Abstract
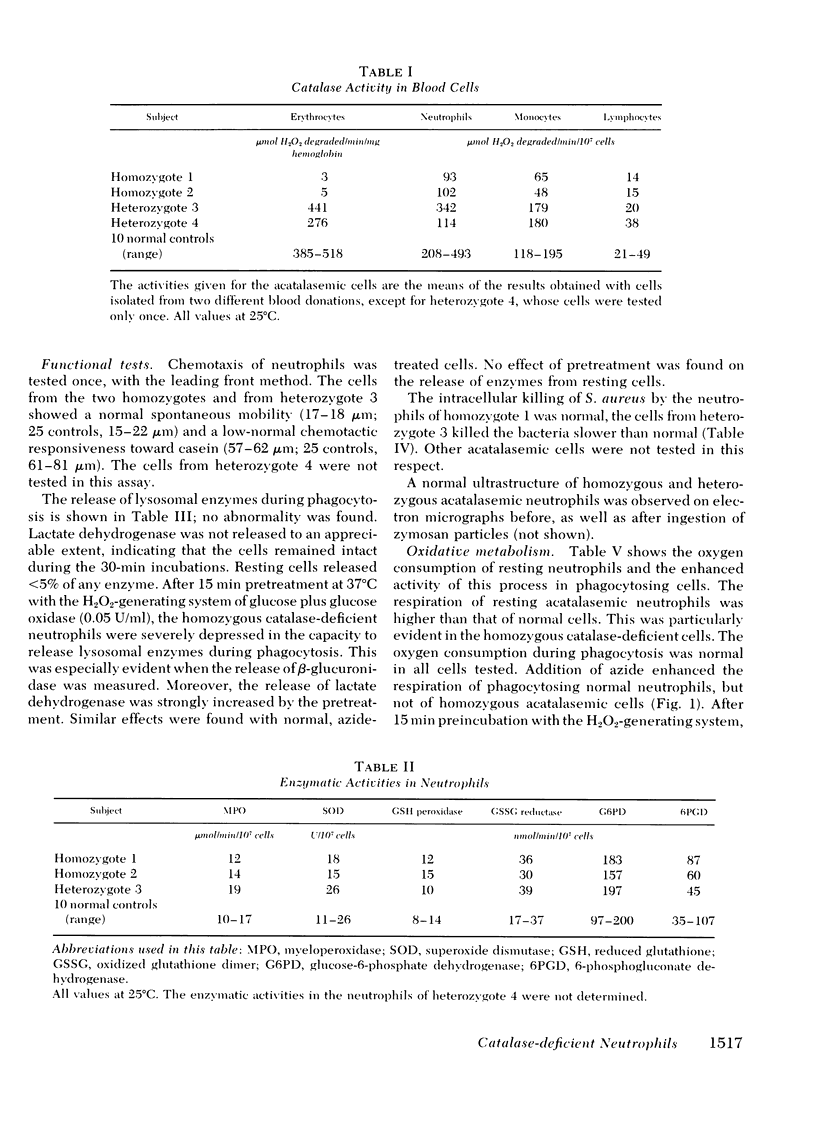
To investigate the importance of catalase as a protecting enzyme against oxidative damage in phagocytic leukocytes, we have tested the functional capacity of neutrophils from two individuals homozygous for Swiss-type acatalasemia and from two individuals heterozygous for this deficiency. In the former cells, 25-30% of residual activity of catalase was present. In the latter cells, the values were close to normal.
