns: not significant at the p<.05 level.
Cortisol responses to stress
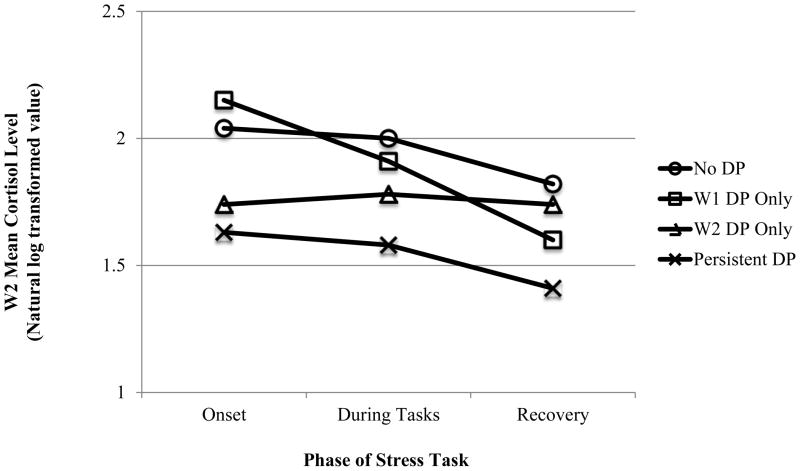
Despite significant associations in the descriptive analyses, neither age nor BMI was significantly related to CortAtOnset, CortDuringTasks, or CortRecovery in the regression analyses. Therefore, these covariates were dropped from the models. In addition, neither the sex main effect nor the sex*DP interaction were significantly associated with the cortisol variables and were therefore removed from the regression as well. Subjects with persistent DP had significantly more blunted HPA-axis responses at every stage of the stress task (at onset, during the tasks, and at recovery) compared to those without the DP at either time point (see Table 5 and Figure 4). Those with the DP at Wave 2 only also had blunted cortisol responding relative to the no-DP group at onset and during the tasks, but not at recovery. Subjects with the DP at Wave 1 only did not display alterations in cortisol levels in response to stress that differed significantly from those without the DP. Table 5 and Figure 4 here
Table 5.
Multivariate regression analysis of CBCL Dysregulation Profile (DP) predicting cortisol response to stress at Wave 2.
| Dependent Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CortAtOnset | CortDuringTasks | CortRecovery | |||||||
| Independent Variable | b (SE) | β | p | b (SE) | β | p | b (SE) | β | p |
| W1 DP Onlya | 0.16 (0.12) | 0.06 | ns | −0.03 (0.12) | −0.01 | ns | −0.09 (0.13) | −0.03 | ns |
| W2 DP Onlya | −0.24 (0.11) | −0.09 | <.05 | −0.26 (0.12) | −0.10 | <.05 | −0.14 (0.12) | −0.05 | ns |
| Persistent DPa | −0.39 (0.14) | −0.13 | <.01 | −0.38 (0.14) | −0.12 | <.01 | −0.31 (0.14) | −0.10 | <.05 |
Note.
No DP is the reference category; CBCL: Child Behavior Checklist; DP: Dysregulation Profile; SE: standard error; β: standardized regression coefficient (beta); ns: not significant at the p<.05 level.
Figure 4.
Dysregulation Profile (DP) predicts Wave 2 cortisol response to stress
Note. W1: Wave 1; W2: Wave 2; DP: Dysregulation Profile
Discussion
To enhance insight into the biology of the Dysregulation Profile (DP) in youth, this study sought to determine whether a stable DP over time was related to blunted HPA axis activity both diurnally and in response to a laboratory stress test. Further, we sought to determine whether the association between DP and HPA axis activity differed between boys and girls.
Our hypothesis that individuals with persistent DP would display blunted HPA axis responses to stress relative to those without the DP was supported. Subjects with the DP at Wave 2 only also showed blunted cortisol reactivity in response to stress, but those with the DP at Wave 1 only did not differ significantly from those without the DP at all in terms of HPA axis reactivity. This is interesting given that overall, youth had higher average DP scores at Wave 1 compared to Wave 2. DP-related differences in HPA axis activity in response to stress may remit as DP remits. It is possible that those with persistent DP have inherently low cortisol levels or blunted HPA axis responses to stress, indicating dysfunction in their stress regulation system which makes these individuals have trouble regulating attention, emotions and behaviors, and also more vulnerable for developing the DP. Alternatively, the observed differences in cortisol levels could be state-dependent and disappear as symptoms remit. Unfortunately, this study is not sufficient to determine whether DP predicts HPA axis response to stress, or vice versa. In other words, because we did not measure cortisol reactivity to stress at Wave 1, additional longitudinal research is needed to determine whether and how changes in DP symptoms parallel changes in HPA axis functioning.
