Abstract
Oxygen depletion (O2) and a decrease in pH are initial pathophysiological events in stroke development, but secondary mechanisms of ischemic cell death are incompletely understood. By patch-clamp recordings of brain slice preparations we show that TASK1 and TASK3 channels are inhibited by pH-reduction (42 ± 2%) and O2 deprivation (36 ± 5%) leading to membrane depolarization, increased input resistance and a switch in action potential generation under ischemic conditions. In vivo TASK blockade by anandamide significantly increased infarct volumes at 24h in mice undergoing 30 min of transient middle cerebral artery occlusion (tMCAO). Moreover, blockade of TASK channels accelerated stroke development. Supporting these findings TASK1−/− mice developed significantly larger infarct volumes after tMCAO accompanied by worse outcome in functional neurological tests compared to wild type mice. In conclusion, our data provide evidence for an important role of functional TASK channels in limiting tissue damage during cerebral ischemia.
Keywords: cerebral ischemia, transient middle cerebral artery occlusion, Two-pore domain potassium channels, TASK channels, thalamic neurons, TASK1−/− mice, electrophysiology
1) Introduction
Ischemic stroke represents a major health care problem associated with a high rate of permanent disability and death but the molecular mechanisms leading to neuronal cell death are still incompletely understood (Dirnagl et al., 1999). On the other hand mild hypoxia can induce neuroprotective signaling cascades within the brain that might counteract harmful pathways that induce neuronal death (Dirnagl et al., 2003; Hallenbeck, 2002; Plant et al., 2002).
Two-pore-domain K+ channels (K2P, KCNK) – often described as “background” channels – play a pivotal role in setting the resting membrane potential and modulating neuronal excitability (Goldstein et al., 2001). To date, fifteen mammalian K2P channels have been characterized. Based on typical properties of the cloned channels, sequence homologies, and functional features they are divided into six subfamilies: TWIK (tandem pore domain in a weakly inwardly rectifying K+ channel), THIK (tandem pore domain halothane-inhibited K+ channel), TREK, TASK, TALK (TWIK-related alkaline pH activated K+ channel), and TRESK (TWIK-related spinal cord K+ channel) (Goldstein et al., 2001; Lesage and Lazdunski, 2000; Patel and Lazdunski, 2004; Sano et al., 2003). The TWIK-related acid-sensitive K+ (TASK) channels form a subgroup of the K2P family consisting of TASK1 (KCNK3), TASK3 (KCNK9) and TASK5 (KCNK15), although only TASK1 and TASK3 represent functional members of that group (Lesage and Lazdunski, 2000; Patel and Honore, 2001). Both channels give rise to outward-rectifying K+ currents (IKSO) that are very similar in kinetics and voltage-dependence. They are regulated by extracellular acidification and O2 restriction, shift the mode of action potential generation in neurons, and therefore are interesting candidates in the context of cerebral ischemia (Heurteaux et al., 2004; Heurteaux et al., 2006; Plant et al., 2002). Based on their inhibition by acidification and hypoxia, both of which occur during artery occlusion, one might predict that TASK channels could contribute to depolarization and susceptibility to brain damage. On the other hand, since they are leak K+ channels, they could help to preserve a negative membrane potential and thus decrease excitability (i.e., be neuroprotective), as long as they can remain active during the insult (i.e., if changes in pH or O2 are not so extreme as to cause their inhibition). The availability of TASK1−/− mice now allows investigations on the role of K2P channels in disorders of the central nervous system (CNS) (Aller et al., 2005; Meuth et al., 2006a; Mulkey et al., 2007). These mice display a significant decrease of IKSO amplitude associated with a depolarized membrane potential in thalamic neurons (Meuth et al., 2006a).
In this study we addressed the role of TASK1 and TASK3 in stroke development. We show that TASK1 and TASK3 are inhibited by pH-reduction and O2 depletion in ex vivo CNS slice preparations. This leads to membrane depolarization and altered action potential generation under ischemic conditions. In vivo, blocking of TASK channels by anandamide accelerated neuronal injury after transient middle cerebral artery occlusion (tMCAO) in mice. In addition, TASK1−/− mice displayed larger infarcts after tMCAO. Our data indicate that, in spite of their inhibition by acidification and hypoxia, TASK1 channels largely serve a neuroprotective function in cerebral ischemia.
2) Materials and Methods
Slice preparation
Thalamic tissue slices including the dorsal lateral geniculate nucleus (dLGN), cortical slices or preparations from the inferior colliculus were prepared from 14 - 22 days old male C57BL6/J mice as described earlier (Meuth et al., 2003). In brief, coronal sections were cut on a vibratome (Vibratome®, Series 1000 Classic, St. Louis, USA) in an ice-chilled solution containing (mM): Sucrose, 200; PIPES, 20; KCl, 2.5; NaH2PO4, 1.25; MgSO4, 10; CaCl2, 0.5; dextrose, 10; pH 7.35 adjusted with NaOH. Prior to the recording procedure, slices were kept submerged in artificial cerebrospinal fluid (ACSF, mM): NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 24; MgSO4, 2; CaCl2, 2; dextrose, 10; pH adjusted to 7.35 by bubbling with a mixture of 95% O2 and 5% CO2.
Electrophysiology
Slices were transferred in a recording chamber and thalamic neurons of the dLGN were visualized with a microscope equipped with infrared-differential interference contrast optics (Dodt and Zieglgansberger, 1990). Whole-cell recording pipettes were fabricated from borosilicate glass (GT150T-10, Clark Electromedical Instruments, Pangbourne, UK; typical resistance 2-3 MΩ) and filled with an intracellular solution containing (in mM): K-gluconate, 95; K3-citrate, 20; NaCl, 10; HEPES, 10; MgCl2, 1; CaCl2, 0.5; BAPTA, 3; Mg-ATP, 3; Na-GTP, 0.5. The internal solution was set to a pH value of 7.25 using KOH and an osmolarity of 295 mOsm/kg. For current clamp recordings a pipette solution containing 5 mM EGTA and 0.5 mM CaCl2 was used. Slices were continuously superfused with a solution containing NaCl 120 mM, KCl 2.5 mM, NaH2PO4 1.25 mM, HEPES 30 mM, MgSO4 2 mM, CaCl2 2 mM and dextrose 10 mM. pH values were adjusted using HCl. Restriction of O2 was achieved by perfusion with an external solution that had been bubbled with nitrogen for at least 60 minutes prior to the recordings (Plant et al., 2002). Whole-cell patch-clamp recordings were measured from relay neurons of the dLGN with an EPC-10 amplifier (HEKA Elektronik, Lamprecht, Germany) and digitially analyzed using Pulse software (HEKA Elektronik). Anandamide was obtained from Calbiochem (Schwalbach/Ts., Germany) and was dissolved in 80% NaCl, 10% ethanol, 10% polyethylene glycol.
