Abstract
Aims
Ca2+ waves are thought to be important in the aetiology of ventricular tachyarrhythmias. There have been conflicting results regarding whether flecainide reduces Ca2+ waves in isolated cardiomyocytes. We sought to confirm whether flecainide inhibits waves in the intact cardiomyocyte and to elucidate the mechanism.
Methods and results
We imaged spontaneous sarcoplasmic reticulum (SR) Ca2+ release events in healthy adult rat cardiomyocytes. Variation in stimulation frequency was used to produce Ca2+ sparks or waves. Spark frequency, wave frequency, and wave velocity were reduced by flecainide in the absence of a reduction of SR Ca2+ content. Inhibition of INa via alternative pharmacological agents (tetrodotoxin, propafenone, or lidocaine) produced similar changes. To assess the contribution of INa to spark and wave production, voltage clamping was used to activate contraction from holding potentials of −80 or −40 mV. This confirmed that reducing Na+ influx during myocyte stimulation is sufficient to reduce waves and that flecainide only causes Ca2+ wave reduction when INa is active. It was found that Na+/Ca2+-exchanger (NCX)-mediated Ca2+ efflux was significantly enhanced by flecainide and that the effects of flecainide on wave frequency could be reversed by reducing [Na+]o, suggesting an important downstream role for NCX function.
Conclusion
Flecainide reduces spark and wave frequency in the intact rat cardiomyocyte at therapeutically relevant concentrations but the mechanism involves INa reduction rather than direct ryanodine receptor (RyR2) inhibition. Reduced INa results in increased Ca2+ efflux via NCX across the sarcolemma, reducing Ca2+ concentration in the vicinity of the RyR2.
Keywords: Na+ current, Ca2+ sparks, Ca2+ waves, Flecainide
1. Introduction
Ca2+ waves are thought to be important in the aetiology of a number of different forms of ventricular tachyarrhythmia, particularly in heart failure (HF) and catecholaminergic polymorphic ventricular tachycardia (CPVT).1 The mechanisms for these arrhythmias are thought to be associated with elevated levels of spontaneous sarcoplasmic reticulum (SR) Ca2+ release for a given SR load.1,2 In other words, the threshold SR Ca2+ content for store-overload-induced Ca2+ release is reduced in both CPVT and in HF,2–4 leading to Ca2+ spark and wave generation. In CPVT, this is related to mutations of the cardiac ryanodine receptor (RyR2) or absence of calsequestrin, whereas in HF this may relate to post-translational modification of the RyR2, such as hyperphosphorylation.2,5
There has been recent interest in pharmacological agents which target potentially arrhythmogenic Ca2+ waves. Flecainide, a drug that has been used for many years clinically for its sodium current (INa)-reducing properties, has shown efficacy in the treatment of CPVT patients.6,7 However, the mechanism of action producing this clinical effect is debated. In a mouse model of CPVT, Knollmann and colleagues7–9 have shown that flecainide reduces Ca2+ wave frequency in both intact and permeabilized myocytes and have provided evidence that this is related to a direct action on the RyR2 via an open-state block of the channel. In contrast, similar experiments in both intact and permeabilized myocytes have been repeated by Liu et al.10 (although in a different mouse model of CPVT), and no effect on Ca2+ wave frequency was found despite similar experimental conditions. The conclusion of Liu et al. was that the reduction in INa caused by flecainide affected the threshold potential, which decreased the number of spontaneous action potentials triggered by delayed after-depolarizations (DADs) associated with Ca2+ waves.
Our aim in this study was to assess whether flecainide had an effect on Ca2+ sparks and waves and to further investigate the mechanism. We observed SR Ca2+ release events in intact rat ventricular cardiomyocytes from healthy rats. We show via a variety of pharmacological and electrophysiological interventions that a reduction in INa during cellular contraction can reduce the frequency of Ca2+ sparks and waves in the diastolic period. We also show that, in the case of flecainide, the INa blocking effects are more relevant to wave reduction under our experimental conditions than RyR2 stabilization. Finally, we explore the mechanism of this wave reduction. We conclude that the most likely explanation for the reduction in the presence of INa blockade is that it prevents an increase in [Na+]i resulting in more effective Na+/Ca2+-exchanger (NCX)-mediated efflux of Ca2+.
2. Methods
2.1. Ventricular myocyte isolation and Ca2+ imaging
Extended methods are available in Supplementary material online. All animal surgical procedures and peri-operative management were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011) under assurance number A5634-01. Imperial College Ethical Review Committee authorized the project licence. Rats were sacrificed by cervical dislocation following exposure to 5% isoflurane until righting reflex was lost. Cardiac myocytes were enzymatically isolated from the left ventricle of healthy adult male Sprague Dawley rats by the Langendorff perfusion technique.11 Intact isolated myocytes were loaded with the Ca2+-sensitive fluorescent dyes fluo-4AM or fura-2AM prior to imaging.
2.2. Intracellular Ca2+ measurements
Experiments were performed with cells undergoing superfusion at 37°C. Transients were assessed during steady-state external field stimulation at 0.5 Hz. Sparks were recorded following cessation of 0.5 Hz contraction during the last 10 s of a 25 s period of quiescence. Diaz et al.12 have previously shown that [Na+]i rises when quiescent cardiomyocytes are stimulated and that this, together with higher SR Ca2+ content, was correlated with increased wave frequency. Similarly, in our experiments, a higher stimulation frequency was associated with an increased wave frequency in a subsequent quiescent period, presumably for similar reasons (see Supplementary material online, Figure S1A). This preliminary series of experiments established that 30 s of 5 Hz stimulation (after 2 min of stable contraction at 0.5 Hz) would consistently produce Ca2+ waves in normal tyrode (NT) in the quiescent interval.
