Abstract
A subpopulation of retinal ganglion cells (RGCs) expresses the photopigment melanopsin, rendering these cells intrinsically photosensitive (ipRGCs). These cells are critical for competent circadian entrainment, pupillary light reflex, and other non-image-forming photic responses. Research has now demonstrated the presence of multiple subpopulations of ipRGC based on the dendritic stratification in the inner plexiform layer (IPL), those monostratified in the Off sublamina (M1), those monostratified in the On sublamina (M2,4,5), and those bistratified in both the On and Off sublaminas (M3). Despite evidence that M1 and M2 cells are distinct subpopulations of ipRGC based on distinct morphological and physiological properties, the inclusion of M3 cells as a distinct subtype has remained controversial. Aside from the identification of M3 cells as a morphological subpopulation of ipRGC, to date there have been no functional descriptions of M3 cell physiology or synaptic inputs. Our data provide the first in-depth description of M3 cell structural and functional properties. We report that M3 cells form a morphologically heterogeneous population, but one that is physiologically homogeneous with properties similar to those of M2 cells.
Keywords: intrinsically photosensitive ganglion cell, circadian entrainment, melanopsin, patch clamp, retina, dendritic arborization, dendrite, synaptic, On pathway, M3 cells
INTRODUCTION
A small subpopulation of retinal ganglion cells (RGCs) express the photopigment melanopsin, rendering them intrinsically sensitive to light (Berson et al., 2002; Hattar et al., 2002)(ipRGCs). These cells project to a variety of brain areas, such as the suprachiasmatic nucleus of the hypothalamus (SCN), the mammalian circadian pacemaker, and the olivary pretectal nucleus (OPN), the structure responsible for driving the pupillary light reflex (Baver et al., 2008; Ecker et al., 2010; Gooley et al., 2003; Hattar et al., 2006; Hattar et al., 2002). ipRGCs are critical for mediation of both circadian photoentrainment and the pupillary light reflex (Goz et al., 2008; Guler et al., 2008; Hatori et al., 2008)
Recent research has demonstrated that there are several morphological subpopulations of ipRGC classified by their dendritic ramification in the inner plexiform layer (IPL): M1 cells, which have dendrites monostratified in the Off sublamina, M2, 4 and 5 cells, which have dendrites monostratified in the On sublamina, and M3 cells, which have dendrites bistratified in both the On and Off sublaminas (Berson et al., 2010; Ecker et al., 2010; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007; Warren et al., 2003). These morphological subpopulations are also physiologically dissimilar, with M1 cells having larger and more sensitive intrinsic light responses as well as a more depolarized resting membrane potential and higher input resistance than M2 cells (Schmidt and Kofuji, 2009). Both M1 and M2 cell dendrites have also now been shown to provide complete tiling of the retina, a criterion traditionally used to define a distinct RGC subtype (Berson et al., 2010; Wassle, 2004). Aside from their identification as a morphological population of ipRGCs, no information regarding M3 cell functional properties has been reported. Furthermore, it has been proposed that because M3 cells are rare relative to M1 and M2 cells and do not provide complete tiling of the retina, that these cells are not a “true” ipRGC subtype but instead are some “anomalous hybrid” or are the result of an incomplete differentiation during ipRGC development (Berson et al., 2010).
We have utilized a transgenic mouse line in which ipRGCs are labeled in vivo with EGFP (Schmidt et al., 2008) to collect physiological and morphological data from the relatively rare and thus-far uncharacterized M3 ipRGC subtype. We provide the first in-depth descriptions of M3 morphology, intrinsic light responses, intrinsic membrane properties, and synaptic light responses. We find that M3 ipRGCs in adult mouse retinas, though morphologically heterogeneous, are physiologically a homogeneous population.
MATERIALS AND METHODS
Animals
Recordings were performed on postnatal (P) 22–40 animals from the Opn4-EGFP mouse line described previously (Schmidt et al., 2008) as well as Opn4-EGFP mice crossed with animals on an Opn4−/− background provided by Dr. King-Wai Yau, Johns Hopkins University (Hattar et al., 2002). Animals were cared for in accordance with guidelines described in Guide for the Care and Use of Laboratory Animals, using protocols approved by the University of Minnesota Institutional Animal Care and Use Committee.
Electrophysiology
Dissections were performed as described previously (Schmidt and Kofuji, 2010b; Schmidt et al., 2008). Briefly, animals were sacrificed by CO2 asphyxiation and the eyes were enucleated in a dark room with minimal ambient light. Retinas were removed from eyecup under a standard dissection scope and placed in 95% O2-5%CO2 bicarbonate buffered Ames’ solution (Sigma, St. Louis, MO) at room temperature. Prior to recording, retinas were treated with Ames’ solution containing collagenase/hyaluronidase (240 and 1000 U/ml, respectively; Worthington Biochemicals, Lakewood, NJ) at room temperature for 15 minutes to aide the removal of any remaining vitreous. Recordings were performed using an Axon 700B Amplifier (Molecular Devices, Union City, CA) with extracellular solution containing 95% O2-5%CO2 bicarbonate buffered Ames’ solution at 32–34°C for intrinsic light response and intrinsic membrane parameter measurements and room temperature for synaptic light response experiments, which allowed for stable and robust synaptic responses to be recorded. Recordings were made with fire-polished borosilicate pipettes (3–7 MΩ; Sutter Instruments, Novato, CA). For current clamp recordings, pipettes were filled with (in mM): 125 K-gluconate, 2 CaCl2, 2 MgCl2, 10 EGTA, 10 HEPES, 0.5 NaGTP, and 2 Na2ATP, pH to 7.2 with KOH. For voltage clamp recordings, pipettes were filled with (in mM): 120 CsMethanesulfonate 2 MgCl2, 5 Hepes, 5 EGTA, 0.5 CaCl2, 1 Na2ATP, 0.5 NaGTP, 2 N-(2,6-dimethylphenyl carbamoylmethyl)triethylammonium bromide (QX314), 5 TEA-Cl, 1 4-aminopyridine (4-AP), pH to 7.2 with CsOH. Intracellular solutions also contained 10–20 μM Alexafluor-594 hydrazide (AF-594)(Invitrogen, Carlsbad, CA) and following recording, dendritic stratification was classified by focusing in the proximal and distal layers of the IPL under epifluorescent illumination with a rhodamine cube.(Schmidt and Kofuji, 2009; Schmidt and Kofuji, 2010b). If a cell’s stratification could not be clearly identified, that cell was excluded from subsequent analyses. Current and voltage acquisitions were performed with a Digidata 1322 D/A and A/D converter connected to a personal computer running pClamp 10 software (Molecular Devices). Liquid junction potentials between the bath and electrode (14 mV for current clamp and 13 mV for voltage clamp solutions) were calculated using the Liquid Junction Potential Calculator (pClamp 10, Molecular Devices) and were corrected for in all recordings. Retinas were allowed to dark adapt for 5 min prior to the first light stimulus, and any stimuli were placed 5 min apart to allow the cell membrane potential to return completely to baseline.