Anandamide is a semi-selective inhibitor of TASK channels (Maingret et al. 2001, Meuth et al., 2006) and other molecular targets of anandamide like CB1, CB2 receptors and TRP1 channels have been described. In a subset of experiments the cannabinoid receptor 1 (CB1) inhibitor AM251 (1 μM, Tocris, Ellisville, USA), CB2 receptor inhibitor AM630 (1 μM, Tocris, Ellisville, USA) and the transient receptor potential vanilloid 1 (TRPV1) inhibitor capsazepine, 100 nM/1 μM, Sigma, Deisenhofen, Germany) were used in the absence and in the presence of anandamide. The solvent concentrations in the final recording solution did not exceed 1‰. Application of the solvent alone (1‰) had no effect on the recorded current. In one set of experiments we used cultured hippocampal neurons for proof of the concept. ZD7288 is a selective inhibitor of Ih an inwardly mixed cationic conductance through HCN channels activated by membrane hyperpolarization. Throughout the experiments, ZD7288 was present in order to eliminate possible contamination by pH-sensitive Ih currents (Munsch and Pape, 1999; Meuth et al., 2003).
All cells had a resting membrane potential negative to −65 mV, the access resistance was in the range of 5-15 MΩ and series resistance compensation of more than 40% was routinely used. A liquid junction potential of 8 ± 2 mV (n = 10) was measured and taken into account when analyzing the data.
Real-time PCR
CNS tissue samples (lateral geniculate nucleus (LGN), cortex and hippocampus), postnatal day 20) were homogenized and total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) following a standard chloroform-isopropanol RNA isolation protocol. cDNA was reversely transcribed in a 100 μl reaction from 500 ng of total RNA. For real-time PCR, pre-developed TaqMan gene expression assays (FAM-labeled Mm00807036_m1 primer for TASK1 and Rn00755967_m1 primer for TASK3, and VIC-labeled 18S rRNA kit as internal control), together with TaqMan universal PCR master mix were used according to the manufacturer's instructions (all from Applied Biosystems, Foster City, CA). A cDNA amount corresponding to 5 ng of total RNA was added to TASK1 and TASK3 samples. In one set of experiments we analyzed TASK1 channel expression in the (total) ischemic and contralateral hemispheres of wild type mice 0, 6, 24 and 48 hours after tMCAO. All samples, including blanks, were studied in triplicates. Real-time PCR cycling was performed in an ABI Prism 7700 sequence detector (Applied Biosystems) under the following thermal conditions: 50 °C for 2 min, 95 °C for 15 min, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. For the LGN samples, TASK1 and TASK3 mRNA expression was assessed by determination of the real-time PCR crossing points (CT) and subtraction of the respective 18S rRNA CT values (ΔCT-method). Data were statistically analyzed in Kruskal-Wallis tests with post-hoc Dunn's Multiple Comparison Tests using Prism 4 (GraphPad Software, San Diego, CA).
Histology and immunohistochemistry
Formalin-fixed brains embedded in paraffin from WT and anandamide treated mice at day 5 after tMCAO (n=5/group) were cut into 4-μm thick sections 0.5 mm anterior from bregma (representing the ischemic territory of the middle cerebral artery) and stained with hematoxylin and eosin.
TASK1 channel staining was performed using rabbit anti-TASK1 antibody (Sigma, 1:200) on 20 μm coronal cryosections of mouse brain tissue that had been cut at the level of the ischemic region (0.5 mm anterior from bregma), thaw-mounted onto slide glasses, air dried, and fixed in 4% PFA/PBS for 10 min. Afterwards slices were incubated with secondary antibody (Cy3-conjugated rabbit anti-goat, 1:100, Dianova). For negative controls, occlusion of the primary antibody from the staining procedure was routinely performed with no positive immunological signal detected (not shown).
Induction of cerebral ischemia
Animal experiments were approved by governmental agencies for animal research and conducted according to the recommendations for research in mechanism-driven basic stroke studies. Focal cerebral ischemia was induced in 6-8-weeks old male C57BL/6J and TASK1−/− mice (Mulkey et al., 2007) weighing 20-25g by transient middle cerebral artery occlusion (tMCAO) as described previously (Clark et al., 1997; Kleinschnitz et al., 2007; Kleinschnitz et al., 2006). Briefly, mice were anesthetized with 2.5% enflurane (Abbott, Wiesbaden, Germany) in a 70% N2O/30% O2 mixture. The TASK1 or TASK3 activity under enflurane showed no differences compared to the activity following intraperitoneal (i.p.) injections of sodium pentobarbital (50 mg/kg) (not shown). Core body temperature was maintained at 37°C throughout surgery using a feedback-controlled heating device. Following a midline skin incision in the neck, the proximal common carotid artery and the external carotid artery were ligated and a standardized silicon rubber-coated 6.0 nylon monofilament (6021; Doccol Corp., CA, USA) was inserted and advanced via the right internal carotid artery to occlude the origin of the right MCA. The intraluminal suture was left in situ for 30 minutes or 1 hour, respectively. Then animals were re-anesthetized and the occluding monofilament was withdrawn to allow reperfusion. After recovery from anesthesia and again after 6 hours, 24 hours or 5 days neurological deficits were scored by two blinded investigators and quantified according to Bederson (Bederson et al., 1986): 0, no deficit; 1, forelimb flexion; 2, as for 1, plus decreased resistance to lateral push; 3, unidirectional circling; 4, longitudinal spinning; 5, no movement. For the gript test, the mouse was placed midway on a string between two supports and rated as follows: 0, falls off; 1, hangs onto string by one or both forepaws; 2, as for 1, and attempts to climb onto string; 3, hangs onto string by one or both forepaws plus one or both hindpaws; 4, hangs onto string by fore- and hindpaws plus tail wrapped around string; 5, escape (to the supports).