2.3. Voltage clamp technique
Cells were voltage-clamped using an amphotericin-perforated patch technique. The switch clamp technique [with an Axoclamp 2B amplifier (Axon Instruments)] was used to overcome any changes in access resistance that may have occurred over the course of an experiment. Myocytes were clamped at −80 or −40 mV and depolarized to 0 mV for 100 ms to cause contraction with and without INa activation, respectively. A 5 Hz stimulation train was followed by a quiescent period during which the membrane potential was held at −80 mV for 30 s, and wave frequency was assessed as before.
2.4. Data-pairing and statistical analysis
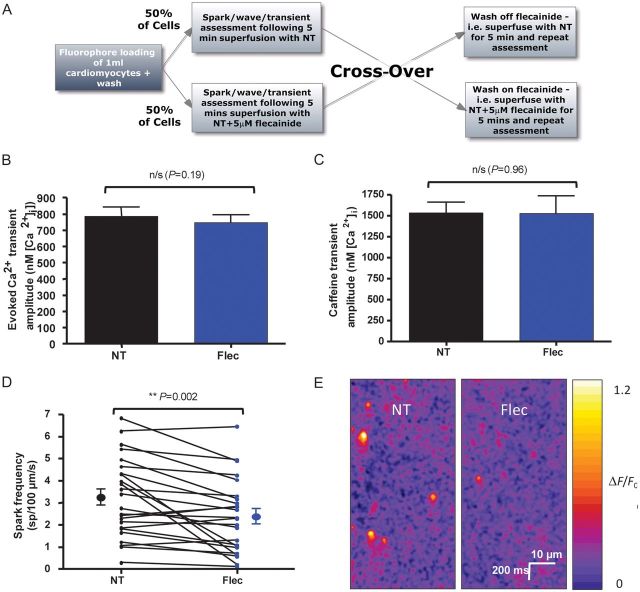
Where possible, data were obtained in a paired fashion and drugs applied or washed off in the form of a cross-over protocol alternating from cell to cell (Figure 1A). For example, the first cell had the drug applied following a control period, whereas the next cell had drug applied first with subsequent wash-off.
Figure 1.
Experimental protocol and effects of flecainide on Ca2+ transients and Ca2+ sparks. (A) Experimental flowchart to explain cross-over protocol used in experiments. Fifty per cent of cells had NT applied first with drug wash-on, whereas 50% had drug applied first and subsequently washed-off. (B) Stimulated Ca2+ transients were assessed using the ratiometric dye fura-2 calibrated to give [Ca2+]i. Transient amplitude was not changed in the presence of 5 μM flecainide (n = 30 cells, P = 0.19). (C) 20 mM caffeine in 0Na+/0Ca2+ solution was used to assess SR Ca2+ load following a 5 Hz contraction train. The amplitude was unchanged in the presence of 5 µM flecainide (n = 13 cells in each group, P = 0.96 by Student's t-test). (D) Spark frequency was reduced following flecainide application (n = 24 cells, P = 0.002). (E) Representative line-scans showing a reduction in spark frequency with flecainide. The same cell is shown before and after flecainide application.
As a result, paired t-tests were used for significance testing unless stated. Depending on the data, Student's t-tests, log-rank, and repeated-measures analysis of variance (ANOVA) were also used to assess effects. Results were considered statistically significant if the P-value was <0.05. Unless otherwise indicated, results are expressed as mean ± standard error of the mean.
3. Results
3.1. Flecainide has no effect on the Ca2+ transient or SR Ca2+ load
We first assessed the effect of 5 µM flecainide on the amplitude of Ca2+ transients evoked by external field stimulation at 0.5 Hz. Stimulation continued at the same rate during the 5 min wash-on or wash-off periods. Ca2+ transient amplitude did not change significantly in the presence of flecainide (Figure 1B). Similarly, transient morphology was unchanged (see Supplementary material online, Figure S1B and C). SR load was measured using a 20 mM caffeine spritz in 0Na+/0Ca2+ solution following field stimulation at 5 Hz to mimic conditions used to assess waves (Figure 1C) and was unchanged by flecainide.
3.2. Flecainide reduces Ca2+ spark and wave frequency and Ca2+ wave velocity
Spark frequency was significantly reduced with exposure to flecainide compared with NT alone from 3.25 ± 0.36 to 2.38 ± 0.34 sp/100 µm/s (Figure 1D and E). Spark morphology was unchanged (see Supplementary material online, Figure S2A–D).
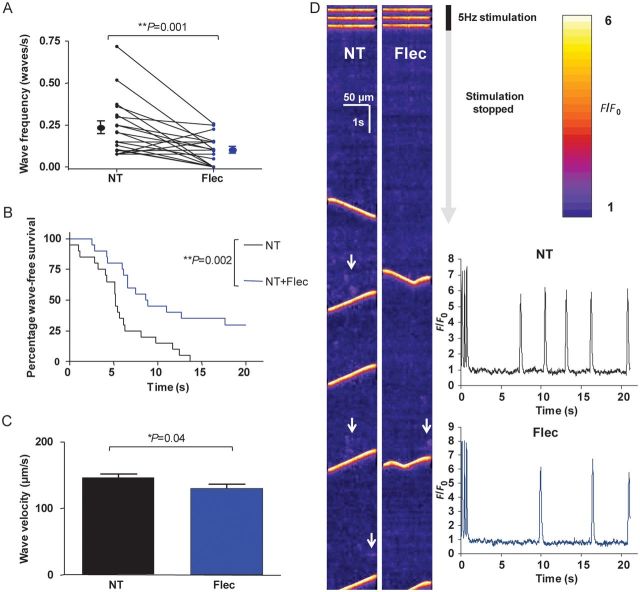
We predicted that the reduction in spontaneous Ca2+ sparks from the SR in the presence of flecainide would result in a reduction in wave frequency. In order to test this hypothesis, 5 Hz stimulation was used to produce waves. There was a reduction in Ca2+ wave frequency in the presence of flecainide (Figure 2A) from 0.23 ± 0.04 to 0.10 ± 0.02 waves/s (P = 0.001). Since it has previously been suggested that a prolonged period of flecainide loading is required to produce SR Ca2+ release reduction,9 we tested whether prolonged exposure would have any additional effect. Thirty minutes of exposure produced no further wave reduction compared to 5 mins (see Supplementary material online, Figure S3A).