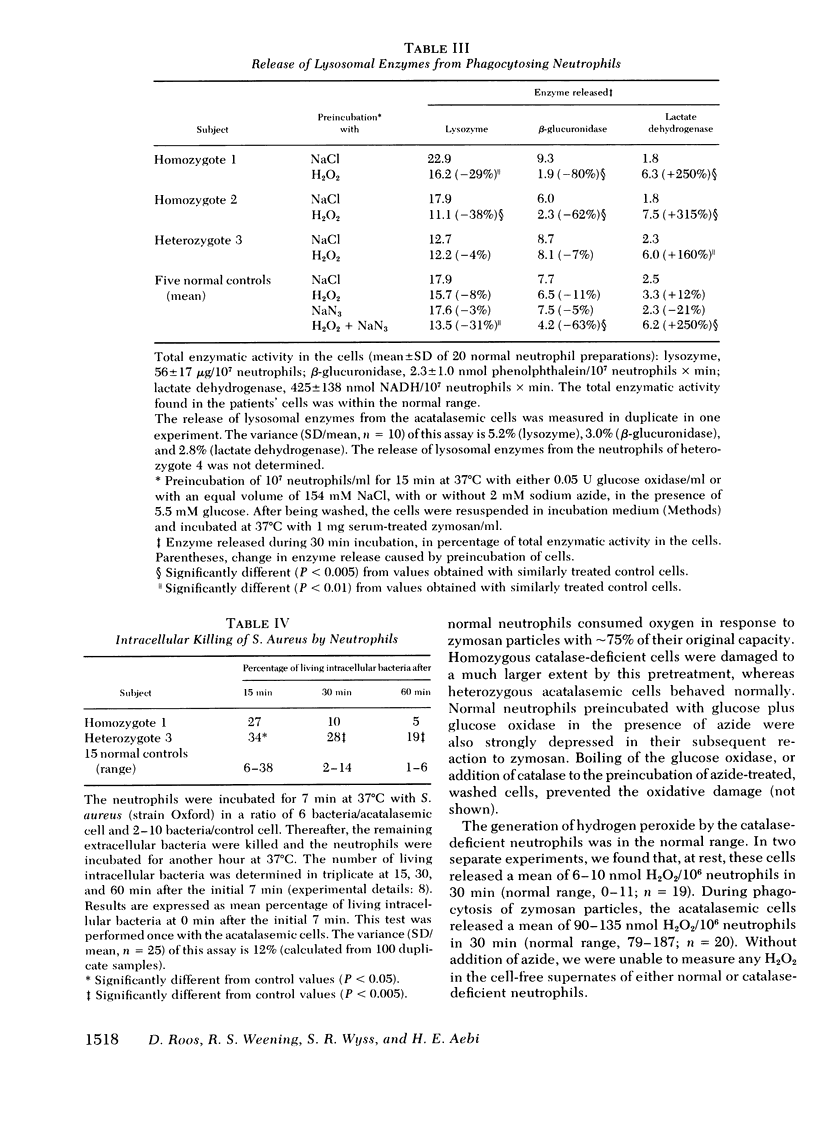
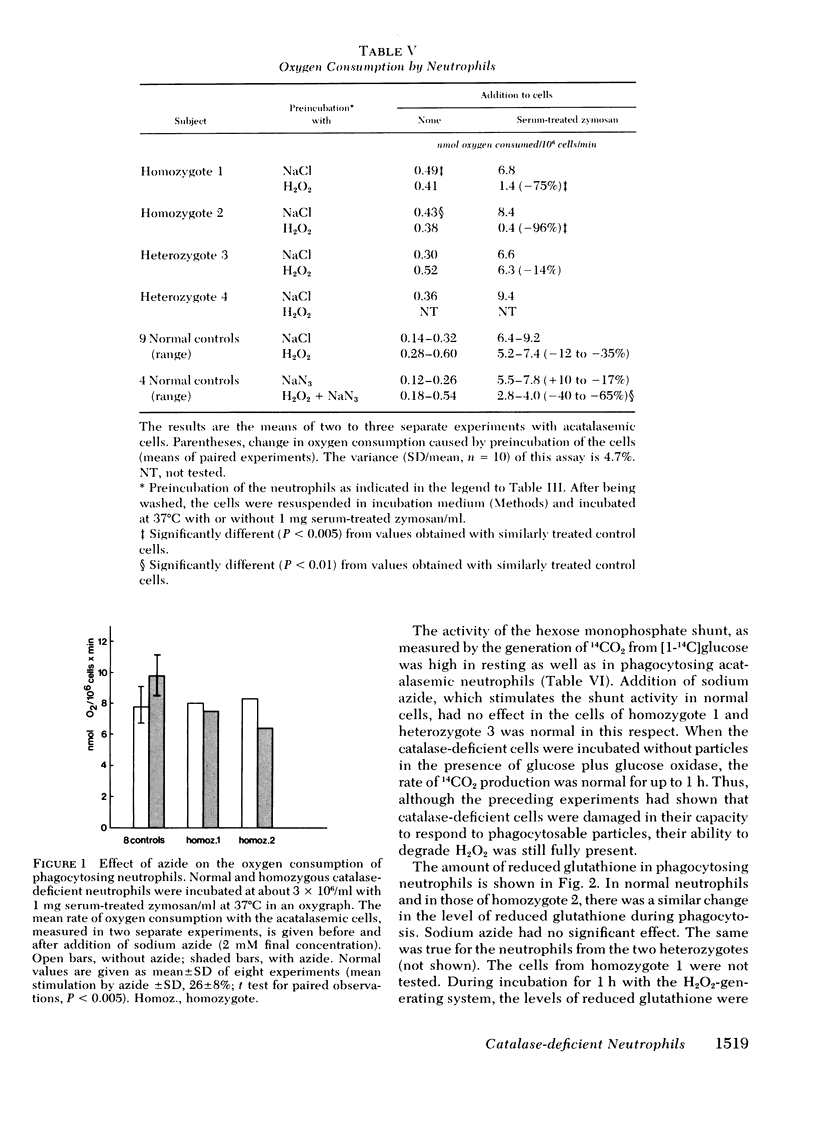
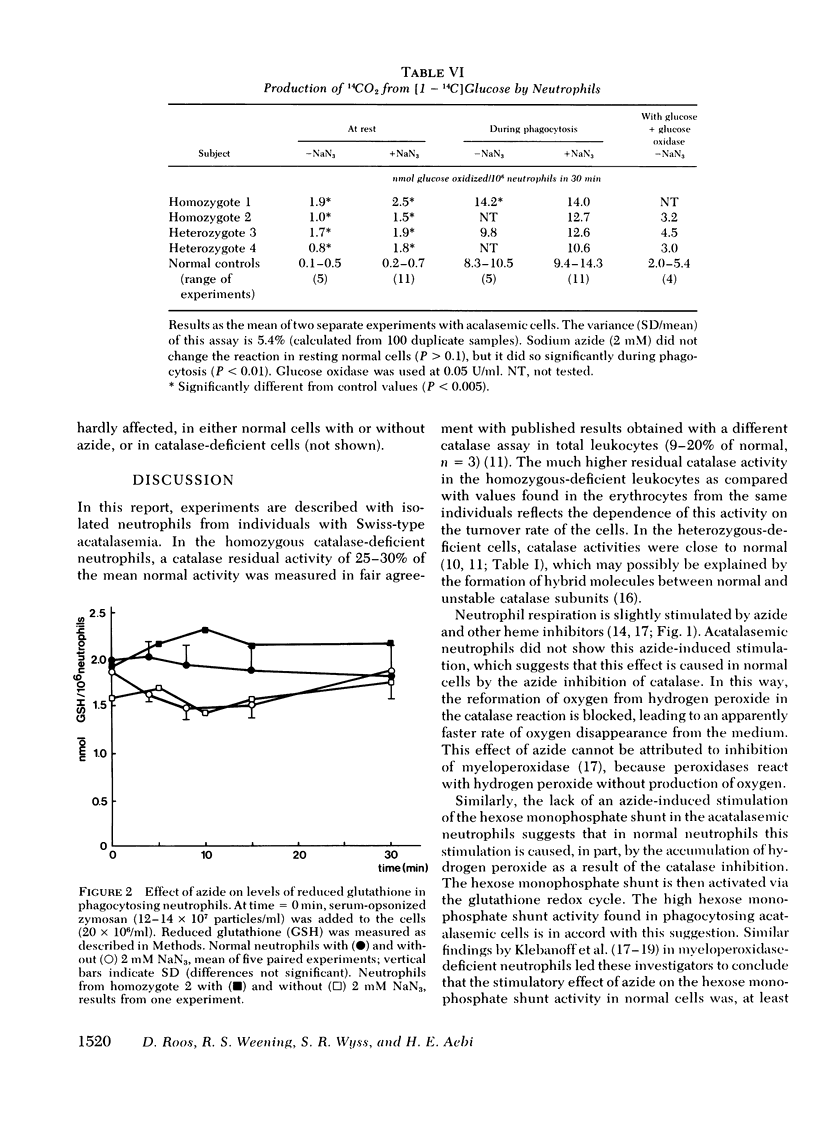
Chemotaxis towards casein, release of lysosomal enzymes and hydrogen peroxide during phagocytosis of zymosan, and intracellular killing of Staphylococcus aureus were normal in all cells tested. Inhibition of heme enzymes with azide (2 mM) enhanced the respiration and hexose monophosphate shunt activity of normal, but not of homozygous acatalasemic, neutrophils. This indicates that the enhancement in normal cells is, at least in part, due to catalase inhibition.
After 15 min preincubation with an H2O2-generating system (glucose plus glucose oxidase), the respiratory response to zymosan phagocytosis was strongly depressed in the homozygous acatalasemic and in normal, azide-treated neutrophils, but not in normal, untreated cells. Under these conditions, the release of lysosomal enzymes was depressed and that of lactate dehydrogenase enhanced, in catalase-deficient and in catalase-inhibited, but not in normal, neutrophils. During prolonged incubation with the H2O2-generating system (30-60 min), the reduction level