Whole cell currents were analyzed off-line with Clampfit (Molecular Devices) or Igor Pro 6.0 (Portland, OR) over a 0.1 s sliding time window, and membrane potential values were measured from raw traces over a 1 s sliding time window to maximize the signal to noise ratio using Igor Pro 6.0 (Portland, OR). Resting membrane potential (Vm) values were calculated by taking the average membrane voltage in the presence of synaptic blockers and TTX (see “Pharmacology” section). For synaptic light response experiments, if a cell’s resting membrane potential (Vm) did not reach −50 mV negative current was injected to bring the resting membrane potential (Vm) to approximately −60mV.
Series resistance was noted in all recordings, but uncompensated and only recordings with series resistance of < 30 MΩ were included for analysis. Cell capacitance (Cm) and input resistance (RN) were calculated from those currents evoked by stepping the cell potential to a 10 mV hyperpolarized value for 20 ms from a holding potential of −60 mV. Charge Q was estimated by time integration of evoked current during the step voltage. RN was estimated from the steady-state evoked current during the step voltage. Light responses were defined as the maximum depolarization of the averaged trace during the first 30 s following light onset.
Irradiance-response experiments were performed and analyzed as described previously (Schmidt and Kofuji, 2009; Schmidt et al., 2008). Curve fits for normalized, averaged irradiance-response data were determined by nonlinear regression using Origin 7.5 (OriginLab Corporation, Northampton, MA) according to the logistic dose-response function: y = A2 + [(A1−A2)/(1+(IR/IR50)^nH)], where A1 is the maximum response plateau, A2 is the minimum response plateau, IR is irradiance, IR50 is the irradiance values that generate half-maximal response, and nH is the Hill slope. Light stimuli for intrinsic light response experiments were full-field, broadband white light delivered from below the retina at 42 × 104 μW.cm−2 (7.6 × 1014 photons.cm−2.s−1). Light stimuli for synaptic and irradiance response experiments were 11 × 103 μW.cm−2 (7.63 × 1014 photons.cm−2.s−1 measured at 480 nm by interposing a narrow bandpass filter) and delivered using a xenon lamp feeding the camera port. A filter wheel fitted with various neutral-density filters (Chroma Technologies, Rockingham, VT) and shutter (Lambda-3, Sutter Instruments) was used to control the intensity and duration of light stimuli. Irradiance measurements were made with a calibrated radiometer model S370 (UDT Instruments, San Diego, CA).
Statistical analyses were performed using Origin 7.5 (OriginLab Corporation). Statistical comparison of means was performed using a Student’s t-test or one-way ANOVA with Tukey’s post-hoc test and significance was concluded when P < 0.05. Data are presented as mean ± SE.
Pharmacology
For intrinsic light response and intrinsic membrane property measurements, synaptic blocker cocktail included: 250 μM DL-2-amino-4-phosphonobutyrate (DL-AP4, a group III metabotropic glutamate receptor agonist); 10 μM 6,7-dinitroquinoxaline (DNQX, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA/kainate receptor antagonist); 0.3 μM strychnine (glycine receptor antagonist); 50 μM picrotoxin (GABA receptor antagonist). Extracellular solution sometimes also included 0.5 μM tetrodotoxin (TTX, sodium channel blocker). DL-AP4, DNQX, and TTX were purchased from Tocris (Ellesville, MO). Picrotoxin, and strychnine were purchased from Sigma (St. Louis, MO). For synaptic recordings 100 μM L-2-amino-4-phosphonobutyrate (L-AP4, a group III metabotropic glutamate receptor (mGluR) agonist) (Tocris, Ellesville, MO) that blocks photoreceptor to On bipolar cell signaling (Slaughter and Miller, 1981), was sometimes included in the bath solution and used to silence light-evoked On pathway responses.
Antibody Characterization
The primary antibodies used in this study can be found in Table 1. Choline acetyl transferase (ChAT) is a marker of cholinergic amacrine cells, the dendrites of which form two plexuses and served as a marker for the On and Off sublaminas of the retina (Kang et al., 2004). The goat polyclonal anti-ChAT antibody (immunogen: human placental enzyme) has been well-characterized and previously demonstrated to recognize 68–70 kDa bands in Western blot analysis of brain extracts from rat and several species of fish that disappeared when the antibody was preincubated with human placental ChAT (Anadon et al., 2000). The same antibody has been used in the mouse retina to recognize cholinergic amacrine cells (Whitney et al., 2008). The anti-Lucifer Yellow antibody has been used previously and shown to specifically label cells filled with Lucifer Yellow (Xu et al., 2008; Zhang et al., 2006).
Table 1.
Primary antibodies used in this study.
| Antigen (what is being stained for) | Immunogen (what the antibody was raised against; full sequence and species) | Manufacturer, species antibody was raised in, mono- vs. polyclonal, catalog or lot number | Dilution used |
|---|---|---|---|
| Choline acetyl transferase (ChAT) | human placental enzyme | Millipore, Bedford, MA; # AB144P | 1:250 |
| Lucifer Yellow | Invitrogen, Carlsbad, CA; #A5750 | 1:500 |
Immunocytochemistry
Immunocytochemistry and neurobiotin filling were performed as published previously (Schmidt et al., 2008). Cells were filled with either 0.3% neurobiotin (Vector Laboratories, Burlingame, CA) or 0.3% Lucifer Yellow (Sigma). For visualization of filled cells and immunostaining of retinas, retinas were then fixed overnight in 4% paraformaldehyde solution at 4°C and washed extensively in PBS. Retinas were then placed in blocking solution containing 10% donkey serum and 0.5% Triton X100 (Sigma) in PBS overnight at 4°C. Retinas were then placed in primary antibody solution containing 5% donkey serum, 0.5% Triton and a combination of goat polyclonal ChAT (1:250; Millipore, Bedford, MA; AB144P), rhodamine-conjugated streptavidin (1:500; Invitrogen, Carlsbad, CA), or rabbit polyclonal anti-Lucifer Yellow (1:500; Invitrogen; A5750) rotating for 3 days at 4°C. Following incubation in primary antibody solution, retinas were again washed extensively in PBS and the placed in secondary antibody solution containing 5% donkey serum, 0.5% TritonX100 and a combination of Alexa-488 conjugated donkey anti-goat or anti-rabbit (1:500; Invitrogen) or rhodamine-conjugated streptavidin (1:500) rotating for 2 days at 4°C. Retinas were then washed extensively in PBS, mounted in Vectashield (Vector Laboratories), coverslipped, and sealed with nail polish. Image acquisition was performed on an upright Olympus Fluoview 1000 laser scanning confocal microscope (Olympus, Center Valley, PA). Image J was used to adjust image brightness and contrast (http://rsb.info.nih.gov/ij/). Multiple confocal stacks of filled neurons were merged and neurons were traced in 3 dimensions using NeuroLucida (Microbrightfield, Williston, VT). Dendritic field size was estimated in NeuroLucida by taking the area of a convex polygon linking the tips of the dendrites from a 2-dimensional tracing of a given cell and the diameter was then expressed as that of a circle having an equal area. Sholl Analysis (Sholl, 1953) was also performed in NeuroLucida with a starting radius of 10 μm from the center of the soma and the number of crossings were counted at circles increasing in radius by 15 μm.