Figure 2.
Effects of 5 µM flecainide on Ca2+ waves. (A) Flecainide was washed on or off via cross-over protocol for 5 min. In the presence of flecainide, wave frequency was significantly reduced (P = 0.001, n = 20 cells). (B) Latency period from the last transient to the first wave is shown in the Kaplan–Meier survival format (i.e. wave-free survival). Cells in the presence of flecainide have an increased wave-free survival period (P = 0.002 by log-rank test, n = 20 cells). (C) Wave velocity is reduced in the presence of flecainide (P = 0.04 by Student's t-test, NT: n = 81 waves; flec: n = 36 waves from 20 cells). (D) Representative line-scans from a cell assessed for waves pre- and post-flecainide application. The end of the 30 s period of 5 Hz stimulation evoking Ca2+ transients can be seen at the top of the scans with subsequent quiescent phase during which waves are observed. Areas of increased spark activity prior to waves are highlighted with white arrows and are more prominent in the absence of flecainide. Inset: line-scans converted into F/F0 plots—reduction of wave frequency and increased latency is apparent.
The time from the last transient to the first wave, defined as the ‘wave-free survival period’ for each cell, and represented in Kaplan–Meier survival curve format in Figure 2B, was also significantly increased in the presence of flecainide. In addition, wave velocity was reduced (Figure 2C) from 146.4 ± 4.7 to 130 ± 5.8 µm/s (P = 0.04 by Student's t-test), suggesting that wave propagation is also altered by flecainide. Confocal line-scanning reveals both the reduction in Ca2+ waves and how this is related to a reduction in spark frequency (Figure 2D). Wave amplitude did not change significantly in the presence of flecainide (see Supplementary material online, Figure S3B).
3.3. Specific INa blockade decreases spark and wave frequency
There are two broad mechanisms which may be responsible for the reduction in Ca2+ waves in the presence of flecainide. First, by blocking Ca2+ release from the RyR2, for which there is conflicting evidence in CPVT myocytes,9,10 and second by inhibiting Na+ influx with subsequent downstream effects. We aimed to assess the latter possibility—namely, whether SR Ca2+ release can be altered by reducing Na+ influx.
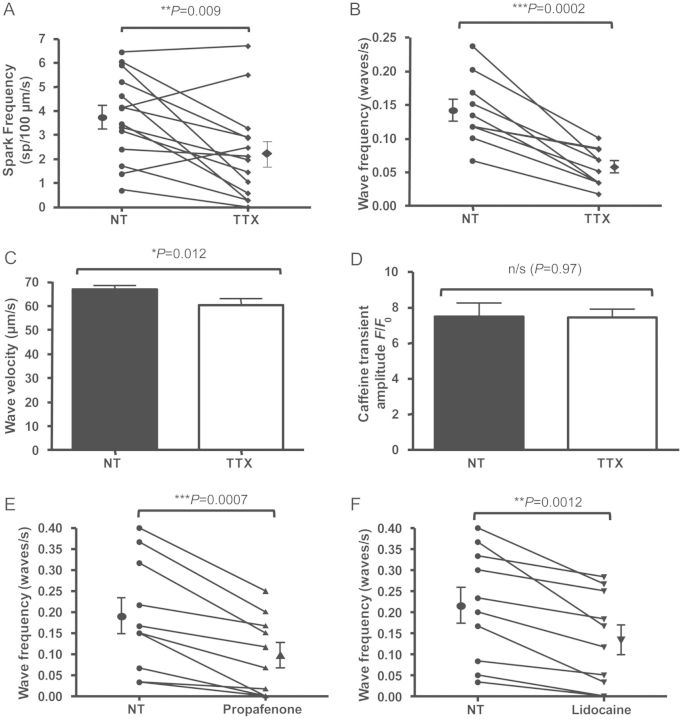
We therefore assessed the effect of specific pharmacological inhibition of INa using 5 µM tetrodotoxin (TTX), a dose which was selected since it provides 25% INa blockade in cardiomyocytes,13 which is similar to that provided by 5 μM flecainide,14 while still allowing Ca2+ transients to occur with external field stimulation. Spark frequency was reduced (Figure 3A) from 3.76 ± 0.48 to 2.24 ± 0.52 sp/µm/s in the presence of TTX (P = 0.009). TTX also significantly reduced wave frequency (Figure 3B) and caused a reduction in wave velocity (Figure 3C) without changing wave amplitude (see Supplementary material online, Figure S3B). Similar to results with flecainide, application of TTX at this concentration resulted in no significant alteration of SR load (Figure 3D). To assess whether this was a general property of other INa blockers, further experiments to assess wave frequency under similar degrees of INa blockade by 5 μM propafenone15 and 200 μM lidocaine16 were carried out. Both agents reduced waves in a similar manner to flecainide and TTX (Figure 3E and F). Together, these results strongly suggest that INa is involved in wave formation.
Figure 3.
Effects of INa inhibition by tetrodotoxin (TTX), propafenone and lidocaine on SR Ca2+ release events. (A) 5 µM TTX applied via similar cross-over protocol to flecainide experiments induced a similar reduction in Ca2+ spark frequency (P = 0.009, n = 14 cells). (B) 5 µM TTX reduced wave frequency (P = 0.0002, n = 10 cells). (C) Wave velocity is significantly reduced in the presence of TTX (P = 0.012 by Student's t-test, NT: n = 84 waves; TTX: n = 34 waves from 10 cells). (D) Similar to flecainide experiments, no significant change in SR load was seen in the presence of 5 µM TTX (P = 0.97 by Student's t-test, n = 20 cells from three isolations). (E) 5 μM propafenone reduced Ca2+ wave frequency in a similar manner (P = 0.0007, n = 10 cells), as did (F) 200 μM lidocaine (P = 0.0012, n = 10 cells).