Immediately before the induction of tMCAO animals received the TASK channel blocker anandamide (1mg/kg or 10mg/kg i. p., respectively) or carrier solution (80% NaCl, 10% ethanol, 10% polyethylene glycol, Sigma, Deisenhofen, Germany). In a subset of animals (n=10) the cannabinoid receptor 1 (CB1) inhibitor AM251, (3mg/kg, Tocris, Ellisville, USA), CB2 receptor inhibitor AM630 (3mg/kg, Tocris, Ellisville, USA) and the transient receptor potential vanilloid 1 (TRPV1) inhibitor capsazepine (10mg/kg, Sigma, Deisenhofen, Germany) were applied intraperitoneally immediately before the induction of MCAO to assure specific TASK channel blockade by anandamide. All substances were dissolved in the carrier solution described for anandamide. Single application of either inhibitor had no effect on infarct size (not shown). Similarly, injection of anandamide did not induce cerebral infarctions in healthy animals.
Laser doppler flowmetry (Moor Instruments, Axminster, United Kingdom) was used to monitor cerebral blood flow (Connolly et al., 1996) in either treatment group or sham-treated animals (n=4 / group) before surgery (baseline), immediately after MCA occlusion, and 5 minutes after removal of the occluding monofilament (reperfusion). Cerebral perfusion did not differ between the groups at any time point (data not shown). Arterial blood gases were analyzed (Rapid lab, Bayer Health care systems, Germany) during the operation in 4 anandamide-treated and 4 vehicle-treated animals after puncturing the right femoral artery and also showed no significant differences (data not shown).
Long-term survival of WT and anandamide treated mice after tMCAO was investigated over a period of 5 days (n=15/group).
Determination of Infarct size
Mice were sacrificed 6 hours, 24 hours or 5 days after tMCAO. Brains were quickly removed and cut in 2-mm thick coronal sections using a mouse brain slice matrix. The slices were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich, St. Louis, MO) in PBS to visualize the infarctions. Planimetric measurements (ImageJ software, National Institutes of Health, Bethesda, MD) blinded to the treatment groups were used to calculate lesion volumes, which were corrected for brain edema as described (Ginsberg et al., 2003; Swanson et al., 1990).
Statistical analysis
Electrophysiological data and results from the animal experiments were analyzed by a modified student's t test for small samples (Dixon and Massey, 1969) or by a Bonferroni-corrected One-way ANOVA in case of multiple comparisons using PrismGraph 4.0 software (GraphPad Software, San Diego, CA) or Origin® (Microcal). P-values < 0.05 were considered statistically significant. For comparison of survival curves (Fig. 7C) the Logrank test was used.
Fig. 7. Long-term effects on stroke outcome after TASK1 blockade.
(A) Mean brain infarct volumes calculated from TTC stained images of control animals (con) and anandamide-treated mice (anand, 10mg/kg) 5 days after 30 min MCAO (n = 7/group). (B) Mean Bederson score from the animals described in A. (C) Survival rates of control and anandamide-treated mice (n=15/group) until day 5 after tMCAO (D) Hematoxylin and eosin stained sections of corresponding territories in the ischemic hemispheres of control and anandamide-treated mice. Note, that infarctions are restricted to the basal ganglia in naive mice but consistently include the neocortex in anandamide-treated animals. Bar represents 200 μm. **p<0.01; Bonferroni's post hoc test (infarct volumes and functional scores), *p<0.05; Logrank test (survival rates) compared to untreated mice.
3) Results
The TASK-related standing outward current of thalamocortical relay neurons in the dLGN is modulated under ischemic conditions
Tissue acidosis and hypoxia are pathogenetic hallmarks of ischemic stroke and occur within minutes after cessation of cerebral blood flow. We therefore analyzed the effect of acidification or O2 restriction on the electrophysiological properties of TASK channels expressed on thalamocortical relay neurons of the dLGN in vitro. At first we studied the expression level of TASK1 and TASK3 channels in brain slices of the recorded animals indicating a comparable expression level for TASK1 and TASK3 mRNA transcripts (Fig. 1A). This implies that the recorded IKSO might be composed of both TASK1 and TASK3 channels as reported previously for thalamic neurons (Meuth et al., 2006a; Meuth et al., 2003; Meuth et al., 2006b). Throughout the experiments, ZD 7288 was present in order to eliminate possible contamination by pH-sensitive Ih channels (Meuth et al., 2006b; Munsch and Pape, 1999). Current-voltage relationships (I/V) were investigated by ramping the membrane potential from −28 mV to −138 mV over 800 ms (Fig. 1B, inset; (Millar et al., 2000; Watkins and Mathie, 1996)). Current traces indicate the outward rectifying TASK channel family and – at more negative potentials – a contribution of inwardly rectifying K+ channels (Fig. 1B) with a standing potassium outward current (IKSO) value of 295 ± 35 pA at −30mV under control conditions. Lowering the extracellular pH from 7.2 (control) to 6.4 significantly reduced IKSO by 42 ± 2% in comparison to control levels (p = 6×10−5; Fig. 1B, upper panel and Fig. 1C). pH-sensitive ramp currents were calculated by numerical subtraction of currents recorded under pH 6.4 from control currents (Meuth et al., 2003). I/V relationship was typical of TASK channels with a strong outward rectification and a reversal potential close to the expected potassium equilibrium potential (EK = −104 mV, data not shown). The current amplitude plotted over time showed a typical U-shape with a steady-state current amplitude under control conditions, a strong current reduction after setting the external solution to pH 6.4 and reversibility following wash out (Fig. 1D, upper panel, grey circles). It is important to note that the observed current reduction was not due to unspecific run-down effects since the current amplitude under control conditions was nearly unchanged over time (Fig. 1D, upper panel, black squares).
Fig. 1. Effects of acidosis and hypoxia on TASK-related currents in dLGN neurons of the thalamus.
(A) RT-PCR analysis of TASK1 and 3 channel mRNA expression in C57Bl6/J mice (postnatal day 20) as used for the electrophysiological recordings. (B) Representative currents evoked by ramping the membrane potential from −28 mV to −138 mV over 800 ms (see inset) under control conditions (con; black traces), during extracellular acidification (pH; grey trace, upper panel), oxygen depletion (O2; grey trace, middle panel) or the combination of both (pH/O2, grey trace, lower panel) in dLGN neurons. (C) Mean TASK current reduction (n = 5/group) at −28 mV induced by acidification (pH), oxygen depletion (O2) or both (pH/O2). (D) Amplitude of the normalized net outward current plotted over time in one relay neuron recorded under voltage-clamp conditions at –28 mV. Note that the stable current amplitude under control conditions (upper panel, black squares) excludes unspecific run down effects. Extracellular acidification (pH 6.4; upper panel, grey circles) or oxygen depletion (lower panel, black squares) strongly reduced the net outward current. This effect was also observed by combining both conditions (lower panel, grey circles) and was reversible after wash out. Periods of acidosis and hypoxia are indicated by the grey or black horizontal line, respectively.