RESULTS
M3 ipRGCs are morphologically heterogeneous
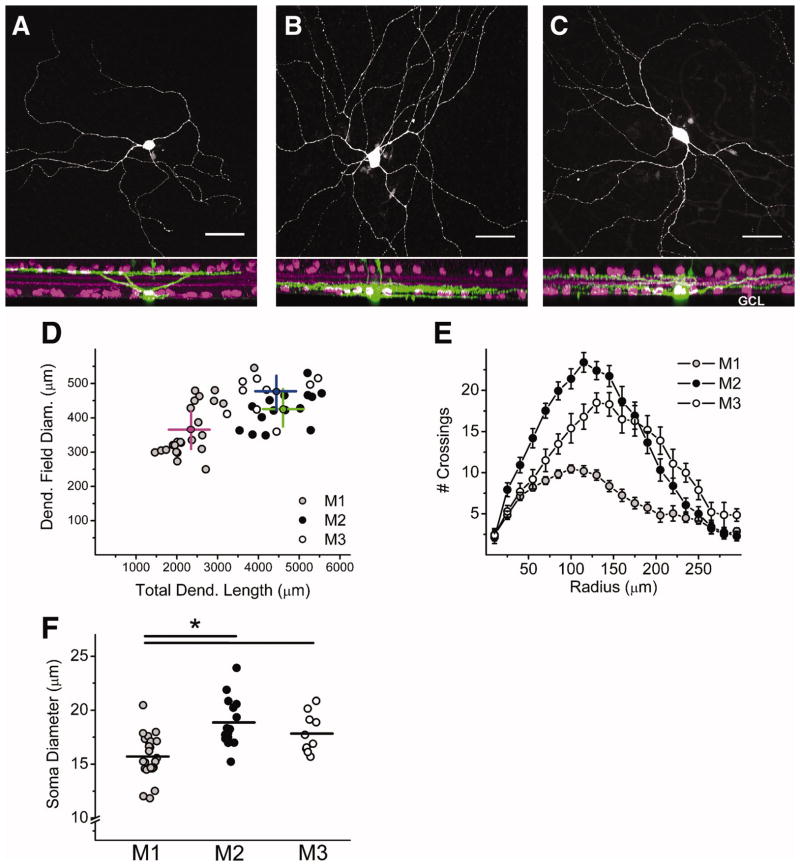
Though the existence of M3 cells has been described previously (Berson et al., 2010; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007; Warren et al., 2003), the morphological properties of M3 cells have not been examined in detail. To provide further morphological characterization of M3 cells, we have filled ipRGCs in adult mouse retinas of a mouse line in which ipRGCs are labeled in vivo with EGFP (Schmidt et al., 2008) with 0.3% neurobiotin or Lucifer Yellow (LY). ipRGCs were classified as M1 if the cell had dendrites terminating in the Off sublamina (Figure 1A), M2 if the cell had dendrites terminating in the On sublamina (Figure 1B), and M3 if the cell had dendrites terminating in both the On and Off sublaminas of the IPL (Figure 1C). M3 ipRGCs had large dendritic arbors (diameter = 477.4 ± 20.1 μm, n = 10; dendritic length = 4441.2 ± 331.4 μm, n = 10). When compared to the arbors of M1 and M2 cells, M3 cells were found to have significantly larger dendritic field diameters and total dendritic length than M1 (diameter = 365.9 ± 16.3 μm, n = 24; dendritic length = 2350.1 ± 110.2 μm, n = 24) but not M2 cells (diameter = 425.4 ± 13.4 μm, n = 15, p < 0.01, ANOVA; dendritic length = 4603.6 ± 164.1 μm, n = 15, p < 0.01, ANOVA) (Figure 1D). Furthermore, when the dendritic arbor complexity was examined using Sholl analysis to quantify the degree of branching, we found that M3 cells had highly branched dendritic arbors, similar to M2 cells, while M1 cells had less branched, relatively simpler dendritic arbors (Figure 1E) (Berson et al., 2010; Schmidt and Kofuji, 2009). When the soma size of M3 cells was quantified (soma diameter = 17.8 ± 0.6 μm, n = 10) these cells were found to have significantly larger somas than M1 (soma diameter = 15.7 ± 0.4 μm, n = 24) but not M2 cells (soma diameter = 18.9 ± 0.6 μm, n = 15, p < 0.0.1, ANOVA)(Figure 1F). Collectively these results indicate that M3 cells are similar to M2 cells in terms of the size and complexity of their dendritic arbors, but differ in terms of their dendritic stratification.
Figure 1. Morphological properties of M3 cells.
(A–C) Confocal stack in which ipRGCs have been filled with neurobiotin (green) and costained for ChAT (magenta), a cholinergic amacrine cell marker. (A) M1 cell with dendrites terminating in the outer (Off) sublamina of the inner plexiform layer (IPL). (B) M2 cell with dendrites terminating in the inner (On) sublamina of the IPL. (C) M3 cell with dendrites terminating in the inner (On) and outer (Off) sublaminas of the IPL. Note: processes visible extending through IPL in A–C are those of Müller cells, which often take up dye during the patching procedure. (D) Plot showing total dendritic length (μm, x-axis) and dendritic field diameter (μm, y-axis) of M1 (gray circles), M2 (black circles), and M3 (open circles). Magenta (M1), green (M2), and blue (M3) lines represent the mean value for each cell type. (E) Sholl analysis (15 μm steps from starting diameter of 10 μm) of M1 (gray circles), M2 (black circles), and M3 (open circles) dendritic arbors. (F) Soma diameter (μm) of M1 (gray circles), M2 (black circles), and M3 (open circles) cells. Lines represent mean values. Scale bar (A–C): 50 μm. *p<0.05 ANOVA. INL, Inner Nucelar Layer, GCL, Ganglion Cell Layer.