3.4. How does INa reduction decrease Ca2+ waves?
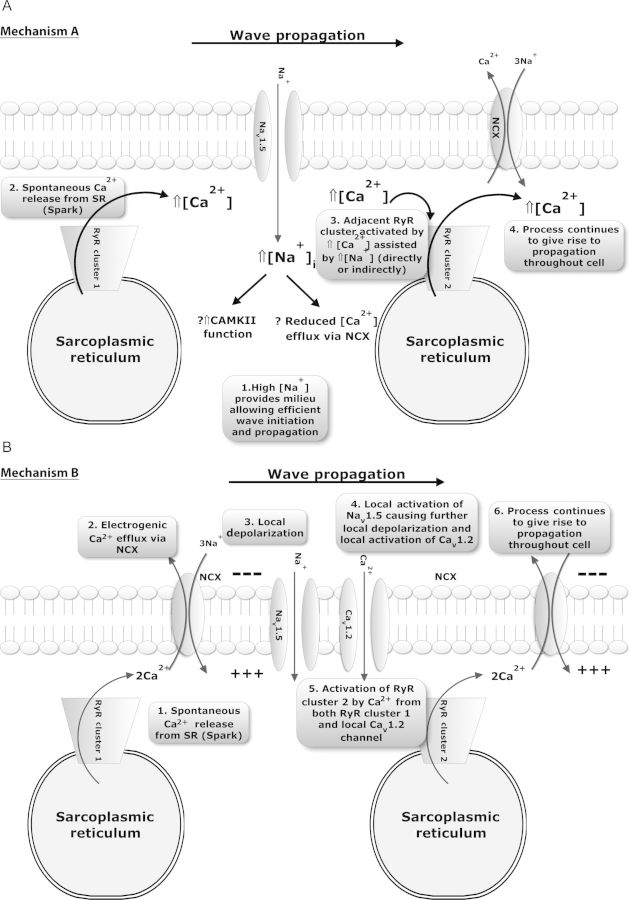
Two main possibilities could explain the involvement of INa in wave formation. The first is that Na+ entry via Nav1.5 channels alters the sub-sarcolemmal ‘fuzzy’ space [Na+] which subsequently modifies wave propagation via a number of possible downstream mechanisms (Mechanism A, Figure 4A). The second is that Nav1.5 channel activation is involved in the process of wave initiation and propagation more directly at the wave front (Mechanism B, Figure 4B).
Figure 4.
Possible hypotheses to explain how INa can contribute to wave initiation and propagation. (A) Entry of Na+ ions occurs via INa and an alteration of wave properties may result from changes in [Na+]i, particularly in the sub-sarcolemmal space. In this proposed mechanism (1) increased fuzzy space [Na+] provides a milieu that enhances the probability of (2) Ca2+ sparks leading to (3) the activation and firing of an adjacent RyR cluster to result in (4) wave initiation and propagation throughout the cell. (B) Alternatively Nav1.5 channels may be involved in wave propagation per se in the intact cardiomyocyte. Such involvement could comprise (1) spontaneous SR Ca2+ release in the form of a spark resulting in (2) local Ca2+ efflux by NCX causing (3) local depolarization of the sarcolemma, which (4) subsequently results in local activation of INa and ICa assisting the rise in local (‘fuzzy space’) [Ca2+]i that can lead to (5) adjacent RyR clusters firing and (6) wave propagation.
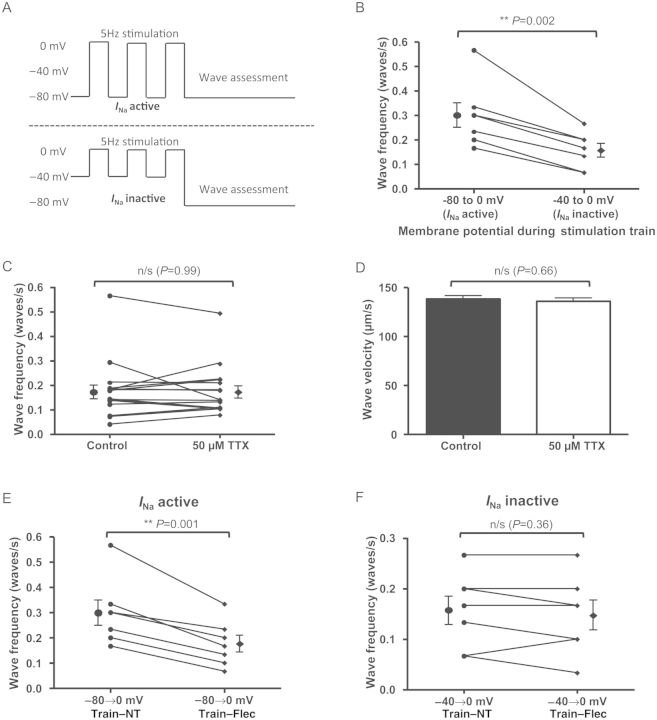
If Mechanism A is accurate, then given its dependence on Na+ influx via Nav1.5, wave frequency should be reduced by an intervention which reduces Na+ influx during the contraction train but leaves Nav1.5 channels available during the quiescent period following the contraction train. Such a scenario was created using a voltage clamp technique to inactivate INa during the stimulation train. Cells were stimulated by a 5 Hz train of clamp pulses (100 ms in duration) from −80 to 0 mV repeatedly for 1 min and waves assessed during a subsequent 30 s quiescent period when the cells were held at −80 mV. The same cell was re-stimulated by another train of pulses from −40 to 0 mV, thereby removing Na+ influx due to INa inactivation. The final holding potential during the quiescent period was −80 mV as before to ensure availability of Nav1.5 channels (Figure 5A). There was a significant reduction in wave frequency from 0.30 ± 0.04 to 0.16 ± 0.03 waves/s following inactivation of INa by voltage clamp (Figure 5B), suggesting greater importance of Mechanism A.