Contrary to our hypotheses, we did not find that the DP was related to impaired diurnal HPA axis functioning. Therefore, differences in HPA axis functioning in youth with the DP appear to be specifically related to stress exposure. This is consistent with other findings from this sample suggesting that HPA axis responses to stress may be a better marker of risk compared to diurnal HPA axis activity (Dieleman, et al., 2010; Evans, et al., 2012). Additional research is needed to determine whether diurnal cortisol levels are no different in those with even longer-lasting, more severe DP symptoms. This was a relatively high-functioning, non-clinical sample, so we might not have had the power to detect differences in diurnal cortisol levels that may only occur in more severely impaired populations.
Consistent with previous literature (Kudielka, et al., 2009; Marsman, et al., 2008), we identified significant sex differences in diurnal cortisol levels. Specifically, girls showed higher cortisol levels diurnally than boys. However, we did not find significant sex differences in the relation between DP and Wave 2 HPA axis functioning. Therefore, the blunted HPA axis responses to stress related to Wave 2 and Persistent DP do not appear to be sex-specific. There is a great deal of literature showing that sex differences in the impact of psychopathology on HPA axis functioning exist (Bouma, Riese, Ormel, Verhulst, & Oldehinkel, 2011; Marsman, et al., 2008; Steen, et al., 2011) though, so our finding that there were no sex differences in cortisol responses to stress is somewhat surprising. To further investigate, it will be useful to examine associations between sex, DP, and cortisol responses to stress within distinct age groups. We included a relatively broad age range of youth in this study, ranging from children to older adolescents. We did not find that age predicted cortisol responses to stress, but were not able to test whether there was a three-way interaction between sex, age, and DP. Our broad age range may have masked age-specific sex differences.
There are many notable clinical and research implications of these findings. First, with increased attention on the identification of endophenotypes (Gottesman & Gould, 2003) for psychiatric disorders, this study may be an important preliminary step in determining endophenotypes for severe and long-lasting difficulties with the regulation of emotion, attention, and behavior, which potentially are manifested as bipolar disorder, borderline personality disorder, substance use disorders, and other highly impairing psychopathology in adulthood (Althoff, et al., 2010; Halperin, Rucklidge, Powers, Miller, & Newcorn, 2011). Prospective studies that test the role of HPA axis functioning as a moderator or mediator of early DP and later forms of severe psychopathology can help to explicate the key etiological processes and developmental pathways involved. Finally, these findings might ultimately help to measure the impact of interventions aiming to reduce problems regulating emotions, attention, and behavior. For example, improvements in HPA axis responses to stress following treatment could be used to corroborate self- or parent-reported symptom reduction.
For this line of study to have maximum impact, however, its limitations must be addressed through future research. A notable weakness of the current study is that HPA axis functioning was not measured at Wave 1. Thus, it was not possible for us to determine whether the same pattern of results would be observed earlier in development, or how HPA axis functioning may have changed over time for youth with the DP at one, both, or no time points, or for boys compared to girls. In addition, this sample was a normative, non-clinical one and thus it is not known whether our results would hold within clinical populations. Because the measurement of cortisol levels required a certain level of motivation and effort (e.g., mailing back saliva samples, collecting them at particular times) youth with more impairing psychopathology may also have been more likely to drop out of the study, although an analysis of the data suggests that this is not the case. If it were the case, however, one would expect research examining the DP in the more severe cases would identify even stronger effects than the ones reported here. Some researchers have suggested that it can be difficult to capture sufficient variability in biological and psychological mechanisms within normative samples because there are typically few non-normative (clinical) cases relative to a large number of normative cases (Beauchaine, 2001). This can make it difficult to detect effects or group differences. Although we were able to detect group differences despite having a non-clinical sample, there is still much to be gleaned from replication within clinical samples. Such studies could reveal interesting and important group differences and similarities that were not detectable in the current study. For example, there could be a difference between youth with the DP at Wave 1 and youth without the DP that we were unable to capture here.
