Abstract
Polycystin-2 (PC2, TRPP2) is a Ca2+-permeable, nonselective cation channel implicated in Ca2+ transport and epithelial cell signaling. Although PC2 may contribute to Ca2+ transport in human term placenta, the regulatory mechanisms associated with Ca2+ handling in this tissue are largely unknown. In this work we assessed the regulation by Ca2+ of PC2 channel function from a preparation of apical membranes of human syncytiotrophoblast (PC2hst) reconstituted in a lipid bilayer system. Addition of either EGTA or BAPTA to the cis hemi-chamber, representing the cytoplasmic domain of the channel, and lowering Ca2+ to ∼0.6–0.8 nM, inhibited spontaneous PC2hst channel activity, with a time response dependent on the chelator tested. EGTA reduced PC2hst channel currents by 86%, with a t1/2 = 3.6 min, whereas BAPTA rapidly and completely (100%) eliminated channel activity with a t1/2 = 0.8 min. Subsequent titration with Ca2+ reversed the inhibition, which followed a Hill-type function with apparent dissociation constants of 1–5 nM, and 4 Ca2+ binding sites. The degree of inhibition by the cis Ca2+ chelator largely depended on increasing trans Ca2+. This was consistent with measurable Ca2+ transport through the channel, feeding the regulatory sites in the cytoplasmic domain. Interestingly, the reconstituted in vitro translated PC2 (PC2iv) was completely insensitive to Ca2+ regulation, suggesting that the regulatory sites are not intrinsic to the channel protein. Our findings demonstrate the presence of a Ca2+ microdomain largely accessible through the channel that controls PC2 function in human syncytiotrophoblast of term placenta.
Introduction
The human syncytiotrophoblast (hST) transfers Ca2+ from mother to the fetus, to meet developmental needs, particularly during the third trimester of pregnancy (1,2). Ca2+ entry by the hST is thought to be mediated by ion channels, whose molecular identity remains to be fully determined. A number of Ca2+-permeable ion channels present in human placenta may contribute to this function, including L-type and T-type voltage-gated channels, and the highly Ca2+ permeable TRP channel isotypes TRPV5 and TRPV6 (3–6). Limited information is presently available, however, regarding the possible contribution of these channels to Ca2+ transport in the placenta. Studies from our laboratory determined the abundant expression of another TRP isotype, polycystin-2 (PC2, TRPP2), in the hST of term placenta (7). PC2 is the product of the gene PKD2, whose mutations cause autosomal dominant polycystic kidney disease (ADPKD) (8). PC2 may be relevant to epithelial Ca2+ transport due to its large nonselective cation conductance and slight selectivity to divalent cations (7).
PC2 is expressed in various epithelial tissues and other organs, where it is largely associated with Ca2+ transport and cell activation (9,10). This channel protein is found in several cell locations, including the primary cilium, intracellular Ca2+ stores, and the plasma membrane (10,11). PC2 shares homology with voltage-gated Na+, and Ca2+ channels (8), both in the pore region, and in putative Ca2+-binding domains such as the EF-hand (8,12), that may contribute to Ca2+-dependent channel regulation (13,14). Cytoplasmic Ca2+ regulation of PC2 function in the plasma membrane may entail direct interactions with the channel protein. Vassilev et al. (15) reported the transient activation of wild-type PC2 by increasing the intracellular Ca2+ concentration from 0.1 to 1 μM in Xenopus laevis oocytes overexpressing the protein. This regulation was absent in the truncated PC2 carrying the mutation R742X, which lacks the cytoplasmic terminus, and causes ADPKD. Cai et al. (16) determined that wild-type PC2 Ca2+-dependent, bell-shaped open probability in response to voltage shifts in the mutated PC2-S812A carrying an alanine substitution in Ser812, which represents a casein kinase phosphorylation site. Previous studies from our laboratory demonstrated that intracellular Ca2+ regulation of PC2 function in the hST implicates interactions with the actin cytoskeleton and actin-associated proteins. The PC2 stimulatory effect of the actin filament disrupter cytochalasin D in reconstituted hST apical membranes was mimicked by addition of gelsolin from the cis (cytoplasmic) site of the reconstitution chamber in the presence, but not the absence, of micromolar Ca2+ (17). Thus, Ca2+ regulation of PC2 may implicate diverse mechanisms, entailing both the channel itself and/or regulatory proteins associated with it.