Figure 5.
Elucidation of Mechanism A as most likely cause for reduction in Ca2+ waves due to INa blockade. (A) Voltage clamp stimulation trains used to assess wave frequency with and without INa activity. Stimulation was induced by stepping from −80 to 0 mv (INa active) or −40 to 0 mV (INa inactive). Pulse duration was 100 ms and pulses were applied at 5 Hz. Waves were assessed in a subsequent 30 s interval during which membrane potential was held at −80 mV. (B) With INa inactive during the stimulation train (but available during the quiescent phase of the experiment), wave frequency was reduced (P = 0.002, n = 7 cells). (C) High-dose (50 µM) TTX was rapidly applied to cells to terminate stimulation following a period of external field stimulation at 5 Hz and compared with the control arm in which stimulation was terminated in the usual fashion at 30 s (see Supplementary material online, Figure S4 for further explanation). This produced the opposite situation to the previous experiment with INa active during the stimulation train but Nav1.5 channels unavailable for stimulation during the quiescent phase. This produced no change in wave frequency (P = 0.99, n = 17 cells). (D) Similarly, there was no change in wave velocity (P = 0.66 by Student's t-test. Control: n = 88 waves; 50 µM TTX: n = 89 waves from 17 cells). (E) Voltage clamp experiments showing effects of flecainide on wave frequency with INa active vs. inactive. With INa active, flecainide reduces wave frequency (P = 0.001, n = 7 cells). (F) However, with INa inactive, no reduction in wave frequency was observed (P = 0.36, n = 7 cells).
To confirm these findings and assess whether Mechanism B might also be playing a role, we designed an experiment which would allow normal Na+ influx during the contraction train but would profoundly reduce availability of Nav1.5 channels during the quiescent phase. A stimulation train was induced by external field stimulation for 30 s at 5 Hz and waves were assessed as before in the control condition (NT + vehicle). The same cell was then exposed to the same protocol but high-dose TTX (50 µM TTX, which blocks >95% of INa13) was superfused over cells rapidly after 30 s stimulation in NT to stop the contraction train. This caused contractions and stimulated Ca2+ transients to cease almost immediately despite continuation of field stimulation at the same voltage (see Supplementary material online, Figure S5). This provided evidence of Nav1.5 blockade during the quiescent period while ensuring the SR loading protocol was identical. Results of these experiments showed that acute, profound Nav1.5 blockade did not alter Ca2+ wave frequency or velocity (Figure 5C and D), suggesting that Mechanism B either does not occur or is of relatively minor importance compared with Mechanism A.
3.5. Mechanism of wave reduction with flecainide
Having shown that INa reduction during the contraction train can reduce the frequency and velocity of Ca2+ waves, we wished to assess whether this effect also played a role in the effects we had observed with flecainide. We first assessed whether, in the absence of INa, flecainide would still reduce wave frequency—potentially through an additional effect on the RyR2. In order to test this possibility, we performed voltage clamp experiments. With a stimulation train of voltage clamp steps from −80 to 0 mV, as expected, there was a significant reduction in Ca2+ waves (Figure 5E) in the presence of flecainide. However, when the stimulation train was induced by voltage steps from −40 to 0 mV (and so INa was inactivated), there was no significant reduction in Ca2+ wave frequency (Figure 5F) in the presence of flecainide. This provided evidence that reduced Na+ influx was crucial in flecainide's mechanism of wave reduction.
To investigate how the changes in Na+ influx into the cytosol altered wave frequency, we identified two possibilities that we felt were most likely to be the cause of the change. First, a reduction in Ca2+/calmodulin-dependent protein kinase II (CamKII) activity as a result of reduced [Na+]i17 or [Ca2+]i, and second as a result of enhanced Ca2+ efflux across the sarcolemma via NCX because of an enhanced [Na+]o:[Na+]i gradient.
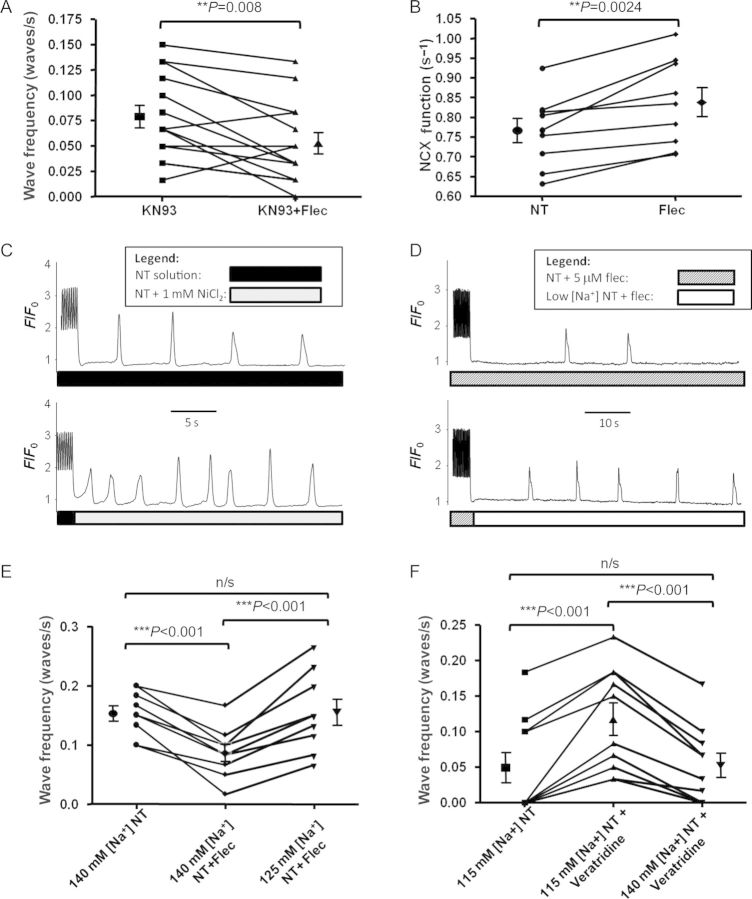
In order to investigate the former possibility, we used 1 μM KN-93 to inhibit CamKII prior to the addition of flecainide. In the presence of either KN-93 (Figure 6A) or KN-92 (see Supplementary material online, Figure S5A), flecainide remained able to reduce Ca2+ wave frequency.