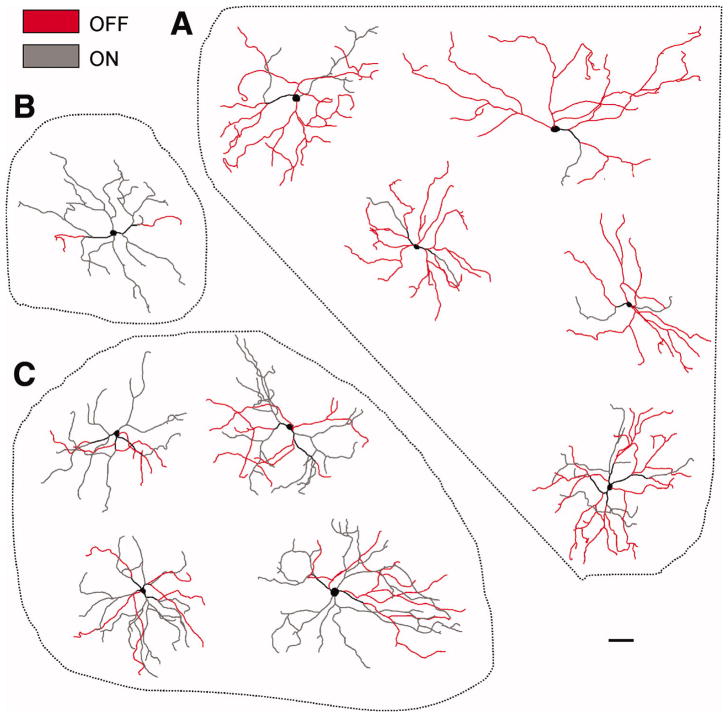
We next examined the proportion of dendrites of M3 cells residing in the On or Off sublaminas of the IPL. Notably, M3 cells had variable proportions of dendritic arbors stratifying in each sublamina. Examples of the dendritic arbors of 10 M3 cells are shown in Figure 2. 5 of 10 M3 cells had dendrites confined primarily to the Off sublamina (Figure 2A), 4 of 10 M3 cells had dendrites stratifying approximately equally in both sublaminas (Figure 2C) and 1 of 10 M3 cells had dendrites confined almost entirely to the On sublamina (Figure 2B). Altogether, these results indicate that M3 cells do not show a uniform pattern of dendritic stratification in either the On or Off sublamina.
Figure 2. Dendritic arbors of M3 cells.
Traced dendritic arbors of neurobiotin or LY-filled M3 cells. Red-colored arbors represent those dendrites terminating in the Off sublamina of inner plexiform layer. Gray colored processes represent dendrites terminating in the On sublamina. (A) M3 cells with dendrites confined mainly to the Off sublamina. (B) M3 cell with dendrites confined mainly to the On sublamina (C) M3 cells with substantial dendritic stratification in both the On and Off sublaminas. LY: Lucifer yellow. Scale bar: 100 μm.
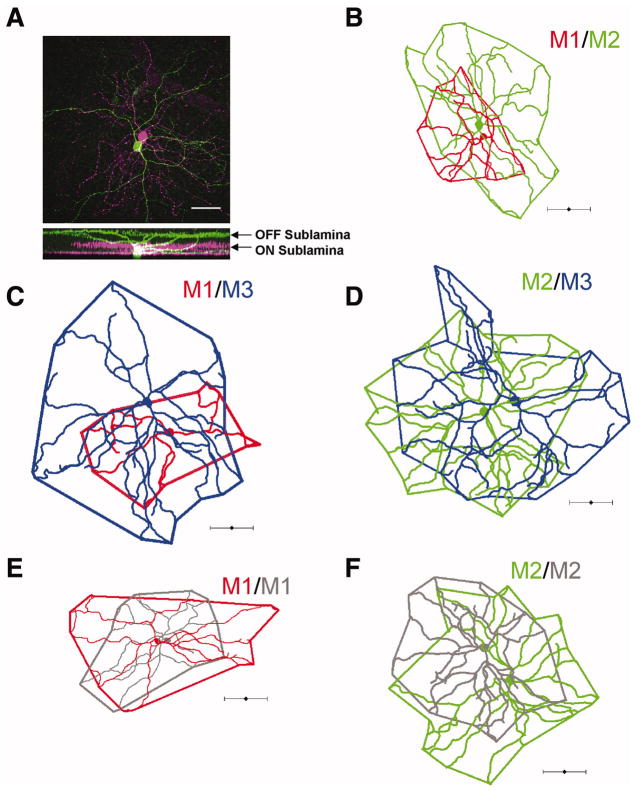
Additionally, when we assessed the dendritic field overlap of ipRGCs in close proximity to each other by filling cells with either neurobiotin or LY, we found that M3 cells overlap extensively with M1 and M2 cells (Figure 3C–D). Furthermore, we found that M1 cell dendritic arbors overlap extensively with those of nearby M2 as well as M1 cells and that the dendrites of M2 cells likewise overlap extensively with nearby M2 cells (Figure 3A–B, E–F). These data are in agreement with previously reported findings (Berson et al., 2010; Schmidt and Kofuji, 2009) of extensive overlap of neighboring ipRGCs of hetero- and homotypic subtypes in mouse, but more extensive than seen in primate ipRGCs (Jusuf et al., 2007). This is contrast to some other ganglion cell subtypes in which dendrites show minimal overlap across the retina (Vaney, 1994). When we analyzed the proportion of M3 ipRGCs across a sample of 213 cells from various experiments, we found that M3 cells formed 20% (43/213 cells) of the dataset while 42% (90/213) off cells were M2 and 38% (80/213) were M1 cells.
Figure 3. Dendritic field overlap of neighboring ipRGCs.
Neighboring ipRGCs were filled with 0.3% neurobiotin or LY and their arbors traced and dendritic field overlap mapped. Extensive dendritic field overlap was observed between heterotypic (A–D) and homotypic (E–F) filled pairs. (A) Confocal image where an M1 cell (green) was filled with LY and an M2 cell (magenta) with neurobiotin. Scale bar 50μm. (B) Neighboring M1 (red) and M2 (green) cell. (C) Neighboring M1 (red) and M3 (blue) cell. (D) Neighboring M2 (green) and M3 (blue) cell. (E) Neighboring M1 cells. (F) Neighboring M2 cells. Scale bar (A) 50 μm (B–F) 100 μm.