Herein, we explored the response to Ca2+ of PC2hst from reconstituted hST apical membranes and the in vitro translated PC2 (PC2iv) after addition of Ca2+ chelators EGTA and BAPTA. Whereas PC2iv was insensitive to changes in Ca2+ concentrations, PC2hst activity decreased to different degrees and kinetics responses depending on the chelator, and the trans Ca2+ concentration. The evidence suggested the presence of cooperative Ca2+ binding sites, which are not intrinsic to, but regulate, PC2hst. The evidence supports a feedback mechanism of a local cytoplasmic Ca2+ pool, with intracellular high-affinity Ca2+ binding sites, which are in turn modulated by Ca2+ entry through the channel in hST.
Materials and Methods
Human placenta membrane preparation
Apical hST plasma membranes from term human placenta were obtained as previously described in González-Perrett et al. (7). Briefly, normal placenta from vaginal deliveries were obtained and immediately processed. The villous tissue was fragmented, washed with ice-cold unbuffered NaCl saline (150 mM), and minced into small pieces. The fragmented tissue was processed, filtered, and centrifuged, as previously reported in González-Perrett et al. (7). The final pellet was resuspended in a buffer solution containing HEPES 10 mM, sucrose 250 mM, and KCl 20 mM and adjusted to pH 7.4, and was then aliquoted and stored frozen until the time of the experiment. Apical hST enrichment usually was higher than 26-fold.
Preparation of in vitro translated PC2
In vitro translated PC2 (PC2iv) was prepared as previously reported in González-Perrett et al. (7), with a reticulocyte lysate system TnT T7 (Promega, Fitchburg, WI). The plasmid pGEM-PKD2 encoding PC2, was in vitro transcribed and translated with a reticulocyte lysate system TnT T7 (Promega) by incubation of plasmid DNA (1 μg) and 50 μL of the reaction mixture for 90 min at 30°C. The PC2iv was introduced by dialysis into liposomes as previously reported in González-Perrett et al. (7).
Ion channel reconstitution
PC2 containing vesicles were reconstituted into lipid bilayers as previously reported in González-Perrett et al. (7) in a KCl chemical gradient (cis/trans, 150:15 mM). PC2 containing vesicles were incorporated into lipid bilayers of a reconstitution system. The lipid mixture was a 7:3 ratio of POPC and POPE (20–25 mg/mL; Avanti Polar Lipids, Birmingham, AL) in n-decane. Unless otherwise stated, the cis chamber contained a solution of KCl 150 mM, CaCl2 10 μM, and HEPES 10 mM, at pH 7.40. The trans side contained a similar solution with lower KCl (15 mM), to create a KCl chemical gradient. PC2hst was identified as previously reported in González-Perrett et al. (7), by a large conductance (∼170 pS), K+-conducting channel, which was inhibited by trans (external) amiloride, and cis (cytoplasmic side of PC2) anti-PC2 antibody, properties that also ensured its orientation in the reconstituted membrane (7).