of intracellular glutathione remained high and the hexose monophosphate shunt continued to operate normally in all cells tested. Thus, although the function of neutrophils without catalase activity was depressed by extracellular hydrogen peroxide, the H2O2 degradation via the glutathione redox system remained operative.
The results indicate that the glutathione redox system by itself efficiently protects phagocytosing neutrophils against their own oxidative products. During heavy external oxidative stress, however, both catalase and the glutathione redox system are needed for adequate protection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H., Wyss S. R., Scherz B., Gross J. Properties of erythrocyte catalase from homozygotes and heterozygotes for Swiss-type acatalasemia. Biochem Genet. 1976 Oct;14(9-10):791–807. doi: 10.1007/BF00485342. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Del Río L. A., Ortega M. G., López A. L., Gorgé J. L. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal Biochem. 1977 Jun;80(2):409–415. doi: 10.1016/0003-2697(77)90662-5. [DOI] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M., Uotila L. Enzymic method for the quantitative determination of reduced glutathione. Anal Biochem. 1974 May;59(1):34–45. doi: 10.1016/0003-2697(74)90006-2. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Herron M., Simmons R. L., Quie P. G. Chemotactic deactivation of human neutrophils: possible relationship to stimulation of oxidative metabolism. Infect Immun. 1979 Feb;23(2):282–286. doi: 10.1128/iai.23.2.282-286.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A., van Schaik M. L., Bot A. A., Meerhof L. J., Loos J. A. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979 May;53(5):851–866. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg S. P., Boxer L. A., Oliver J. M., Allen J. M., Schulman J. D. Oxidative damage to neutrophils in glutathione synthetase deficiency. Br J Haematol. 1979 Jun;42(2):215–223. doi: 10.1111/j.1365-2141.1979.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Wyss S. R., Aebi H. Properties of leukocyte catalase in Swiss type acatalasemia: a comparative study of normals, heterozygotes and homozygotes. Enzyme. 1975;20(5):257–268. doi: 10.1159/000458948. [DOI] [PubMed] [Google Scholar]