M3 cells have uniform intrinsic physiological characteristics
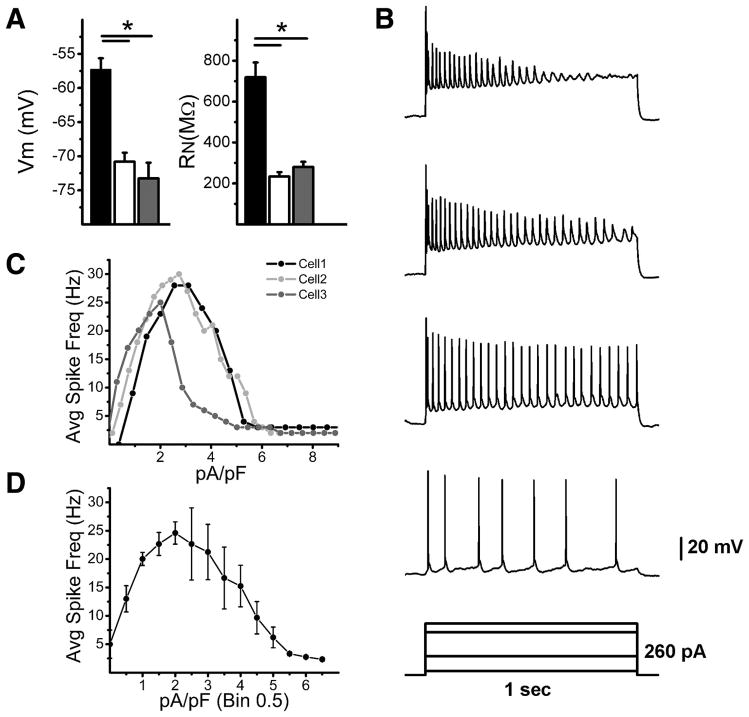
The functional properties of M3 cells have not yet been examined and compared to M1 and M2 cells in any study to date. We therefore examined the resting membrane potential and input resistance of M3 cells in the presence of a synaptic blocker cocktail and TTX (see methods). We found that M3 cells had a significantly more hyperpolarized resting membrane potential (Vm) (Vm = −73.3 ± 2.3 mV, n = 9) than M1 (Vm = −57.3 ± 1.7 mV, n = 21) but not M2 cells (Vm = −70.8 ± 1.3 mV, n = 23, p < 0.05, ANOVA) (Figure 4A). Furthermore, M3 cells had an input resistance (RN= 280.3 ± 25.8 MΩ, n = 11) that was significantly lower than M1 cells (RN=718.9 ± 72.5 MΩ, n = 21) but similar to M2 cells (RN=233.7 ± 21.8 MΩ, n = 23, p < 0.05, ANOVA) (Figure 4A). Collectively, these results indicate that M3 cells have intrinsic membrane properties that are similar to M2 cells.
Figure 4. Intrinsic membrane properties of M3 cells.
(A) Recordings were performed in the presence of synaptic blockers and TTX. (left panel) Mean ± SE Vm (mV) of M1 (black bar), M2 (white bar), and M3 cells (gray bar). (right panel) RN (MΩ) of M1 (black bar), M2 (white bar), and M3 (gray bar) cells. (B–D) Recordings were performed in the presence of synaptic blockers. (B) Representative response of M3 cell to 1 s depolarizing current injection over a range of 260 pA. (C) Average firing frequencies of 3 M3 cells to increasing amounts of depolarizing current injection. (D) Mean ± SE average firing frequency of M3 cells to increasing amounts of 1 s depolarizing current injections. Current injection for each cell was divided by its capacitance (in D, Bin = 0.5 pA/pF) to facilitate averaging. Vm: resting membrane potential, RN: input resistance. *p<0.05 ANOVA
We also examined the spiking properties of M3 cells (again in the presence of synaptic blockers, but without TTX). To examine the spiking evoked by depolarizing current, we performed current clamp experiments where we first injected steady state current to bring the Vm to ~−70mV and then injected increasing amounts of depolarizing current (Figure 4B). We recorded from 3 M3 cells and found that, like M1 and M2 cells, M3 cells respond to increasing amounts of current injection with increasing spike frequency (Figure 4C). We found that M3 cells reached maximum average spike frequencies (27.6 ± 1.5 Hz, n = 3) comparable to those reported previously for M2 cells (38.4 ± 4 Hz) and higher than reported previously for M1 cells (10.1 ± 1.3 Hz) (Figure 4C–D)(Schmidt and Kofuji, 2009) (Figure 4D). However, M3 cells reached depolarization block at lower amounts of current injection (2.6 ± 0.3 pA/pF), defined as the first current injection after which a submaximal spike frequency was attained, than M2 cells (4.4 ± 0.6 pA/pF) but higher than that for M1 cells (1.2 ± 0.3 pA/pF) (Figure 4C–D) (Schmidt and Kofuji, 2009). These results demonstrate that M3 cells respond to depolarizing current injections by attaining spike frequencies similar to those reported for M2 cells, but appear to reach depolarization block at lower frequencies than those reported previously for M2 cells, indicating that M3 cells have spiking properties unique to M1 and M2 cells.
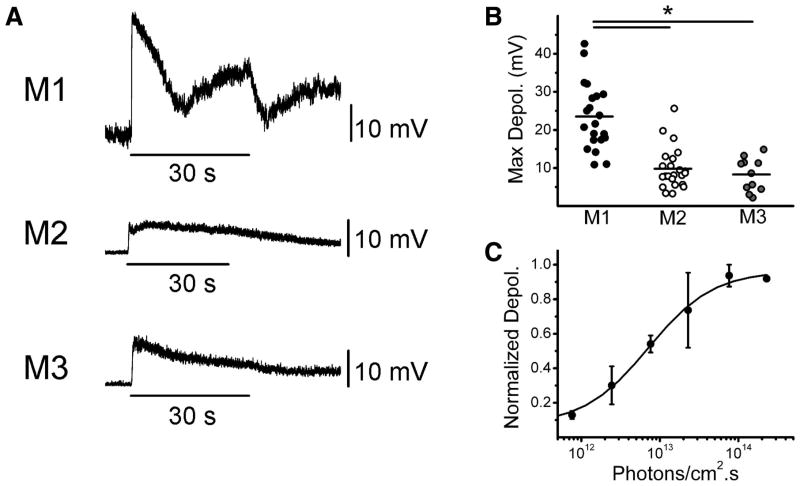
We next sought to examine the properties of the intrinsic light response of M3 cells. We recorded light responses in current clamp mode to a 30 s bright, full-field white light stimulus from M1, M2, and M3 ipRGCs in the presence of a cocktail of synaptic blockers (see methods) and TTX. We found that M3 cells, like M1 and M2 cells, responded to light stimulation with a sustained depolarization that persisted following stimulus offset (Figure 5A). M3 cells responded to light with a significantly smaller depolarization (8.3 ± 1.3 mV, n = 11) than M1 cells (23.5 ± 1.9 mV, n = 21) and with a similar maximum depolarization to M2 cells (9.8 ± 1.1 mV, n = 23)(Figure 5B). M1 cells are ~1 log unit more sensitive to 480 nm light, the ~λmax for melanopsin, than M2 cells, but this has not been examined for M3 cells (Berson et al., 2002; Dacey et al., 2005; Lucas et al., 2001; Qiu et al., 2005; Schmidt and Kofuji, 2009). We obtained full irradiance response curves for 3 M3 cells to 480 nm light. We found that the fitted LogIR50 for M3 cells of 12.8 Log photons/cm2.s ( range 12.5–13.2) was in between that reported previously for M1 and M2 cells, shifted ~0.3 log units higher in intensity relative to that of M1 cells of 12.5 Log photons/cm2.s (range 12–12.8) and ~0.7 log units lower in intensity relative to M2 cells of 13.5 Log photons/cm2.s (range 13–14.1) collected under identical conditions (Schmidt and Kofuji, 2009) (Figure 5C). These results indicate that while M3 cells respond to light with a maximum intrinsically-evoked depolarization similar to that of M2 cells, these cells may display a unique sensitivity to 480 nm light compared to both M1 and M2 cells.