Reagents and Ca2+ chelation
EGTA was dissolved in NaOH and titrated with HCl to reach pH 7.1. BAPTA was dissolved in dimethylsulfoxide. The concentrated reagents (16 μL and 8 μL, respectively) were diluted in either cis (1600 μL) or trans (1000 μL) chambers and buffered at pH 7.4 (10 mM HEPES), to reach final concentrations of 1 mM and 2 mM, respectively (see Results). Neither chelator nor vehicle alone affected the final pH. The Ca2+ concentration was calculated by
| (1) |
where KQ, is the dissociation constant of the Ca2+-chelator complex, [Q] is the concentration of the free chelating agent, and [CaQ] is the concentration of Ca2+ bound to Q. The final free Ca2+ concentration was estimated to be either 0.6 or 0.8 nM (pH ∼7.4) in the presence of EGTA or BAPTA, respectively (more details in the Supporting Material).
Data acquisition and analysis
Single channel currents obtained at 40–60 mV with a PC501A patch-clamp amplifier (Warner Instruments, Hamden, CT) with a 10 gigaOhm feedback resistor and signal were driven and processed with the software pCLAMP 6.2 (7). Output (voltage) signals were low-pass-filtered at 700 Hz (3 dB) with an eight-pole, Bessel-type filter (Frequency Devices, Haverhill, MA). Single channel current tracings were further filtered for display purposes only. Unless otherwise stated, the software pCLAMP, Ver. 10.0 (Axon Instruments, Foster City, CA), was used for data analysis and the software SIGMAPLOT, Ver. 11.0 (Jandel Scientific, Corte Madera, CA), was used for statistical analysis and graphics. Unless otherwise stated, all tracings shown in this study were obtained at holding potentials between 40 and 60 mV. PC2 channel identification was conducted as previously reported in González-Perrett et al. (7). Statistical significance was obtained by unpaired Student’s t-test comparison of sample groups of similar size, and accepted at p < 0.05. Average data values were expressed as the mean ± SE (N) under each condition, where n represents the total number of experiments analyzed.
Results
Effect of Ca2+ chelation on PC2hst channel function
To assess the effect of cytoplasmic Ca2+ on PC2hst channel function, hST apical vesicles were reconstituted in the presence of a KCl chemical gradient, (150 mM KCl in cis, and 15 mM trans), with symmetrical Ca2+ (10 μM), and pH 7.4 (Fig. 1 a, Ctrl). Once spontaneous channel activity was observed, EGTA (1 mM) was added to the cis chamber to reduce the free Ca2+ concentration to ∼0.6 nM. Ca2+ chelation decreased the PC2hst-mediated K+ mean currents by 86% (6.25 ± 1.8 vs. 0.88 ± 0.08 pA, N = 24, p < 0.05) with a t1/2 of 3.6 min (Fig. 1, a and c). The EGTA inhibition was never complete, leaving a remainder current (∼14%, Fig. 1 c). A lag in the response was usually observed, before a decrease in current was noticeable after EGTA addition (Fig. 1 c). The inhibitory effect of lowering cis Ca2+ on PC2hst channel function was confirmed with another Ca2+-chelating agent, BAPTA (2 mM), which produced a faster (t1/2 = 0.4 min) and complete (100%, N = 8) inhibition of channel function (Fig. 1 c). It is important to note that higher concentrations of EGTA never reproduced the effect of BAPTA, nor achieved complete inhibition but instead generated membrane instability (data not shown). Thus, only 1 mM EGTA data are reported herein.
Figure 1.
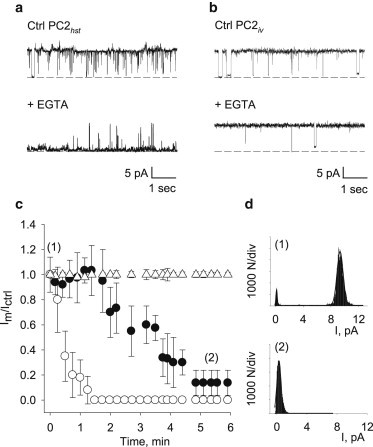
Effect of Ca2+ chelation on PC2 channel function. (a) Representative single channel tracings of reconstituted PC2hst. Addition of cis EGTA (1 mM) inhibited channel function after 5 min (N = 24). (b) Representative single channel tracings of reconstituted PC2iv as in panel a. Addition of EGTA (1 mM) to the cis chamber had no effect on channel function (N = 5). (c) Relative current (Im/Ictrl) after addition of either EGTA (1 mM, solid circles) or BAPTA (2 mM, open circles) to PC2hst and EGTA (1 mM, triangles) to PC2iv. Experimental data are the mean ± SE of 24, 8, and 5 experiments, respectively. (d) All-point histograms of single channel tracings from panel a.