The use of self-obtained saliva samples to compute diurnal cortisol is a limitation as well, since it is difficult to verify whether all subjects followed instructions regarding the timing of collection. Also because this was not a clinical sample, we ended up with relatively small cell sizes for the analysis of main effects of DP and interactions with sex, which may have limited our ability to detect smaller effects. The prevalence of the DP in this sample is consistent with other general population studies and twin studies (Althoff, et al., 2006; Althoff, et al., 2010), and our power calculations suggested that we had sufficient power to detect relatively small effects, but replication in larger samples is still needed. In addition, our sample included a broad age range of youth. We did not have sufficient sample size to determine whether associations between the DP and HPA axis function differed at various developmental stages, and therefore more research is needed to test whether our findings generalize across age groups. Similarly, we do not know whether our findings generalize across clinical subgroups with various types of co-occurring conditions, such as anxiety, depression, and ADHD. More research with larger samples is necessary to explore these subgroups. Collecting these types of data on large samples is time and resource-intensive, however. Future studies should consider oversampling individuals with clinical levels of the psychiatric conditions of interest, or focusing on clinical samples entirely.
Another limitation to our study was our inability to include IQ data in our analyses. It is possible that IQ may interact with risk for DP as well as HPA axis functioning in children with DP. More research will be needed to investigate these relations. Finally, because the participants in this study overall displayed high baseline/anticipatory cortisol levels prior to the stress task (see Figure 4), we did not observe the “typical” initial rise in cortisol levels. This is not unusual, especially in youth samples (Ouellet-Morin et al., 2011), and our subjective stress measure suggested that the stress tasks did elicit a response.
Despite these limitations, the current study contained many strengths which we believe make it informative and useful for future research in this area. First, this is the only study to our knowledge that has examined HPA axis activity in relation to the DP. Second, the sample we used was quite large, and because it was a normative population it may have relatively strong generalizability at the population level. Third, we implemented a prospective design to examine the effects of the DP on later HPA axis functioning. We hope that the current study can spur further investigation into the biological processes associated with the development and persistence of the DP and its role as a key risk factor for severe problems with emotion, attention, and behavior regulation later in life.
Acknowledgments
This project was supported by NIH grants MH082116, and 5RC2MH089995-02, ERAB grant 0609 and ZonMW grant 3116.0002.
Footnotes
Financial Disclosures:
All authors declare to have no conflicts of interest.
References
- Aardal E, Holm AC. Cortisol in saliva - Reference ranges and relation to cortisol in serum. European Journal of Clinical Chemistry and Clinical Biochemistry. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont Center for Children, Youth, and Families; 2001. [Google Scholar]
- Althoff RR, Rettew DC, Faraone SV, Boomsma DI, Hudziak JJ. Latent class analysis shows strong heritability of the child behavior checklist-juvenile bipolar phenotype. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Biological psychiatry. 2006;60(9):903–911. doi: 10.1016/j.biopsych.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Althoff RR, Verhulst FC, Rettew DC, Hudziak JJ, van der Ende J. Adult outcomes of childhood dysregulation: a 14-year follow-up study. [Research Support, N.I.H., Extramural] Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(11):1105–1116. doi: 10.1016/j.jaac.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardslee WR, Chien PL, Bell CC. Prevention of mental disorders, substance abuse, and problem behaviors: a developmental perspective. Psychiatric services. 2011;62(3):247–254. doi: 10.1176/appi.ps.62.3.247. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. [Research Support, U.S. Gov’t, P.H.S. Review] Development and psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Monuteaux MC, Evans M, Parcell T, Faraone SV, Wozniak J. The Child Behavior Checklist-Pediatric Bipolar Disorder profile predicts a subsequent diagnosis of bipolar disorder and associated impairments in ADHD youth growing up: a longitudinal analysis. [Research Support, N.I.H., Extramural] The Journal of clinical psychiatry. 2009;70(5):732–740. doi: 10.4088/JCP.08m04821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Wozniak J, Kiely K, Ablon S, Faraone S, Mick E, Kraus I. CBCL clinical scales discriminate prepubertal children with structured interview-derived diagnosis of mania from those with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(4):464–471. [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Self-assessed parental depressive problems are associated with blunted cortisol responses to a social stress test in daughters. The TRAILS Study. [Research Support, Non-U.S. Gov’t] Psychoneuroendocrinology. 