** p < 0.01 compared to controls
Under hypoxic conditions IKSO amplitudes were significantly reduced by 36 ± 5% in comparison to controls (Fig. 1B, middle panel and Fig. 1C, p = 0.003). The O2-sensitive current obtained by graphical subtraction revealed outward rectification and a reversal potential of −102 ± 3 mV (not shown) which was close to that expected for K+ currents (EK = −104 mV). The outward current amplitude recorded over time was stable under control conditions but clearly reduced following O2 depletion. This effect was fully reversible (Fig. 1D, lower panel, black squares). Combination of extracellular acidification and O2 depletion reduced the IKSO by 56 ± 6% (Fig. 1B, Fig. 1C, p = 1.2×10−5) and the sensitive current component again displayed features indicative of TASK channels. As in the case of hypoxia or acidification alone, the current amplitude recorded over time under both conditions was U-shaped (Fig. 1D, lower panel, grey circles).
Taken together these findings show that ischemic conditions (pH lowering and/or hypoxia) subsequently and reversibly reduce IKSO - a current mainly mediated by TASK channels (Meuth et al., 2003) - in thalamic slice preparations. Despite thalamic neurons represent an ideal model to study these effects due to their predominant TASK1 and TASK3 channel expression we further proofed our concept in other central nervous system neurons. We found anandamide-sensitive outward potassium currents in cortical, hippocampal and inferior colliculus neurons (see supplementary Fig. 1) illustrating the general value of the observed effects.
Acidosis and hypoxia modulate resting membrane potential, input resistance and interburst frequency of thalamic neurons in dLGN in a TASK-dependent manner
Next we investigated the effect of extracellular acidification on the resting membrane potential (Vrest) of thalamic relay neurons. In accordance with earlier reports (Meuth et al., 2006b) Vrest under control conditions was significantly more negative (−72 ± 1 mV) than in pH 6.4 adjusted (−64 ± 4 mV, p = 0.003) or O2 restricted extracellular solutions (−65 ± 1 mV; p= 0.002; Fig. 2A) or both (pH 6.4/O2; −58 ± 2 mV, p= 2×10−4). In all groups the depolarizing pH-sensitive current through HCN channels (Ih) was blocked by ZD 7288 to minimize contaminations of TASK currents as described previously (BoSmith et al., 1993; Meuth et al., 2006b). ZD 7288-induced membrane depolarization was counterbalanced by setting Vrest to control values. Consistent with these findings acidification and hypoxia significantly increased the neuronal input resistance (Fig. 2B). These were calculated by approximating voltage ramp evoked currents to straight lines for 10 mV positive and negative to the cell resting potential, respectively (Plant et al., 2002). Input resistances were significantly increased under extracellular acidosis (pH 6.4, 566 ± 29 MΩ, p = 3.8×10−4), hypoxia (562 ± 37 MΩ, p = 5.7×10−4) or combined conditions (pH/O2, 624 ± 28 MΩ, p = 7×10−5) compared to controls (266 ± 36 MΩ; Fig. 2B).
Fig. 2. Functional impact of TASK current modulation on the resting membrane potential, the input resistance and the interburst frequency of thalamic relay neurons.
(A) Mean values (n = 5/group) of the resting membrane potentials under different recording conditions (black square: controls; open circle: pH 6.4; grey triangle: O2 deprivation; black triangle: pH 6.4/O2 deprivation; open diamond: anandamide 30 μM; black diamond: anandamide/O2 deprivation. (B). The input resistance under different recording conditions (con: black column, n = 7; pH 6.4: light grey column, n = 5; O2: dark grey column, n = 5; pH/O2: white column, n = 5) was calculated from the slope of current ramps (approximated to a straight line) evoked between 10 mV positive to and 10 mV negative to the resting membrane potential. (C) Effects of hypoxia (O2, upper panel) and acidosis (pH 6.4, lower panel) on the firing mode of thalamocortical relay neurons. Cells were recorded under whole-cell current-clamp conditions and stimulated using a 100 - 200 pA depolarizing current pulse (300 ms duration, see inset), which induced robust burst firing (left panel). Lowering of extracellular pH values or O2 restriction depolarized the membrane potential and generated tonic trains of action potentials (right panel) in response to the same depolarizing current pulse. (D) Instantaneous firing frequencies under different recording conditions (con: black column, n = 10; pH 6.4: dark grey column, n = 8; O2: light grey column, n = 5). The firing frequency was determined from the first two action potentials elicited by the depolarizing pulse.
** p < 0.01 compared to controls
The functional impact of extracellular acidification and hypoxia on the firing patterns of thalamic relay neurons was investigated using current-clamp techniques. Recordings were obtained by starting from the resting membrane potential (−72 ± 1 mV, n = 10) using direct current (DC) injection if necessary. Under control conditions depolarizing current pulses (Fig. 2C, inset) induced typical thalamic burst firing with one to five action potentials riding on top of a low threshold Ca2+ Spike (Fig. 2C, left panel). A frequency analysis including the first two action potentials (instantaneous firing frequency) revealed an intra-burst frequency of 130 ± 5 Hz. Hypoxia and acidosis depolarized the membrane potential (to −65 ± 1 mV or −64 ± 4 mV, respectively) and switched the firing mode to a tonic pattern (Fig. 2C, upper and lower right panel) with an instantaneous firing frequency of 22 ± 6 Hz or 28 ± 4 Hz, respectively (Fig. 2D; p = 0.001 or p = 0.002).
Taken together, these findings show that ischemic conditions (decreased extracellular pH, hypoxia) immediately induce hyperactivity of thalamocortical neurons as indicated by (i) depolarized resting membrane potentials, (ii) increased cell input resistance and (iii) promoting tonic firing behavior.