Figure 5. Intrinsic light responses of M3 cells.
All light responses recorded in the presence of synaptic blockers and TTX. (A) Response in current clamp mode of M1 (top panel), M2 (middle panel), and M3 (bottom panel) cells to 30 s full-field, bright, white light stimulus. (B) Maximum depolarization evoked by single 30 s white light stimulus measured in current clamp mode of M1 (black circles), M2 (white circles), and M3 (gray circles) cells. Black bars represent mean maximum depolarization. (C) Irradiance response curve fitted with logistic dose-response function for M3 cells generated by stimulating cells with increasing intensities of a 5 s 480 nm light stimulus. *p<0.05 ANOVA.
The On pathway forms the primary synaptic input to M3 cells
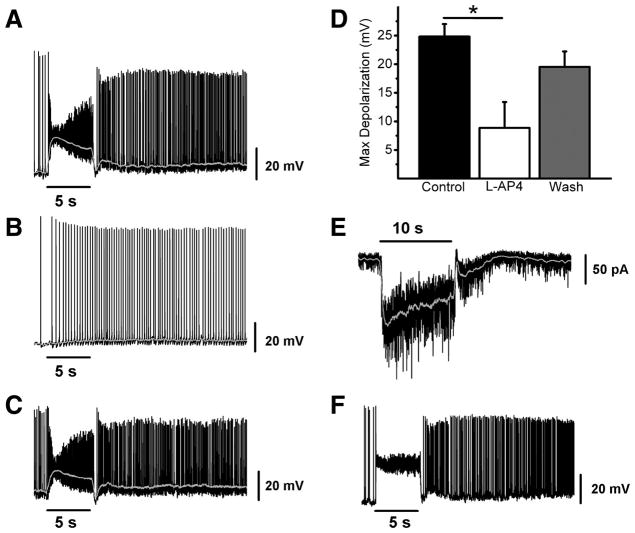
We next examined the synaptic light response of M3 cells. M1 and M2 cells both receive synaptic input from the On pathway in response to light stimulation, and this On pathway input forms the primary light-evoked synaptic input to these two ipRGC subpopulations at bright light intensities(Schmidt and Kofuji, 2010a). In order to study the light-evoked synaptic inputs to M3 cells, we recorded light responses in current or voltage-clamp mode to a 5 or 10 s bright white light stimulus (see methods) in the absence of synaptic blockers. In current clamp mode, M3 cells responded to light with fast (< 1 sec onset) and sustained light-evoked depolarizations (Figure 6A). We next applied L-AP4, a Group III mGluR receptor agonist that selectively silences signaling at the photoreceptor-On bipolar cell synapse (Slaughter and Miller, 1981). Following L-AP4 application, M3 cells responded with a smaller, sluggish depolarization that persisted following stimulus offset (Figure 6B). This effect was reversible upon washout (Figure 6C). When we quantified maximum depolarization in control (24.8 ± 2.2 mV, n = 4), L-AP4 (8.9 ± 4.5 mV, n = 4), and upon washout (19.5 ± 2.7 mV, n = 4) we found that in the presence of L-AP4, the maximum light-evoked depolarization of M3 cells was significantly reduced relative to control (p < 0.05, ANOVA) (Figure 6D). Light responses in voltage clamp (n = 2) paralleled those seen in current clamp, with M3 cells showing a fast and sustained inward current that terminated quickly following light offset (Figure 6E). The effect of L-AP4 application on light-evoked depolarization of M3 cells was virtually identical to the effect of application of a cocktail of glutamatergic, GABAergic, and glycinergic blockers (Supplemental Figure 1), indicating that, similar to M1 and M2 cells, the On pathway forms the dominant synaptic input to M3 cells. Despite the fact that the bulk of dendrites for many M3 cells are almost entirely confined to the Off sublamina (Figure 2), M3 cells show robust On pathway-evoked depolarizations that are consistently similar to the synaptic light responses of M2 cells (Figure 6F)(Schmidt and Kofuji, 2010a).
Figure 6. Synaptically-evoked light response of M3 cells in WT mice.
(A–C) Example of typical synaptic light response of an M3 cell recorded in current clamp mode to a 5 s, full-field, bright white light stimulus first in control conditions (A), in the presence of 100 μM L-AP4 (B), and following washout (C). (D) Mean ± SE maximum depolarization evoked by 5 s full-field bright white light stimulus in control (black bar), 100 μM L-AP4 (white bar), and after washout (gray bar). (E) Voltage clamp recording of M3 cell response to 10 s full field, bright, white light stimulus. (F) Example of typical synaptic light response of an M2 cell recorded in current clamp mode to a 5 s, full-field, bright white light stimulus in the absence of synaptic blockers. Gray lines (A–C) represent 0.1 s smoothing of membrane voltage. * p < 0.05 ANOVA.
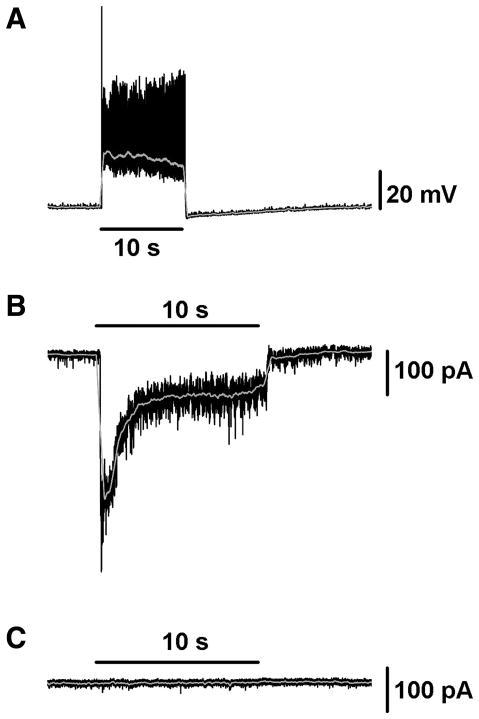
In order to measure synaptic responses in isolation from the intrinsic photosensitivity of M3 cells, we crossed our EGFP reporter mouse line with a mouse line in which the Opn4 gene coding region has been replaced with that of TauLacZ (Hattar et al., 2002), to generate a melanopsin null mouse line (Opn4−/−) in which ipRGCs are labeled with EGFP. When we recorded from M3 cells in the Opn4−/− mouse line we found that, in current clamp, the response of these cells to light was virtually identical to that seen in wild-type (WT) mice (n = 2, maximum depolarization 35.0 mV and 22.1 mV), with a fast onset and offset depolarization, but lacking the after-discharge often seen several seconds after light offset in the WT (Figure 7A). In voltage clamp mode, M3 cells responded to 10 s, bright, white light stimuli with fast and sustained inward currents that terminated quickly following light offset (n = 4). An example of a typical M3 cell light response in Opn4−/− mice recorded in voltage clamp mode is shown in Figure 7B. Furthermore, all light-evoked currents were completely abolished in this cell upon application of L-AP4, providing further support for the idea that the On pathway provides the dominant light-evoked synaptic input to M3 cells (Figure 7C). This is similar to both M1 and M2 cells in which the synaptic light response of ipRGCs in Opn4−/− mice is completely abolished following L-AP4 application (Schmidt and Kofuji, 2010a). Given the similarity of the light-evoked response in WT and Opn4−/− M3 cells, we conclude that the synaptic input via the On pathway is primarily responsible for shaping the integrated light response of WT M3 cells under control conditions. The robust synaptic influence on the M3 cell light response is similar to that seen in WT M2 cells (Figure 6A,F) but not in M1 cells (Schmidt and Kofuji, 2010a).