Effect of cis Ca2+ chelation on PC2iv channel activity
The above results indicated the presence of Ca2+ regulatory sites in PC2hst that control its function. Thus, PC2iv was also reconstituted in a lipid bilayer system. Spontaneous PC2iv single channel currents were neither modified by addition of EGTA (1 mM) to the cis side (8.1 ± 0.4 pA vs. 8.2 ± 0.3 pA, N = 5, Fig. 1, b and c) nor by BAPTA (2 mM) (data not shown). Actually, the PC2iv protein did not respond to high Ca2+ in the trans compartment either (see Table S1 in the Supporting Material). Thus, in contrast to PC2hst, Ca2+ chelation did not affect single channel currents through the PC2iv isolated protein.
PC2hst function recovery by Ca2+addition to the cis chamber
To determine whether the inhibition of PC2hst by Ca2+ chelation was reversible, the cis chamber was titrated with increasing concentrations of Ca2+ in the presence of chelator (Fig. 2 a). The cis free Ca2+ was correlated with the recovery of PC2hst channel function that was obtained as fractional currents after either EGTA or BAPTA conditions (Fig. 2, a and b). Inhibition from either chelator was entirely reversible. Mean recovery data as a function of [Ca2+]cis for both chelating agents were best fitted with a Hill-type equation (18) (Fig. 2 b) that followed
| (2) |
where KD is the Ca2+ apparent dissociation constant for the binding sites associated with the nH, namely the Hill coefficient, and Ib and Imax are the basal (Ca2+ insensitive) and maximal currents, respectively. The results showed a KD = 5.01 ± 0.06 nM (Ib = 0.08, nH = 4.0 ± 0.3) in the presence of EGTA, and a KD = 1.75 ± 0.13 nM (Ib = 0.04, nH = 4.0 ± 0.2) in the presence of BAPTA (Fig. 2 b). These results suggested the presence of at least four regulatory Ca2+ binding sites of PC2hst channel function, although nH does not represent the actual number of binding sites. In its simplified form, the Hill equation describes an allosteric interaction between originally identical, unoccupied binding sites (18,19). Changes in affinity occur as these sites become increasingly occupied. Thus, nH is, strictly speaking, a measurement of cooperation, not of the actual number of binding sites (or their intrinsic binding affinity). The simplified Hill equation will have an nH, which will only represent the actual number of binding sites, as positive cooperativity becomes very strong (nH → n). Strong cooperation was suggested by the nonlinear Scatchard and Hanes plots (Fig. 2, c and d) and calculation of Rs which, as defined by Bisswanger (19), is the ratio of ligand concentration at 90 and 10% saturation, respectively, with a value of 81 for a normal hyperbolic saturation curve. The value Rs decreases with the strength of positive cooperativity. We defined Rs as the ratio between 90 and 10% saturation values from the titration curves, respectively (18,19). The Rs values, 4.31 and 3.44 obtained after recovery from EGTA and BAPTA inhibition, respectively, were in agreement with a strong positive cooperativity (Rs ≪ 81 (18,19)).
Figure 2.