2011;36(6):854–863. doi: 10.1016/j.psyneuen.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. [Comparative Study Meta-Analysis Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S. Review] Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Cappadocia MC, Desrocher M, Pepler D, Schroeder JH. Contextualizing the neurobiology of conduct disorder in an emotion dysregulation framework. [Review] Clinical psychology review. 2009;29(6):506–518. doi: 10.1016/j.cpr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Cents RA, Tiemeier H, Luijk MP, Jaddoe VW, Hofman A, Verhulst FC, Lambregtse-van den Berg MP. Grandparental anxiety and depression predict young children’s internalizing and externalizing problems: the generation R study. [Research Support, Non-U.S. Gov’t] Journal of affective disorders. 2011;128(1–2):95–105. doi: 10.1016/j.jad.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. [Research Support, Non-U.S. Gov’t Review] Psychological bulletin. 2008;134(6):829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. [Research Support, Non-U.S. Gov’t Review] Biological psychology. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Child development. 2010;81(1):252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot A, Koot HM, Verhulst FC. Cross-cultural generalizability of the Youth Self-Report and Teacher’s Report Form cross-informant syndromes. [Multicenter Study Research Support, Non-U.S. Gov’t] Journal of abnormal child psychology. 1996;24(5):651–664. doi: 10.1007/BF01670105. [DOI] [PubMed] [Google Scholar]
- Dieleman GC, van der Ende J, Verhulst FC, Huizink AC. Perceived and physiological arousal during a stress task: can they differentiate between anxiety and depression? [Evaluation Studies] Psychoneuroendocrinology. 2010;35(8):1223–1234. doi: 10.1016/j.psyneuen.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Franken IH, Huizink AC. The relation between hypothalamic-pituitary-adrenal (HPA) axis activity and age of onset of alcohol use. [Research Support, Non-U.S. Gov’t] Addiction. 2012;107(2):312–322. doi: 10.1111/j.1360-0443.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. [Research Support, U.S. Gov’t, P.H.S. Review] The American journal of psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greaves-Lord K, Ferdinand RF, Oldehinkel AJ, Sondeijker FE, Ormel J, Verhulst FC. Higher cortisol awakening response in young adolescents with persistent anxiety problems. [Research Support, Non-U.S. Gov’t] Acta psychiatrica Scandinavica. 2007;116(2):137–144. doi: 10.1111/j.1600-0447.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- Greaves-Lord K, Huizink AC, Oldehinkel AJ, Ormel J, Verhulst FC, Ferdinand RF. Baseline cortisol measures and developmental pathways of anxiety in early adolescence. [Research Support, Non-U.S. Gov’t] Acta psychiatrica Scandinavica. 2009;120(3):178–186. doi: 10.1111/j.1600-0447.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to prduce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Rucklidge JJ, Powers RL, Miller CJ, Newcorn JH. Childhood CBCL bipolar profile and adolescent/young adult personality disorders: a 9-year follow-up. [Research Support, N.I.H., Extramural] Journal of affective disorders. 2011;130(1–2):155–161. doi: 10.1016/j.jad.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: cortisol reactivity to psychosocial stress from preschool through middle adolescence. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Biological psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Fortier I, Utendale WT, Simard LR, Robaey P. Adrenocortical functioning in boys with attention-deficit/hyperactivity disorder: examining subtypes of ADHD and associated comorbid conditions. [Research Support, Non-U.S. Gov’t] Journal of abnormal child psychology. 2009;37(4):565–578. doi: 10.1007/s10802-008-9292-y. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. [Review] Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Buchmann AF, Esser G, Schmidt MH, Banaschewski T, Laucht M. The Child Behavior Checklist-Dysregulation Profile predicts substance use, suicidality, and functional impairment: a longitudinal analysis. [Multicenter Study] Journal of child psychology and psychiatry, and allied disciplines. 2011;52(2):139–147. doi: 10.1111/j.1469-7610.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Duketis E, Goth K, Poustka L, Boelte S. Severe affective and behavioral dysregulation in youth is associated with increased serum TSH. Journal of affective disorders. 2010;121(1–2):184–188. doi: 10.1016/j.jad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. [Research Support, U.S. Gov’t, P.H.S. Review] Endocrine reviews. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. [Review] Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. [Comparative Study Research Support, Non-U.S. Gov’t] Psychosomatic medicine. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. [Research Support, Non-U.S. Gov’t Review] Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. [Research Support, N.I.H., Extramural] Psychoneuroendocrinology. 2009;34(9):1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen YH, Chen H, Liu YY, Wang YX. The function of hypothalamus-pituitary-adrenal axis in children with ADHD. [Comparative Study Research Support, Non-U.S. Gov’t] Brain research. 2011;1368:159–162. doi: 10.1016/j.brainres.2010.10.045. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman R, Swinkels SH, Rosmalen JG, Oldehinkel AJ, Ormel J, Buitelaar JK. HPA-axis activity and externalizing behavior problems in early adolescents from the general population: the role of comorbidity and gender The TRAILS study. [Research Support, Non-U.S. Gov’t] Psychoneuroendocrinology. 2008;33(6):789–798. doi: 10.1016/j.psyneuen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Carlson GA, Youngstrom E, Ronsaville DS, Martinez PE, Gold PW, Radke-Yarrow M. Long-term outcomes of youth who manifested the CBCL-Pediatric Bipolar Disorder phenotype during childhood and/or adolescence. [Research Support, N.I.H., Intramural] Journal of affective disorders. 2009;113(3):227–235. doi: 10.1016/j.jad.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide: Sixth Edition. 5. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- Nater UM, Bohus M, Abbruzzese E, Ditzen B, Gaab J, Kleindienst N, Ehlert U. Increased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorder. [Research Support, Non-U.S. Gov’t] Psychoneuroendocrinology. 2010;35(10):1565–1572. doi: 10.1016/j.psyneuen.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. [Review] Psychological bulletin. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- O’Connell ME, Boat T, Warner KE. Preventing mental, emotional, and behavioral disorders among young people: Progress and possibilities. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Bowes L, Shakoor S, Ambler A, Pariante CM, Arseneault L. A discordant monozygotic twin design shows blunted cortisol reactivity among bullied children. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Twin Study] Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(6):574–582. e573. doi: 10.1016/j.jaac.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Dusing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Psychoneuroendocrinology. 2011;36(6):898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. [Research Support, U.S. Gov’t, P.H.S. Review] Endocrine reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Faraone SV, Surman CB, Petty C, Clarke A, Batchelder H, Biederman J. Toward defining deficient emotional self-regulation in children with attention-deficit/hyperactivity disorder using the Child Behavior Checklist: a controlled study. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Postgraduate medicine. 2011;123(5):50–59. doi: 10.3810/pgm.2011.09.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler C, Kroeger A, Weyers P, Grasmann D, Horschinek M, Freitag C, Clement HW. Cortisol reactivity in boys with attention-deficit/hyperactivity disorder and disruptive behavior problems: the impact of callous unemotional traits. Psychiatry research. 2011;187(1–2):204–209. doi: 10.1016/j.psychres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Steen NE, Lorentzen S, Barrett EA, Lagerberg TV, Hope S, Larsson S, Andreassen OA. Sex-specific cortisol levels in bipolar disorder and schizophrenia during mental challenge- Relationship to clinical characteristics and medication. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011 doi: 10.1016/j.pnpbp.2011.03.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Tick NT, van der Ende J, Verhulst FC. Twenty-year trends in emotional and behavioral problems in Dutch children in a changing society. [Research Support, Non-U.S. Gov’t] Acta psychiatrica Scandinavica. 2007;116(6):473–482. doi: 10.1111/j.1600-0447.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Tick NT, van der Ende J, Verhulst FC. Ten-year trends in self-reported emotional and behavioral problems of Dutch adolescents. [Research Support, Non-U.S. Gov’t] Social psychiatry and psychiatric epidemiology. 2008;43(5):349–355. doi: 10.1007/s00127-008-0315-3. [DOI] [PubMed] [Google Scholar]
- van West D, Claes S, Deboutte D. Differences in hypothalamic-pituitary-adrenal axis functioning among children with ADHD predominantly inattentive and combined types. [Comparative Study Research Support, Non-U.S. Gov’t] European child & adolescent psychiatry. 2009;18(9):543–553. doi: 10.1007/s00787-009-0011-1. [DOI] [PubMed] [Google Scholar]
- Velders FP, Dieleman G, Henrichs J, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. Prenatal and postnatal psychological symptoms of parents and family functioning: the impact on child emotional and behavioural problems. [Research Support, Non-U.S. Gov’t] European child & adolescent psychiatry. 2011;20(7):341–350. doi: 10.1007/s00787-011-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. [Review] Annual review of clinical psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zepf FD, Vloet TD, Polier GG, Baurmann D, Bubenzer S, Helmbold K, Wockel L. No association between affective and behavioral dysregulation and parameters of thyroid function in youths. [Research Support, Non-U.S. Gov’t] Journal of affective disorders. 2011;134(1–3):478–482. doi: 10.1016/j.jad.2011.05.040. [DOI] [PubMed] [Google Scholar]
- Zepf FD, Wockel L, Poustka F, Holtmann M. Diminished 5-HT functioning in CBCL pediatric bipolar disorder-profiled ADHD patients versus normal ADHD: susceptibility to rapid tryptophan depletion influences reaction time performance. [Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Human psychopharmacology. 2008;23(4):291–299. doi: 10.1002/hup.934. [DOI] [PubMed] [Google Scholar]