Figure 7. Synaptic input to M3 cells recorded in Opn4−/− mice.
(A) Current clamp recording of M3 cell light response to 10 s full-field, bright, white light stimulus in Opn4−/− mouse. (B–C) Voltage clamp recording of M3 cell light response to 10 s full field, bright, white light stimulus in Opn4−/− mouse first in control conditions (B) and then in the presence of 100 μM L-AP4 (C). In the presence of L-AP4 the light response of this cell is completely abolished. Gray lines (A–C, E) represent 0.1 s smoothing of membrane voltage.
DISCUSSION
ipRGCs are a distinct subpopulation of ganglion cell within the mammalian retina. It has become clear that ipRGCs are not a homogeneous population, but one that contains several morphological subtypes, each with distinct physiological characteristics (Ecker et al., 2010; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Tu et al., 2005; Viney et al., 2007; Warren et al., 2003). In this study we demonstrate for the first time that the mouse retina contains a population of ipRGCs that are homogenous in regard to their physiological properties and yet are heterogeneous in regard to key morphological features. These ipRGCs, termed M3 cells, with a bistratified dendritic arborization in the inner plexiform layer (IPL), respond to light stimulation via intrinsic and synaptic mechanisms in a manner reminiscent of M2 cells. Intrinsic membrane properties and spiking properties are also more similar to M2 than M1 cells (Schmidt and Kofuji, 2009).
We first analyzed the detailed morphological properties of M3 cells. These cells have large and highly branched dendritic arbors and relatively large somas, similar to the arbors of M2 cells. The defining morphological characteristic of the M3 subpopulation is the bistratification of the dendrites within the innermost and outermost sublaminas of the IPL. What is perhaps most striking about the dendritic arbors of M3 cells is the lack of uniformity with regards to the proportion of dendrites stratifying in the On and Off sublaminas. It appears that M3 cell morphology forms a continuum with regard to the proportion of dendrites stratifying in either the On or Off sublaminas, with some cells having the vast majority of their dendrites confined to the Off or On sublamina and some stratifying in both sublaminas in roughly equal proportions. Indeed this sparse branching into either the On or the Off sublamina could be at least partially responsible for some of the lower estimates of M3 cell density or lack of M3 cell projections reported in studies using immunostaining methods to identify various subtypes (Baver et al., 2008; Berson et al., 2010).
It has been proposed that M3 cells are some kind of developmental “anomalous hybrid” of the M1 and M2 subtypes (Berson et al., 2010). However, if this were the case, then one might expect to see a range of intrinsic properties and intrinsic light responses, some of which correlate with those of M1 cells and some that correlate with those of M2 cells. However, despite variation in the dendritic arborization of M3 cells, this was not observed in our experiments. The magnitude of the intrinsic light response, spike frequencies attained, resting membrane potential, and input resistance are all not only relatively consistent across the M3 cell population, but are also similar to the physiological properties of M2 cells. The apparent functional homogeneity of M3 cells, and the similarity of M3 functional properties to those of M2 cells is surprising, especially given that these cells displayed marked variability in the proportion of their dendritic arbors branching in the On and Off sublaminas of the IPL. Perhaps most surprising is that, regardless of the relative proportion of the M3 arbor stratifying in the Off sublamina, M3 cells had remarkably uniform On-pathway mediated synaptically driven light responses. This seems to indicate that the synaptic inputs to M3 cells reside not only on the dendrites branching in the On sublamina (which are often very sparse) but are perhaps distributed between the On and Off sublaminas. It has recently been demonstrated that M1 cell dendrites in the Off sublamina form ectopic synapses with On bipolar cells (Dumitrescu et al., 2009; Hoshi et al., 2009). M3 cell Off arbors costratify with the arbors of M1 cells and thus it is feasible that these cells receive a combination of both conventional and atypical On pathway inputs.
M3 cell dendrites that terminate in the Off sublamina stratify in the same sublamina as the dendrites of both M1 cells as well as the dopaminergic amacrine cells (DACs) (Dumitrescu et al., 2009; Hoshi et al., 2009; Vugler et al., 2008; Zhang et al., 2008). Bidirectional signaling between M1 cells and DACs has been proposed (Zhang et al., 2008). The close proximity of the dendritic arbor of M3 cells to DACs could expose M3 cells to levels of dopaminergic signaling that M2 cells would not experience. Furthermore, whether M3 cells might also contribute to the centrifugal flow of information from ipRGCs to the DACs (Zhang et al., 2008) is an intriguing possibility that has yet to be explored.
It is unclear whether this M2-like photic information conveyed by M3 cells is particularly relevant to a specific non-image forming (NIF) behavior. It has recently been demonstrated that the SCN and OPN receive distinct proportions of innervation of M1 and M2 cells (Baver et al., 2008). M3 cell projections were not reported in this study, perhaps because these cells do not project to these areas in high enough proportions or because it might be difficult to identify these cells using immunostaining methods as there are often only sparse dendritic branching in the On or Off sublaminas. If M3 cells do not project to the SCN or OPN, however, it will be important to determine whether any other NIF nuclei receive preferential projections from M3 cells. Whether M3 cells project in specific proportions to different NIF nuclei or play a specialized role in certain NIF behaviors will be an important question in future research.
Are M3 cells a “true” ipRGC subtype? RGCs are divisible into over a dozen morphological subtypes in the mammalian retina and a key feature of RGCs is that their dendrites create a mosaic that covers the surface the retina and exhibit consistent dendritic morphology (Coombs et al., 2006; Sun et al., 2002; Wassle, 2004). By using this criterion, we would expect M3 cell dendrites to cover the retina completely. However, a recent study by Berson et al. (2010) shows that M3 cell dendrites do not completely tile the retina, and that M3 cells are relatively rare. We find that the morphological features of M3 cells are consistently similar to M2 cells in terms of their dendritic length, dendritic field size, soma size, and dendritic arbor complexity. However, M3 cells show marked variation with regards to the proportion of dendrites stratifying in either the On or the Off sublamina. Stratification in the On or Off sublamina is an important indicator of synaptic connectivity for RGCs (Wassle, 2004), but we find that the On channel provides the primary synaptic input to M3 cells regardless of the proportion of dendrites stratifying in the On or Off sublamina for a given cell.