Figure 6.
Role of CaMKII and NCX in wave reduction by flecainide. (A) Despite incubation of cells with 1 μM CaMKII inhibitor KN-93, flecainide was still able to significantly reduce Ca2+ wave frequency. Magnitude of reduction was similar in the presence of inactive analogue KN-92 (see Supplementary material online, Figure S5A), suggesting CaMKII inhibition is not the mechanism of wave reduction with flecainide. (B) NCX function in terms of Ca2+ efflux efficacy was significantly improved following a 5 Hz contraction train in the presence of flecainide. (C) Direct partial inhibition of NCX by 1 mM Ni2+ applied after the contraction train increased Ca2+ wave frequency. (D) Reduction of [Na+]o after the contraction train can reverse the reduction in wave frequency seen with flecainide. (E) Pooled data from experimental protocol shown in (D) revealing that a reduction in wave frequency induced by flecainide can be reversed by reducing [Na+]o to 125 mM. (F) 0.5 μM veratridine can increase Ca2+ wave frequency via enhancing INa. This effect was abolished by increasing [Na+]o from 115 to 140 mM.
We subsequently assessed NCX function by observing the rate constant of Ca2+ efflux following a caffeine transient in NT under the same conditions as waves were assessed (see Supplementary material online). There was a significant increase in Ca2+ efflux via NCX following a contraction train in the presence of flecainide (Figure 6B). We subsequently assessed how such efflux would affect diastolic [Ca2+]i in the period following the last field-stimulated contraction and the first Ca2+ wave, using the ratiometric dye fura-2. We found that there was a significant reduction in diastolic Ca2+ by 12% (P = 0.005, see Supplementary material online, Figure S5B).
In order to assess whether the opposite effect would occur with the inhibition of Ca2+ efflux via NCX, we assessed the effects of partial NCX inhibition18 with 1 mM NiCl2 following the contraction train and found an increase in waves (Figure 6C). Flecainide enhances Ca2+ efflux via a reduction of [Na]i, enhancing the [Na+]o:[Na+]i gradient; however, this gradient can also be altered by changing [Na+]o. We sought to do this in the presence of flecainide to reverse the reduction in wave frequency. We found that a reduction of [Na+]o after the contraction train from 140 to 125 mM was sufficient to reverse the wave reduction seen with flecainide (Figure 6D and E). In order to ascertain whether the opposite effects would occur with INa enhancement, we assessed whether 0.5 μM veratridine could increase Ca2+ wave frequency. There was a significant increase in waves in the presence of veratridine which was reversed by increasing [Na]o from 115 to 140 mM.
4. Discussion
4.1. Main findings
The main finding of this study is that a reduction of INa can reduce the frequency of Ca2+ sparks and waves and the velocity of Ca2+ waves. This holds true whether INa is pharmacologically reduced by a variety of agents or reduced by voltage clamp techniques. Initially, we wished to clarify whether this occurred via altering the intracellular ionic milieu (Mechanism A, Figure 4A) or whether Na+ influx was involved in the process of wave propagation itself (Mechanism B, Figure 4B). A series of experiments inactivating INa either during the stimulation train or the quiescent phase (Figure 5) confirmed that a reduction in Na+ influx is the most important mechanism involved in reducing Ca2+ waves rather than implicating a role for Nav1.5 channels at the Ca2+ wave front. In further support of the importance of changes of cytosolic ionic milieu is the fact that very different INa blockers including the neurotoxin TTX, class 1c drugs flecainide and propafenone, and the class 1b drug lidocaine produce a similar reduction in Ca2+ wave frequency when concentrations producing similar degrees of INa blockade are used.
We used voltage clamp to assess whether a reduction in INa was crucial for this effect. In the absence of INa, flecainide is not able to reduce Ca2+ waves, suggesting dominance of this mechanism over RyR2 blockade under our conditions.
The question of how the alteration in cellular ionic milieu reduces Ca2+ waves is complex and may be multifactorial. A reduction in [Na+]i is expected to increase [Ca2+] efflux across the sarcolemma via NCX and so there is additional complexity since both [Na+]i and [Ca2+]i may be altered. We went on to investigate how such changes contribute to wave reduction.
4.2. Mechanism of wave reduction does not depend on CaMKII
First, Ca2+/calmodulin complex (CaMKII), a major regulator of SR Ca2+ leak,19 is affected both by [Ca2+]i and directly by [Na+]i.17 We investigated the efficacy of flecainide in Ca2+ wave reduction in the presence of KN-93, an inhibitor of CaMKII, and its inactive analogue KN-92. Wave reduction still occurred in the presence of either compound. In addition, the efficacy of wave reduction was unchanged whether KN-93 or KN-92 was present (35 vs. 37% reduction, respectively), suggesting that CaMKII inhibition does not have a major role in wave reduction due to INa inhibition.