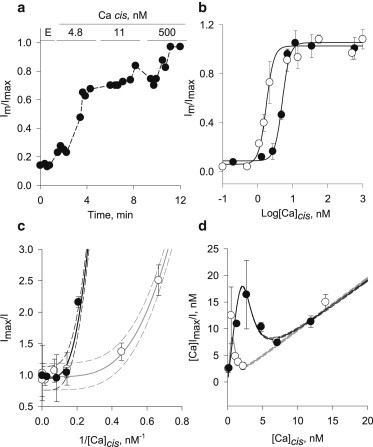
Recovery by Ca2+ of PC2hst channel function after Ca2+ chelation. (a) Time response of current change after addition of different Ca2+ concentrations to the cis chamber after inhibition by EGTA. (b) Recovery curves as a function of cis Ca2+ concentration, after addition of either EGTA (solid circles) or BAPTA (open circles). Experimental data are the mean ± SE of seven and five experiments, respectively. (Solid lines) Best fitted Hill-type equation. Scatchard (c), and Hanes (d) plots of fitted EGTA (black line) and BAPTA (shaded line) recovery curves. (Dashed lines) Mean ± SE of fitted results.
We further evaluated an expanded Hill equation considering that if ligand binding cooperativity is very marked, then the fraction of all channel-ligand states containing fewer than n molecules of ligand would be negligible at any concentration that is appreciable compared to the intrinsic constant, Ks (18). In such cases, the Hill equation may be rewritten with the assumption that nH = 4, such that
| (3) |
where a, b, and c are factors by which the intrinsic binding constants have been increased in each step, and a3b2cKs4 = KD4, in Eq. 3. The parameters obtained from the recovery dose-response fitted data to Eq. 3 are shown in Table S2, where the highest dissociation constant for the first binding site of either EGTA or BAPTA conditions were 111.6 and 105 nM, respectively.
Diffusional limitation corrections
Although Ca2+ binding interactions to the putative binding sites after recovery from inhibition by either chelator were almost identical (see Table S2), current decay (Fig. 1 c) was faster and stronger in the presence of BAPTA as compared with EGTA (7:1). This difference could not be explained by either binding interaction or by the forward rate constants of the chelators, which relate as 100:1 (20). Thus, we explored the possibility that a diffusional limitation of the chelators could be limited to the Ca2+ binding sites. Thus, we corrected the recovery curves by a transport coefficient (h) ((19), also see the Supporting Material), considering the diffusive properties of EGTA and BAPTA (20,21). This coefficient represented the existence of a diffusional layer between the channel and bulk Ca2+, which would have distinct diffusive properties for either chelator. Under these conditions, diffusional Ca2+ flow (Jdiff) from the bulk solution (C2) to the diffusional limitation layer (C1) would be described as
| (4) |
where δ represents the layer thickness, and DC the diffusion constant of the chelating agent (20). Once Ca2+ accesses the regulatory sites, PC2hst activation will follow the function described by Hill (Eq. 2). Under steady-state conditions, Jdiff and the activation process would occur at the same velocity (19), such that
| (5) |
To estimate h, we plotted Im/Imax versus time after Ca2+ addition to the cis chamber following either EGTA or BAPTA inhibition, respectively (see Fig. S1 a in the Supporting Material). The slopes of the curves were plotted versus the cis Ca2+ concentration (see Fig. S1 b). The transport coefficients obtained, 4.3 ± 1.3 × 10−3 s−1 nM−1 (r = 0.82) and 2.5 ± 1.7 × 10−3 s−1 nM−1 (r = 0.58) for EGTA and BAPTA, respectively, were not statistically different from each other (p > 0.05), indicating that the two chelators did not display any relevant diffusional differences. Even further correction taking into account the actual diffusional coefficient ratio of the chelators (DEGTA/DBAPTA = 1.1), based on their difference in molecular size (molecular mass 380.35 vs. 476.43 g/mol for EGTA and BAPTA, respectively), did not render further differences, but actually favored slightly EGTA over BAPTA (data not shown). These coefficients were corrected considering that Ca2+ was added at an average distance (d) of 500 μm from Ca2+ regulatory sites (approximately center of the cuvette), and h could then be calculated as
| (6) |
where i represents either chelator.