The endogenous cannabinoid anandamide inhibits IKSO in the presence and absence of CB1, CB2 and TRPV1 antagonists indicating significant effects on TASK channels
Next, the semi-selective TASK channel inhibitor anandamide (arachidonyl-ethanolamide, 50μM) was applied under current-clamp and voltage-clamp conditions (Maingret et al., 2001; Meuth et al., 2006a; Veale et al., 2007). Anandamide significantly depolarized the membrane potential to −62 ± 4 mV (p = 0.004; Fig. 2A). This was accompanied by an anandamide induced reduction of IKSO amplitude at −28 mV by 33 ± 4% (p = 0.001; Fig. 3A, left inset and Fig. 3B) indicating that at least one third of IKSO is carried by TASK channels. Depolarization was synergistically and significantly enhanced by combining anandamide and hypoxia (−55 ± 5 mV; p = 0.002; Fig. 2A) indicating an additional O2 effect on Vrest in thalamic neurons of dLGN. Since anandamide is a semi-selective TASK channel blocker but also exerts effects on CB1, CB2 and TRPV1 receptors we further analyzed anandamide actions in the presence of the CB1 receptor blocker AM251 (1 μM) (Chambers et al., 2004; Chen et al., 2006; Hill et al., 2006), CB2 receptor inhibitor AM630 (1 μM) (Moezi et al., 2006; Werner and Koch, 2003) and the TRPV1 antagonist capsazepine (10 μM) (Holt et al., 2005; Jakab et al., 2005). While application of the blockers alone had no significant effect on IKSO (6.3 ± 2.8%; p = 0.07; Fig. 3A, right panel and Fig. 3B) co-application of anandamide still reduced the current amplitude by 28 ± 3.1% (p = 0.003; Fig. 3A and Fig. 3B).
Fig 3. Effects of anandamide on IKSO in the presence and absence of CB1, CB2 and TRPV1 inhibitors in thalamic relay neurons.
(A) Representative currents evoked by ramping the membrane potential from −28 mV to −138 mV over 800 ms under control conditions (con; black traces), after application of anandamide (anand, left inset) and in the presence of CB1, CB2 and TRPV1 inhibitors (blocker, right inset). Co-application of anandamide and CB1, CB2 and TRPV1 inhibitors results in a significant reduction of IKSO (anand + blocker). Scale bars in the insets represent 500 pA and 200 ms. (C) Mean bar graph representation of TASK current reduction under control conditions (black column), after application of the blockers (blocker; CB1, CB2, TRPV1; light grey column), after application of anandamide (anand, dark grey column) and after co-application of anandamide and CB1, CB2 and TRPV1 inhibitors (anand + blocker; white column).
** p < 0.01 compared to controls, ns – not significant
TASK1 channel expression following cerebral ischemia
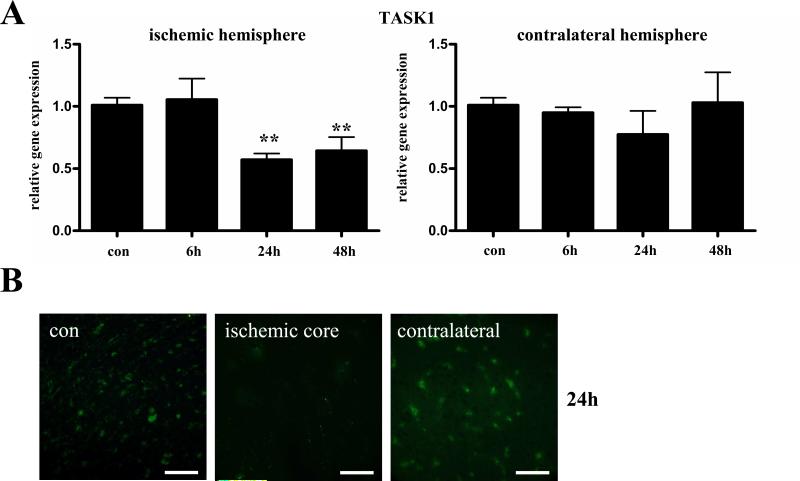
Next we used Real-Time RT-PCR to investigate the time course of TASK1 mRNA expression in the (total) ischemic and contralateral hemispheres of wild type mice 0, 6, 24 and 48 hours after tMCAO (Fig. 4A). While no difference in TASK1 channel expression was observed in the ischemic hemisphere 6h after MCAO a significant downregulation of TASK1 mRNA was found 24h and 48h after tMCAO compared to controls. In the contralateral hemisphere stable TASK1 mRNA expression was found over the investigated time course (Fig. 4A). In line with our PCR data and the findings described in the literature (Meuth et al., 2003; Bean, 2007) TASK1 displayed a predominantly neuronal expression pattern in the contralateral hemisphere at day 1 after tMCAO as revealed by immunohistochemistry (Fig. 4B). However, immunohistochemistry was too insensitive to clearly differentiate TASK1 expression in the infarct core and the penumbra since 24 h after tMCAO most of the TASK1 signal had vanished in the ischemic core region probably due to massive neuronal cell damage (Fig. 4B).
Fig. 4. TASK1 channel expression following tMCAO.
(A) Real-Time RT-PCR data indicate the time course of TASK1 expression in the (total) ischemic (left panel) and contralateral hemispheres (right panel) of wild type mice 0, 6, 24 and 48 hours after tMCAO. (B) Immunohistochemical staining revealed neuronal TASK1 channel expression under control conditions (con, left panel) and in the contralateral hemisphere (contralateral, right panel) at day 1 after tMCAO. TASK1 immunoreactivity was nearly absent in the ischemic core after 24 h probably due to significant cell damage (infarct core, middle panel). Scale bar represents 200 μm.
Blockade of TASK channels in vivo accelerates development and increases the definite extent of ischemic brain damage
We next investigated the functional role of TASK channels in vivo during experimental cerebral ischemia. In a first set of experiments we induced mild cerebral ischemia by 30 min of tMCAO and subsequent reperfusion for 23.5 hours. This leads to small infarcts at 24 hours after tMCAO which are restricted to the basal ganglia in sham-treated/normal mice (Fig. 5A) and do not affect the neocortex as revealed by TTC staining. In contrast, animals treated with anandamide for semi-selective TASK channel inhibition (10 mg/kg) developed larger infarcts involving the neocortex (90.3 ± 17 mm3 versus 16.1 ± 11.4 mm3; p<0.0001; Fig. 5 A, B). To further prove specificity of anandamide actions on TASK channels we co-applied the CB1 receptor blocker AM251 (3mg/kg), CB2 receptor inhibitor AM630 (3mg/kg) and the TRPV1 antagonist capsazepine (10mg/kg) with anandamide. All these inhibitors failed to prevent anandamide actions on stroke severity (Fig. 5A, right column; Fig. 5B, right column) underlining the specific effect of anadamide on TASK channels.
Fig. 5. Infarct volumes and functional outcomes 24 h after 30 min MCA occlusion (A, B) and 6 h after 60 min MCA occlusion (C-E).
(A) Representative TTC-stained images of three corresponding coronal sections of control animals (con), anandamide treated (anand) mice (10 mg/kg body weight) and animals treated with anandamide in the presence of CB1 receptor inhibitor AM251, CB2 receptor blocker AM630 and TRPV1 antagonist capsazepine 24 h after induction of cerebral ischemia (30 min MCAO) (anand+blocker). (B) Mean brain infarct volumes calculated from (A) (n = 7/group). (C) Representative TTC-stained images of three corresponding coronal sections of control animals (con) and anandamide treated (anand) mice (10 mg/kg body weight) 6 h after induction of cerebral ischemia (60 min MCAO). (D) Mean brain infarct volumes calculated from (C) (n=7/group). (E) Mean Bederson score from the animals depicted in (C) (n=7/group).