4.3. Wave reduction does not result from reduced SR Ca2+ load
Another major possibility was that reduced [Na+]i resulted in enhanced Ca2+ efflux via NCX. This has the potential to decrease SR luminal [Ca2+]; however, we found that neither 5 µM flecainide nor 5 μM TTX had significant effects on SR Ca2+ content. This is consistent with the work of previous investigators using similar doses of flecainide.9,10 Altered NCX function could reduce waves by mechanisms unrelated to SR load, however. For example, let us assume that almost maximal SR load was produced by our experimental conditions in the rat species, and that a tightly controlled SR luminal Ca2+ threshold exists beyond which sparks and waves occur. In this case, if INa blockade enhances Ca2+ efflux via NCX, then SR load may reach threshold for spark and wave release less frequently since the SR Ca2+-ATPase would have more competition for Ca2+ ions in the fuzzy space. Since the threshold per se would not change in this situation (no RyR2 modification), one may not observe lower SR load but simply less frequent SR Ca2+ release.
4.4. INa reduction increases Ca2+ efflux via NCX, which reduces Ca2+ waves
We performed experiments to assess the possibility of an NCX-mediated effect on Ca2+ waves despite the absence of SR Ca2+ load reduction. We assessed NCX function using the decay constant of NCX-mediated [Ca2+]i decline in the presence of caffeine and confirmed that Ca2+ efflux via NCX was increased after a contraction train in the presence of flecainide (Figure 6B). This resulted in a slight reduction in diastolic [Ca2+]i in the quiescent period following our contraction train as assessed by fura-2 fluorescence (see Supplementary material online, Figure S5B). In order to confirm the relevance of this mechanism, we modulated NCX function in other ways. Direct partial inhibition of NCX18 with 1 mM Ni2+ applied after the contraction train increased Ca2+ waves (Figure 6C), suggesting that NCX is functioning predominantly in the inward mode under our experimental conditions. Impairing NCX increases waves by reducing Ca2+ efflux. This helps to clarify how INa blockade might reduce Ca2+ waves. In the presence of lower [Na+]i, NCX would provide more effective Ca2+ efflux at resting membrane potentials.20 On the other hand, a lower [Na+]o would shift the reversal potential of NCX in the negative direction. As such, if altered NCX function resulting from reduced [Na+]i was the cause of wave reduction in the presence of flecainide, we expected that such an effect could be abrogated by a reduction in [Na+]o. Indeed, we found that reducing [Na+]o from 140 to 125 mM in the period following the contraction train completely reversed the reduction in Ca2+ waves seen with flecainide (Figure 6E).
Finally, we provide evidence that an increase in INa can increase Ca2+ wave frequency, using the Nav1.5 channel activator veratridine (Figure 6F). The subsequent reduction in wave frequency by increasing [Na]o shows that increasing Ca2+ efflux via NCX can reverse this effect.
Direct blockade of NCX function using a selective NCX blocker may have been a useful approach to highlight the importance of [Na+]i on waves. However, most NCX blockers have off-target effects. Even when these are limited, such as in the case of SEA-0400, they still produce a reduction of ICa via intracellular accumulation of Ca2+ which causes inhibition of the L-type Ca2+ current via Ca2+-dependent inactivation.21 Hence, it was felt that direct NCX blockade with small molecule inhibitors may yield results that could be more difficult to interpret than modulating NCX function via alterations in [Na+]o to counteract the changes in [Na+]o:[Na+]i gradient caused by INa blockade.
4.5. INa blockers and SR Ca2+ release
Although it is accepted that Na+ influx can, via subsequent efflux by NCX, cause Ca2+ entry and generation of contractile force,22 and even that Ca2+ entry via the exchanger can induce Ca2+ sparks,23 NCX has been largely neglected in the investigation of how INa inhibitors can reduce SR Ca2+ release. This is largely because, at high concentrations (e.g. 20 μM flecainide), some INa inhibitors have direct effects on RyR2 in permeabilized cells and lipid bilayer experiments.7–9 It is not possible to compare our experiments directly with such previous work since ventricular myocytes from mouse models of CPVT were used. In these studies, contrasting results were presented, with Knollman and co-workers7,9 reporting a reduction in wave frequency but increased spark frequency in both intact Casq−/− and permeabilized normal rat ventricular myocytes and Liu et al.10 finding no changes in sparks or waves with flecainide in either intact or permeabilized ventricular cardiomyocytes from RyR2R4496C+/− mice.
This inconsistency led us to investigate further despite the provision by Knollman and co-workers7,9' of multiple lines of evidence that RyR2 inhibition rather than altered Na+ flux is the predominant mechanism of action in their experiments. In contrast, we find that without an active Na+ current, no reduction in waves can be observed with flecainide. In addition, reduction in INa alone, via various pharmacological agents and voltage clamp techniques, is sufficient to cause a reduction in wave frequency via enhancement of Ca2+ efflux by NCX. Contributory to the differences between our work and other studies may be: (i) species difference and lack of CPVT model in our experiments; (ii) use of supra-therapeutic flecainide concentrations to obtain effects in permeabilized cells and lipid bilayer experiments by Knollman and co-workers while we used a therapeutically relevant concentration throughout; and (iii) lack of paired data in other studies which may reduce the power to detect differences in wave frequency (perhaps explaining the lack of efficacy seen with alternative INa blockers such as TTX and lidocaine by Hwang et al.24).
4.6. Limitations
Rapid application of caffeine is a well-accepted technique to assess SR load but may be insensitive to subtle changes in store Ca2+ content. We attempted to minimize inaccuracies by using a ratiometric dye and applying caffeine in the presence of Na+-free/Ca2+-free solution to obtain an accurate peak [Ca2+]i.
Our voltage clamp trains from −80 to 0 mV vs. −40 to 0 mV, designed to eliminate INa, could also alter NCX function during the contraction train; however, this would promote Ca2+ entry at −40 vs. −80 mV and thus increase Ca2+ waves rather than reduce them. Hence, this is not responsible for the reduction in wave frequency in the absence of INa.