The h values obtained were 2.15 μm s−1 and 1.25 μm s−1, for EGTA and BAPTA, respectively. The corrected PC2hst reactivation curves (see Fig. S1 c), following Bisswanger (19), showed a left shift in the corrected KD (KD′) that was greater in the case of EGTA than of BAPTA, with corrected values of 4.70 ± 0.02 nM (nH = 4.1 ± 0.07), and a KD of 1.26 ± 0.03 nM (nH = 4.5 ± 0.04) for Ca2+, respectively. Thus, the values fell within the experimental error, such that the diffusional contribution would be negligible for either chelator.
Effect of external Ca2+ on PC2hst channel function
To explore whether external Ca2+ also had an effect on PC2hst channel function, we increased the Ca2+ concentration from the trans side after channel inhibition with EGTA. A rise in trans Ca2+ rapidly increased PC2hst channel function, which reached a peak current higher (240%) than control (before EGTA addition) at ∼5 mM Ca2+ (Fig. 3 a). Further addition of external Ca2+ (10–50 mM CaCl2) decreased the mean currents through PC2hst. In the presence of cis BAPTA, however, increasing concentrations of trans Ca2+ did not restore PC2hst channel function (Fig. 3 b). These findings raised the hypothesis that the differences in inhibition elicited by either chelator may be associated with a remainder channel function, as in the case of EGTA, which should drive Ca2+ through, from the trans compartment. This was explored in the next section.
Figure 3.
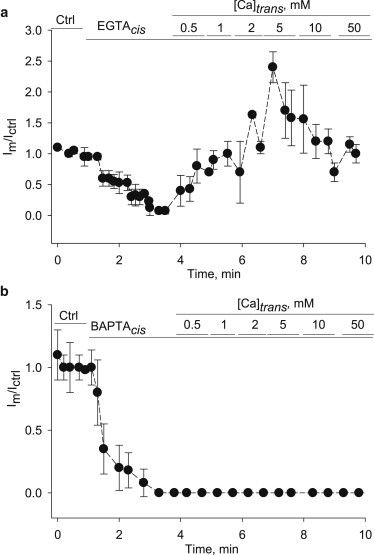
Effect of trans Ca2+ on PC2hst function. (a) Kinetics of the Im/Ictrl ratio under control conditions (Ctrl), after cis EGTA addition, followed by increasing Ca2+ concentrations added to the trans side. Experimental data (solid circles) are the mean ± SE (N = 5). (b) Similar data as in panel a, after addition of BAPTA. Experimental data (solid circles) are the mean ± SE (N = 3).
Effect of Ca2+ chelation at different initial trans Ca2+ concentrations
To further explore the changes observed in PC2hst channel function by titration of external Ca2+, experiments were also designed to test the effect of the cis chelator at a given initial trans Ca2+ concentration. The experiment was initiated with a given trans Ca2+ already present in the chamber before addition of the cis chelator. In the presence of low external Ca2+ (0.6 nM), addition of EGTA (Fig. 4 a) elicited a rapid and almost complete inhibition with t1/2 = 0.4 min, which was rather similar to the BAPTA effect with 10 μM Ca2+ trans. Conversely, in the presence of high (1 mM) external Ca2+, cis EGTA only had a transient and incomplete effect, reaching 50% inhibition (Fig. 4 a). Because the BAPTA effect was fast and complete in symmetrical 10 μM Ca2+, we also tested the effect of BAPTA while in the presence of high (1 mM) trans Ca2+, which only elicited a transient inhibition of the currents by 20%, returning to control values in ∼1 min (Fig. 4 b). Thus, the inhibitory effect of cis Ca2+ chelation (with either chelator) on PC2hst channel function strongly depended on the trans Ca2+ concentration. This would suggest that the Ca2+ chemical gradient, and thus permeation through the channel pore, modifies the availability of Ca2+ in the proximity to the regulatory sites in the cis compartment.