While tiling and morphological homogeneity are valid arguments for a “regular” RGC to be consider a distinct subtype, these stringent criteria need not necessarily apply to ipRGCs because of their unique functional features. First, because ipRGCs function primarily as irradiance detectors (Berson et al., 2002; Dacey et al., 2005), spatial discrimination as provided by the orderly and complete tiling of ganglion cell dendrites may not be required to mediate various NIF functions (but see (Ecker et al., 2010). The extensive overlap of ipRGC dendritic fields with nearby ipRGCs of the same or different subtype indicates that spatial segregation of the receptive field is not important for the mediation of ipRGC function. Furthermore, we find that 20% (43 cells) of a sample of 213 ipRGCs are bistratified by our criteria, in agreement with ~21% reported by (Viney et al., 2007) where the ipRGC population was randomly virally labeled with GFP, and less than the 26% we have reported previously based on single-cell neurobiotin filling (Schmidt et al., 2008). A recent study reported extremely low density of M3 cells of <10% though this study used immunostaining and the authors acknowledge that the bistratified arbors were not easily identifiable using their methods (Berson et al., 2010). Even if areas of the retina are not sampled by the dendritic arbors of a particular ipRGC subtype, collectively they still may function as efficient irradiance detectors. A relatively small number of ipRGCs is still expected to exert a large physiological impact as cell ablation studies have indicated that 17% percent of ipRGCs are able to sustain a significant proportion of NIF behaviors such as pupillary light reflex and circadian photoentrainment (Guler et al., 2008).
In this study, we have provided the first comprehensive assessment of the morphological and physiological properties of M3 ipRGCs. We find that though M3 cells show heterogeneity in the proportion of their dendritic arbors stratifying in either the On or Off sublaminas, these cells show remarkable homogeneity in their physiological properties. Many of M3 cell morphological features as well as many, though not all, of their functional features are similar to those of the previously described M2 cells (Schmidt and Kofuji, 2009). Whether these properties of M3 cells warrant their classification as a “true” ipRGC subtype depends on the criterion for classification used. Further analysis of whether M3 cells receive distinct patterns of synaptic inputs, receive differing amounts of inhibitory or excitatory synaptic inputs, or play distinct roles in mediating various NIF behaviors will be important questions for future research. Thus, we propose that since further research will be necessary to conclusively answer the question of whether M3 constitute a distinct ipRGC subtype, the M3 nomenclature continue to be used to differentiate these cells from other ipRGC subtypes.
Supplementary Material
(A–C) Example of typical light response of an M3 cell recorded in current clamp mode to a 5 s, full-field, bright white light stimulus first in control conditions (A), in the presence of a cocktail of synaptic blockers (B), and following washout (C). (D) Mean ± SE maximum depolarization evoked by 5 s full-field bright white light stimulus in control (black bar), in the presence of a synaptic blocker cocktail (white bar), and after washout (gray bar). * p < 0.05, ANOVA. Synaptic blocker cocktail included: 100 μM L-AP4, 10 μM DNQX (Tocris, Ellesville, MD), 50 μM picrotoxinin (Sigma), and 5 μM strychnine.
Acknowledgments
This work was supported in part by grants from the NIH R01EY012949, R21-EY018885, T32 EY0707133. We thank Darwin Hang for his technical assistance.
LITERATURE CITED
- Anadon R, Molist P, Rodriguez-Moldes I, Lopez JM, Quintela I, Cervino MC, Barja P, Gonzalez A. Distribution of choline acetyltransferase immunoreactivity in the brain of an elasmobranch, the lesser spotted dogfish (Scyliorhinus canicula) The Journal of comparative neurology. 2000;420(2):139–170. doi: 10.1002/(sici)1096-9861(20000501)420:2<139::aid-cne1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. The European journal of neuroscience. 2008;27(7):1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. The Journal of comparative neurology. 2010;518(13):2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science (New York, NY. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140(1):123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. The Journal of comparative neurology. 2009;517(2):226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67(1):49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23(18):7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3(9):e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3(6):e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. The Journal of comparative neurology. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science (New York, NY. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29(28):8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. The European journal of neuroscience. 2007;26(10):2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- Kang TH, Ryu YH, Kim IB, Oh GT, Chun MH. Comparative study of cholinergic cells in retinas of various mouse strains. Cell and tissue research. 2004;317(2):109–115. doi: 10.1007/s00441-004-0907-5. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature neuroscience. 2001;4(6):621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433(7027):745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29(2):476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci. 2010a doi: 10.1523/JNEUROSCI.3656-10.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. An isolated retinal preparation to record light responses from genetically labeled retinal ganglion cells. Journal of Visualized Experiments. 2010b doi: 10.3791/2367. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. Journal of neurophysiology. 2008;100(1):371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. Journal of anatomy. 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science (New York, NY. 1981;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. The Journal of comparative neurology. 2002;451(2):115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J Neurosci. 1994;14(11 Pt 1):6301–6316. doi: 10.1523/JNEUROSCI.14-11-06301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17(11):981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Vugler AA, Semo M, Joseph A, Jeffery G. Survival and remodeling of melanopsin cells during retinal dystrophy. Visual neuroscience. 2008;25(2):125–138. doi: 10.1017/S0952523808080309. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. The European journal of neuroscience. 2003;17(9):1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nature reviews. 2004;5(10):747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Whitney IE, Keeley PW, Raven MA, Reese BE. Spatial patterning of cholinergic amacrine cells in the mouse retina. The Journal of comparative neurology. 2008;508(1):1–12. doi: 10.1002/cne.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Vasudeva V, Vardi N, Sterling P, Freed MA. Different types of ganglion cell share a synaptic pattern. The Journal of comparative neurology. 2008;507(6):1871–1878. doi: 10.1002/cne.21644. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang AJ, Wu SM. Immunocytochemical analysis of GABA-positive and calretinin-positive horizontal cells in the tiger salamander retina. The Journal of comparative neurology. 2006;499(3):432–441. doi: 10.1002/cne.21116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–C) Example of typical light response of an M3 cell recorded in current clamp mode to a 5 s, full-field, bright white light stimulus first in control conditions (A), in the presence of a cocktail of synaptic blockers (B), and following washout (C). (D) Mean ± SE maximum depolarization evoked by 5 s full-field bright white light stimulus in control (black bar), in the presence of a synaptic blocker cocktail (white bar), and after washout (gray bar). * p < 0.05, ANOVA. Synaptic blocker cocktail included: 100 μM L-AP4, 10 μM DNQX (Tocris, Ellesville, MD), 50 μM picrotoxinin (Sigma), and 5 μM strychnine.