** p < 0,001; *** p < 0,0001 compared to controls; Bonferroni corrected One-way ANOVA.
In a second set of experiments we analyzed infarct development at an earlier time point at 6h after more severe ischemia induced by 60 min of tMCAO (Fig. 5C, D). While normal mice developed very small infarctions (11.0 ± 1.7 mm3) restricted to the basal ganglia at this early time point, anandamide treated animals already suffered from large strokes involving most parts of the temporoparietal cortex as revealed by TTC staining (53.2 ± 14.0 mm3, p=0.0066; Fig. 5C, D). This indicates that neuronal damage developed more rapidly after in vivo blockade of TASK channels. In confirmation of increased tissue damage, neurological outcome in anandamide treated mice was significantly worse (Bederson score assessing global neurological function of 3.7 ± 0.8 versus 2.6 ± 0.4; p=0.0273, Fig. 5E). In a third set of experiments stroke volumes were assessed at 24h after 60 min tMCAO. According to the more advanced stroke development at 6 hrs after 60 min tMCAO, final infarct volumes at 24 hours were also significantly increased in anandamide treated compared to control mice (85 ± 12 mm3 versus 50 ± 22 mm3, respectively; p=0.034; Fig. 6A, B). This increase in infarct size was again functionally relevant as the Bederson score was significantly higher after high-dose anandamide treatment (4.0 ± 0.35 versus 3.3 ± 0.5, p=0.047; data not shown). In contrast, a lower dose of anandamide (1 mg/kg) did not change infarct size or neurological outcome after 24h (Fig. 6A, B).
Fig. 6. Infarct volumes 24 h after 60 min MCA occlusion.
(A) Representative TTC-stained images of three corresponding coronal sections of control animals (con) and anandamide treated (anand) mice (1 or 10 mg/kg body weight, respectively). (B) Mean brain infarct volumes calculated from (A) (n=7/group).
* p < 0.05 compared to controls; Bonferroni corrected One-way ANOVA.
To investigate the impact of TASK channel modulation for disease end points we also performed long-term experiments after tMCAO. In line with our findings after 6 hours and 24 hours (Fig. 7) TTC staining 5 days after 30 minutes tMCAO revealed significantly larger infarct volumes in anandamide-treated animals compared to controls (56.76 ± 9.95 mm3 and 31.75 ± 5.44 mm3; p = 0.007; Fig. 7A). The increase in infarct size was of functional relevance as the Bederson score at that time point was significantly higher in anandamide-treated compared to control animals (3.3 ± 0.4 versus 2.1 ± 0.1, p=0.0264; Fig. 7B). Importantly, this was paralleled by decreased long-term survival in these animals: While 3 out of 15 untreated mice (20%) died until day 5 after infarct induction, 60% (9 out of 15) of anandamide-treated animals died over the same period (p = 0.041; Fig. 7C). Consistent with the TTC stains and the functional data, histological analysis at day 5 after tMCAO revealed ischemic infarctions which were mostly restricted to the basal ganglia in control animals, while infarct areas in anandamide-treated animals regularly involved the basal ganglia and the neocortex (Fig. 7D).
Taken together, these findings indicate that TASK channels critically contribute to the limitation of tissue damage at early and advanced stages after transient cerebral ischemia.
TASK1−/− mice develop larger infarcts after tMCAO
To further prove the specific neuroprotective role of functional TASK1 in stroke development TASK1−/− mice underwent 60 min of tMCAO and stroke volumes were determined 24h later. Control animals showed infarct volumes of 55.6 ± 8.7 mm3 (Fig. 8A and B). As expected from our pharmacological experiments TASK1−/− mice displayed significantly enlarged ischemic areas (89.3 ± 10.2 mm3, p = 0.0009; Fig. 8A and B). This increase in infarct size was functionally relevant as indicated by a higher Bederson score (3.75 ± 0.76 versus 2.94 ± 0.3, p = 0.001; Fig. 8C) and worse performance in the grip test (TASK1−/−: 2.25 ± 0.53 versus wt: 3.28 ± 0.71, p = 0.00067; Fig. 8D). Application of anandamide in TASK1−/− mice did not result in larger infarct volumes compared to TASK1−/− mice receiving PBS (n = 3; data not shown).
Fig. 8. Infarct volumes 24 h after 60 min MCA occlusion in wild type and TASK1−/− mice.
(A) Representative TTC-stained images of three corresponding coronal sections of wild type animals (WT) and TASK1−/− mice. (B) Mean brain infarct volumes calculated from (A) (WT group: n=10; TASK1−/− mice: n = 8). (C) Mean Bederson score and (D) grip test from the animals shown in (B).
** p < 0,001; compared to controls; Bonferroni corrected One-way ANOVA.
4) Discussion
Our findings demonstrate that TASK channels are critically involved in the control of neuronal excitability and contribute to neuroprotection during acute cerebral ischemia. TASK channel inhibition by administration of anandamide – an endogenous cannabinoid acting on TASK channels – resulted in accelerated stroke formation, larger infarcts at 24h after 1h of transient middle cerebral artery occlusion and worse neurological outcome. Moreover, even mild ischemia of 0.5 h MCAO leading to small infarcts restricted to basal ganglia in control mice, caused large hemispheric infarcts in anandamide-treated animals. Finally, long term outcome 5 days after tMCAO was also significantly influenced by TASK channel modulation. To assure specificity and minimize actions of the semi-selective inhibitor anandamide aside of TASK channel inhibition we co-applied the CB1 inhibitor AM251, the CB2 receptor blocker AM630 and the TRPV1 antagonist capsazepine. In the presence of these inhibitors anandamide treatment again resulted in significantly larger brain infarcts indicating that anandamide predominantly altered TASK channel function. This notion could be substantiated using TASK1−/− mice (Mulkey et al., 2007) which also developed larger infarcts after tMCAO. In line with our observations lack of another K2P channel TREK1 increased mortality in a model of global cerebral ischemia (Heurteaux et al., 2004). After transient spinal cord ischemia, TREK1−/− mice developed severe hind limb paralysis at the onset of reperfusion, while wild type mice recovered without any neurological deficits (Buckler and Honore, 2005; Franks and Honore, 2004; Heurteaux et al., 2004). Importantly, animals deficient for another K2P channel TRAAK showed no increased sensitivity to ischemia. This indicates that the conspicuous vulnerability of TREK−/− and TASK1−/− mice is not simply an effect attributable to lack of any member of the K+ channel family (Heurteaux et al., 2004).