Figure 4.
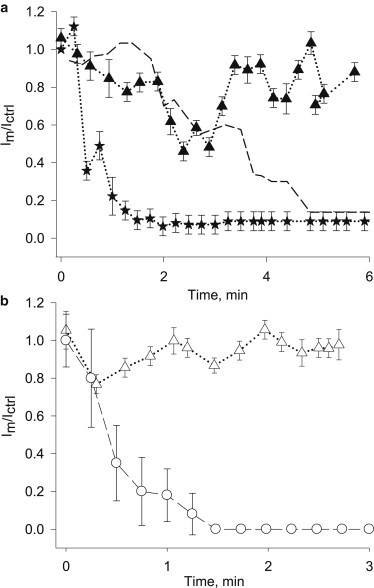
Effect of trans Ca2+ on PC2hst channel inhibition. (a) Kinetics of channel recovery (Im/Ictrl) after EGTA addition at different initial trans Ca2+ concentrations. Experimental data were obtained in 1 mM (triangles) and 0.6 nM (stars) Ca2+ conditions, respectively. Experimental data are expressed as mean ± SE (N = 3). (b) Kinetics of channel recovery (Im/Ictrl) after BAPTA addition in the presence of 1 mM trans Ca2+ concentration (triangles). Experimental data are expressed as mean ± SE (N = 3). (Dashed lines) Values obtained in the presence of 10 μM trans Ca2+ (Fig. 1c).
Calculation of Ca2+ flux coefficient through PC2hst
The results obtained in the presence of different trans Ca2+ concentrations allowed us to determine the contribution PC2hst made to Ca2+ transport and its delivery to the cis side. Ca2+ transport through the channel was calculated as the rate of PC2hst channel recovery. To this end, we increased the trans Ca2+ concentration after cis EGTA channel inhibition, and then obtained the fractional recovery current Im/Imax as a function of [Ca2+]trans (Fig. 5 a, left). The relationship was well approximated by a Michaelis-Menten-type function with an apparent dissociation constant of 2.8 ± 0.92 mM and n = 1.0 ± 0.4. The relationship t1/2 versus [Ca2+]trans evidenced a linear function (Fig. 5 b). Because the results with cis Ca2+ titration indicated that the regulatory effect of Ca2+ on PC2hst was only possible from the cis side, we compared the Im/Imax for both [Ca2+]trans and [Ca2+]cis titrations, thus obtaining the ratio between trans and cis Ca2+ to reach similar recovery values (i.e., Fig. 5 a, dashed line). This recovery ratio was thus associated with a Ca2+ influx (JCa) for each trans Ca2+ concentration. Further, the trans Ca2+ concentration was inversely correlated with the average time it took for the channel to recover to a given value. We next obtained a curve of cis Ca2+ versus trans Ca2+ (to obtain the same fractional current) divided by the time necessary to reach a steady recovery response (Fig. 5 c). The best fitted theoretical curve had a slope of 2 × 10−5 s (Fig. 5 c), which represents the Ca2+ flux coefficient (tCa) through the PC2hst channel. A summary of conclusions obtained from these calculations, including the Ca2+ permeability at physiological Ca2+ gradients, is given in the Discussion (see below).
Figure 5.