4.7. Conclusions
Reducing Na+ influx during contraction in the intact cardiomyocyte reduces spontaneous diastolic SR Ca2+ release both in the form of Ca2+ sparks and waves. Given that SR load is unchanged, this is the result of reduced [Ca2+]i in the vicinity of the RyR2 (due to enhanced efflux via NCX), which reduces the open probability of the channel. In the intact rat cardiomyocyte, this is the predominant mechanism of action for the reduction in Ca2+ waves seen with flecainide at therapeutic concentrations. Other means of reducing Na+ influx, such as INa,L reduction, would be expected to reduce SR Ca2+ leak via similar mechanisms.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflicts of interest: none declared.
Funding
This work was primarily funded by the Wellcome Trust (WT092852) and the British Heart Foundation (PG/11/87/29158). ARL is supported by a British Heart Foundation Intermediate Research Fellowship (FS/11/67/28954) and the National Institute for Health Research-funded Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital. Open access funding is jointly provided by the Wellcome Trust and the British Heart Foundation.
Supplementary Material
References
- 1.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. doi:10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. doi:10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 3.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. doi:10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLennan DH, Chen SR. Store overload-induced Ca2+ release as a triggering mechanism for CPVT and MH episodes caused by mutations in RYR and CASQ genes. J Physiol. 2009;587:3113–3115. doi: 10.1113/jphysiol.2009.172155. doi:10.1113/jphysiol.2009.172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kushnir A, Marks AR. The ryanodine receptor in cardiac physiology and disease. Adv Pharmacol. 2010;59:1–30. doi: 10.1016/S1054-3589(10)59001-X. doi:10.1016/S1054-3589(10)59001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. doi:10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. doi:10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galimberti ES, Knollmann BC. Efficacy and potency of class I antiarrhythmic drugs for suppression of Ca2+ waves in permeabilized myocytes lacking calsequestrin. J Mol Cell Cardiol. 2011;51:760–768. doi: 10.1016/j.yjmcc.2011.07.002. doi:10.1016/j.yjmcc.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. doi:10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N, Denegri M, Ruan Y, Avelino-Cruz JE, Perissi A, Negri S, et al. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ Res. 2011;109:291–295. doi: 10.1161/CIRCRESAHA.111.247338. doi:10.1161/CIRCRESAHA.111.247338. [DOI] [PubMed] [Google Scholar]
- 11.Sato M, O'Gara P, Harding SE, Fuller SJ. Enhancement of adenoviral gene transfer to adult rat cardiomyocytes in vivo by immobilization and ultrasound treatment of the heart. Gene Ther. 2005;12:936–941. doi: 10.1038/sj.gt.3302476. doi:10.1038/sj.gt.3302476. [DOI] [PubMed] [Google Scholar]
- 12.Diaz ME, Cook SJ, Chamunorwa JP, Trafford AW, Lancaster MK, O'Neill SC, et al. Variability of spontaneous Ca2+ release between different rat ventricular myocytes is correlated with Na(+)-Ca2+ exchange and [Na+]i. Circ Res. 1996;78:857–862. doi: 10.1161/01.res.78.5.857. doi:10.1161/01.RES.78.5.857. [DOI] [PubMed] [Google Scholar]
- 13.Baer M, Best PM, Reuter H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature. 1976;263:344–345. doi: 10.1038/263344a0. doi:10.1038/263344a0. [DOI] [PubMed] [Google Scholar]
- 14.Nitta J, Sunami A, Marumo F, Hiraoka M. States and sites of actions of flecainide on guinea-pig cardiac sodium channels. Eur J Pharmacol. 1992;214:191–197. doi: 10.1016/0014-2999(92)90118-n. doi:10.1016/0014-2999(92)90118-N. [DOI] [PubMed] [Google Scholar]
- 15.Kohlhardt M. Block of sodium currents by antiarrhythmic agents: analysis of the electrophysiologic effects of propafenone in heart muscle. Am J Cardiol. 1984;54:13D–19D. doi: 10.1016/s0002-9149(84)80279-9. doi:10.1016/S0002-9149(84)80279-9. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa T, Koumi S, Sakakibara Y, Singer DH, Jia H, Arentzen CE, et al. An analysis of lidocaine block of sodium current in isolated human atrial and ventricular myocytes. J Mol Cell Cardiol. 1995;27:831–846. doi: 10.1016/0022-2828(95)90090-x. doi:10.1016/0022-2828(95)90090-X. [DOI] [PubMed] [Google Scholar]
- 17.Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, et al. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–C586. doi: 10.1152/ajpcell.00125.2011. doi:10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 18.Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. doi:10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka S, Hilgemann DW. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Ion and voltage dependencies of the transport cycle. J Gene Physiol. 1992;100:963–1001. doi: 10.1085/jgp.100.6.963. doi:10.1085/jgp.100.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoons G, Willems R, Sipido KR. Alternative strategies in arrhythmia therapy: evaluation of Na/Ca exchange as an anti-arrhythmic target. Pharmacol Ther. 2012;134:26–42. doi: 10.1016/j.pharmthera.2011.12.001. doi:10.1016/j.pharmthera.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Larbig R, Torres N, Bridge JH, Goldhaber JI, Philipson KD. Activation of reverse Na+-Ca2+ exchange by the Na+ current augments the cardiac Ca2+ transient: evidence from NCX knockout mice. J Physiol. 2010;588:3267–3276. doi: 10.1113/jphysiol.2010.187708. doi:10.1113/jphysiol.2010.187708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter M, Sui Z, Philipson KD, Li F, Spitzer KW, Ishida H, et al. Ca2+ sparks induced by Na/Ca exchange. Cell Calcium. 2003;34:11–17. doi: 10.1016/s0143-4160(03)00017-4. doi:10.1016/S0143-4160(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 24.Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, et al. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–135. doi: 10.1161/CIRCEP.110.959916. doi:10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.