It is widely accepted that central nervous system neurons have developed a broad array of immediate and long term homeostatic protective mechanisms “on demand” to suppress hyperexcitability induced by brief hypoxic and/or ischemic episodes. Other potassium channels such as KATP channels (Ballanyi, 2004), Ca2+-activated BK channels (Runden-Pran et al., 2002) or the voltage-gated potassium channel 2.1 (Misonou et al., 2005) could already be linked to the depression of neuronal excitability during hypoxia/ischemia. We now add evidence that TASK1 a member of the two-pore domain potassium channel family similarly plays an important role in intrinsic defence mechanisms against ischemic brain injury. Thereby the hyperpolarizing effect of the functional leak potassium channel probably represents one major mechanism especially at early stages after tMCAO. This is corroborated by the findings that protection through TASK channels is accentuated in experimental settings with shorter ischemia induction (30 min) or earlier evaluation of stroke volumes after tMCAO (6h). Contrastingly, activation of sodium channels causes detrimental effects after cerebral ischemia (Benveniste and Dingledine, 2005; Xiong et al., 2004). The detailed mechanisms by which TASK1 channels mediate neuroprotection in ischemic stroke need to be further elucidated and, besides their hyperpolarizing effect, might include altered excitotoxicity and peri-infarct depolarizations, as well as less inflammation and apoptosis (Dirnagl, 1999).
Although TASK channels are widely expressed throughout the central nervous system (CNS) as demonstrated by our RT-PCR data and immunohistochemical findings as well as other groups (Karschin et al., 2001) and are key components to adjust the resting membrane potential in a number of different cell types (Bean, 2007; Meuth et al., 2006b; Talley et al., 2003) we decided to use thalamic slice preparations for the following reasons: I.) In thalamic neurons IKSO is dominated by TASK1 and TASK3 channels (Meuth et al., 2006a; Meuth et al., 2003; Meuth et al., 2006b) while other CNS neurons express a diversity of K2P channels (e.g. cerebellar granule cells (Aller et al., 2005)) II.) Action potential generation of thalamic neurons (burst versus tonic activity) is highly dependent on the resting membrane potential and influences on particular ion channels can directly be linked to the functional impact. However, recordings from other central nervous system neurons (cortex, hippocampus, inferior colliculus) also showed anandamide-sensitive potassium outward currents supporting the general value of the observed effects. Mimicking pathological hallmarks of cerebral ischemia, namely decrease in extracellular pH and hypoxia caused inhibition of TASK channels in thalamic relay neurons followed by membrane depolarization, increased input resistance and a switch of action potential generation from burst activity to tonic action potential generation (Meuth et al., 2003). In thalamocortical (TC) relay neurons (dLGN) and other central nervous system neurons TASK1 and TASK3 channels are detectable at substantial levels and generate whole-cell outward rectifying K+ currents (IKSO) that are very similar concerning kinetic features and voltage dependence (Bayliss et al., 2003; Brickley et al., 2001; Meuth et al., 2003). These channels are key components in shaping neuronal excitability. Characteristic features of the TASK channel mediated currents are the outward rectification, reversal near the K+ equilibrium potential and a characteristic pharmacological profile, e.g. current inhibition by pH-lowering, muscarine or anandamide vs. current increase by inhalational anesthetics (Goldstein et al., 2001; Meuth et al., 2006a). Our current data confirm and extend these findings by showing the modulation of TASK currents in the presence of decreased pH and O2 restriction in vital brain slices. However, changes in extracellular pH can influence a variety of different conductances and receptors and vice versa. Therefore, we cannot exclude that additional channels might have contributed to the observed pH effects. Anandamide - originally described as an endogenous cannabinoid - significantly reduced IKSO in thalamic brain slice preparations. It is known that anandamide also acts on CB1, CB2 and TRPV1. However, co-application of the appropriate inhibitors had no significant influence on IKSO reduction by anandamide indicating that the observed effect was specifically related to TASK channel inhibition. The notion that anandamide also acts on T-type calcium channels is important (Chemin et al., 2007; Lambert et al., 2006), since it was recently published that inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage (Nikonenko et al., 2005). In the current study we found no evidence for a contribution of T-type channels to the described effects. In vitro recordings focusing on IKSO can mainly be linked to TASK channels, because T-type calcium channels do not contribute to IKSO. Since a concomitant blockade of T-type calcium channels in anandamide treated animals could not be formally excluded, we used TASK1−/− mice. Accelerated stroke development in TASK1−/− mice provides the direct link between TASK channel function and neuroprotection under ischemic conditions.
In summary, this work provides evidence for a major involvement of TASK channels (TASK1) in the control of neuronal excitability and endogenous defence against hypoxia and ischemia. Our in vivo experiments indicate that inhibition or lack of TASK1 channels accelerates and worsens ischemic brain damage. Stabilization of TASK channel function appears as a novel therapeutic target to protect CNS tissue from ischemic damage.
Supplementary Material
Supplementary Fig 1: Expression of TASK1 and 3 channels and effects of anandamide on IKSO in cortical, hippocampal and colliculus inferior neurons. (A) Expression of TASK1 and TASK3 channels in the dorsal lateral geniculate nucleus, cortex and hippocampus. (B) Currents from cortical neurons evoked by ramp protocols (−28 mV to −120 mV over 800 ms, left panel). Application of anandamide (30 μM) resulted in a current reduction of 32 ± 2.9% (n=3, p<0.01, inset). Anandamide-sensitive currents (after substraction) displayed features indicative of TASK channels: outward rectification and a reversal potential of ~ −104mV (close to the K+ equilibrium potential; right panel). (C) Currents recorded from cultured hippocampal and (D) from colliculus inferior neurons also showed anandamide sensitivity.
Acknowledgement
We thank Mrs. A. Schmitt and Mrs. A. Jahn for excellent technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 688/B1 to G.S.; BU1019/7-1 to T.Bu.; Me3283/1-1 to SGM) and the Interdisciplinary Clinical Research Center (IZKF) Würzburg (to SGM and HW: IZKF A-39-N). T.Br. was a fellow of the Boehringer Ingelheim Fonds.
References
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K. Protective role of neuronal KATP channels in brain hypoxia. J Exp Biol. 2004;207:3201–3212. doi: 10.1242/jeb.01106. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Benveniste M, Dingledine R. Limiting stroke-induced damage by targeting an acid channel. N Engl J Med. 2005;352:85–86. doi: 10.1056/NEJMcibr045010. [DOI] [PubMed] [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea- pig dissociated sinoatrial node cells. Br J Pharmacol. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Honore E. The lipid-activated two-pore domain K+ channel TREK-1 is resistant to hypoxia: implication for ischaemic neuroprotection. J Physiol. 2005;562:213–222. doi: 10.1113/jphysiol.2004.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004;82:863–869. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chemin J, Nargeot J, Lory P. Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J Biol Chem. 2007;282:2314–2323. doi: 10.1074/jbc.M610033200. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Frassetto A, Fong TM. Effects of the CB1 cannabinoid receptor inverse agonist AM251 on food intake and body weight in mice lacking muopioid receptors. Brain Res. 2006;1108:176–178. doi: 10.1016/j.brainres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr., Winfree CJ, Stern DM, Solomon RA, Pinsky DJ. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery. 1996;38:523–531. doi: 10.1097/00006123-199603000-00021. discussion 532. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Dixon W, Massey F. Introduction to statistical analysis. McGraw-Hill; New York: 1969. [Google Scholar]
- Dodt HU, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–608. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Becker DA, Busto R, Belayev A, Zhang Y, Khoutorova L, Ley JJ, Zhao W, Belayev L. Stilbazulenyl nitrone, a novel antioxidant, is highly neuroprotective in focal ischemia. Ann Neurol. 2003;54:330–342. doi: 10.1002/ana.10659. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang- Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. Embo J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Laigle C, Blondeau N, Jarretou G, Lazdunski M. Alpha-linolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience. 2006;137:241–251. doi: 10.1016/j.neuroscience.2005.08.083. [DOI] [PubMed] [Google Scholar]
- Hill MN, Froese LM, Morrish AC, Sun JC, Floresco SB. Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology (Berl) 2006;187:245–259. doi: 10.1007/s00213-006-0421-4. [DOI] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler CJ. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab B, Helyes Z, Varga A, Bolcskei K, Szabo A, Sandor K, Elekes K, Borzsei R, Keszthelyi D, Pinter E, Petho G, Nemeth J, Szolcsanyi J. Pharmacological characterization of the TRPV1 receptor antagonist JYL1421 (SC0030) in vitro and in vivo in the rat. Eur J Pharmacol. 2005;517:35–44. doi: 10.1016/j.ejphar.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K(+) channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renne C, Gailani D, Nieswandt B, Renne T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert RC, Bessaih T, Leresche N. Modulation of neuronal T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:611–627. doi: 10.2174/187152706779025544. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. Embo J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth SG, Aller MI, Munsch T, Schuhmacher T, Seidenbecher T, Meuth P, Kleinschnitz C, Pape HC, Wiendl H, Wisden W, Budde T. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol. 2006a;69:1468–1476. doi: 10.1124/mol.105.020594. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci. 2003;23:6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth SG, Kanyshkova T, Meuth P, Landgraf P, Munsch T, Ludwig A, Hofmann F, Pape HC, Budde T. Membrane resting potential of thalamocortical relay neurons is shaped by the interaction among TASK3 and HCN2 channels. J Neurophysiol. 2006b;96:1517–1529. doi: 10.1152/jn.01212.2005. [DOI] [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci U S A. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci. 2005;25:11184–11193. doi: 10.1523/JNEUROSCI.3370-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moezi L, Gaskari SA, Liu H, Baik SK, Dehpour AR, Lee SS. Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br J Pharmacol. 2006;149:898–908. doi: 10.1038/sj.bjp.0706928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch T, Pape HC. Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. J Physiol 519 Pt. 1999;2:493–504. doi: 10.1111/j.1469-7793.1999.0493m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonenko I, Bancila M, Bloc A, Muller D, Bijlenga P. Inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage. Mol Pharmacol. 2005;68:84–89. doi: 10.1124/mol.104.010066. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Anesthetic-sensitive 2P domain K+ channels. Anesthesiology. 2001;95:1013–1021. doi: 10.1097/00000542-200110000-00034. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M. The 2P-domain K+ channels: role in apoptosis and tumorigenesis. Pflugers Arch. 2004;448:261–273. doi: 10.1007/s00424-004-1255-8. [DOI] [PubMed] [Google Scholar]
- Plant LD, Kemp PJ, Peers C, Henderson Z, Pearson HA. Hypoxic depolarization of cerebellar granule neurons by specific inhibition of TASK-1. Stroke. 2002;33:2324–2328. doi: 10.1161/01.str.0000027440.68031.b0. [DOI] [PubMed] [Google Scholar]
- Runden-Pran E, Haug FM, Storm JF, Ottersen OP. BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: a study in organotypical hippocampal slice cultures. Neuroscience. 2002;112:277–288. doi: 10.1016/s0306-4522(02)00092-1. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003;278:27406–27412. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Talley EM, Sirois JE, Lei Q, Bayliss DA. Two-pore-Domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist. 2003;9:46–56. doi: 10.1177/1073858402239590. [DOI] [PubMed] [Google Scholar]
- Veale EL, Buswell R, Clarke CE, Mathie A. Identification of a region in the TASK3 two pore domain potassium channel that is critical for its blockade by methanandamide. Br J Pharmacol. 2007;152:778–786. doi: 10.1038/sj.bjp.0707436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CS, Mathie A. A non-inactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. J Physiol. 1996;491:401–412. doi: 10.1113/jphysiol.1996.sp021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner NA, Koch JE. Effects of the cannabinoid antagonists AM281 and AM630 on deprivation-induced intake in Lewis rats. Brain Res. 2003;967:290–292. doi: 10.1016/s0006-8993(02)04274-9. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1: Expression of TASK1 and 3 channels and effects of anandamide on IKSO in cortical, hippocampal and colliculus inferior neurons. (A) Expression of TASK1 and TASK3 channels in the dorsal lateral geniculate nucleus, cortex and hippocampus. (B) Currents from cortical neurons evoked by ramp protocols (−28 mV to −120 mV over 800 ms, left panel). Application of anandamide (30 μM) resulted in a current reduction of 32 ± 2.9% (n=3, p<0.01, inset). Anandamide-sensitive currents (after substraction) displayed features indicative of TASK channels: outward rectification and a reversal potential of ~ −104mV (close to the K+ equilibrium potential; right panel). (C) Currents recorded from cultured hippocampal and (D) from colliculus inferior neurons also showed anandamide sensitivity.