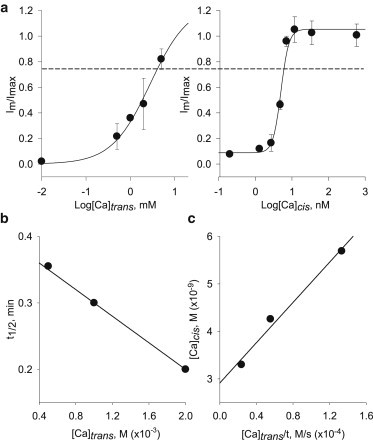
Recovery of PC2hst channel activity by either cis or trans Ca2+. (a) (Left) PC2hst function (Im/Imax) recovery curve in the presence of increasing trans Ca2+ concentrations. (Right) Recovery curve in the presence of increasing cis Ca2+ concentrations (from Fig. 2b). (Dashed line) Im/Imax = 0.75 reached in both graphs. (b) Experimental t1/2 as a function of trans Ca2+ concentration. The curve was fitted with t1/2 = m [Ca2+]trans + t0, where m = −100 min.M−1 and t0 = 0.40 min. (c) Correlation between cis and trans Ca2+ concentrations generating similar Im/Imax ratios.
Phenomenological model
The encompassed results were explored in terms of a phenomenological model (Eq. 7) that approximated the temporal response of the PC2hst currents (Im/Ictrl) after addition of either EGTA or BAPTA for the various cis and trans Ca2+ concentrations, as
| (7) |
where the parameters A, B, C, and D are constants, and t is the time after addition of the chelator. The fitting parameters are shown in Table S3. This model considered that the overall kinetics of current decay after chelation would occur due to two simultaneous processes taking place after addition of the cis chelator. The first contribution would be a deficit in local Ca2+ which, in turn, modified the Ca2+ bound to the regulatory sites associated with, but not intrinsic to, the PC2hst channel. A second contribution to this local pool is associated with an entry step feeding Ca2+ through the open channel. Thus, the Ca2+ associated with the regulatory binding sites would primarily be determined by:
-
1.
The initial Ca2+ concentration (represented by A, relative value);
-
2.
Ca2+ entry through the channel (Bt); and
-
3.
Ca2+ retrieval from the regulatory sites as the result of the chelating reaction and diffusion (Cte−Dt).
This equation best approximated all experimental data from the various conditions tested (Fig. 6, a and b).
Figure 6.
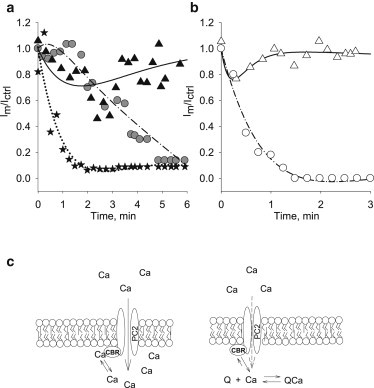
Phenomenological model. (a) Fitted curves obtained with the phenomenological model (Eq. 7) for EGTA additions. Experimental data were obtained after cis EGTA addition, for different trans Ca2+ concentrations, 1 mM, 10 μM, and 0.6 nM (triangles, circles, and stars, respectively). (b) Fitted curves obtained for BAPTA conditions and two different trans Ca2+ concentrations, 1 mM, and 10 μM (triangles and circles, respectively). The fitting parameters obtained are listed in Table S3 in the Supporting Material. (c) Diagram of Ca2+ interactions with PC2hst. Ca2+ flows through the channel pore. The mobile chelating agents (Q) compete for a local pool of Ca2+ (fixed buffer) on cis side, contained in a microdomain accessible from the trans side while the channel is open. PC2hst channel function will depend on the Ca2+ level reached in the cis microenvironment by interaction with the Ca2+ binding region (CBR).
Discussion
The human placenta transfers ∼30 grams of Ca2+ during late gestation. The mechanisms implicated in this transepithelial transport are the subject of active research (4,22,23). PC2 is implicated in Ca2+ transport in Ca2+ handling epithelia including the kidney and the placenta. The first functional characterization of PC2 as a Ca2+-permeable nonselective cation channel was made in hST from term placenta (7). Little is known, however, as to how Ca2+ controls PC2 function in this epithelium. Herein, we explored the regulation by Ca2+of PC2hst from apical hST membranes we have previously characterized (7). We observed that a decrease in free cis Ca2+ to subnanomolar levels obtained with either EGTA or BAPTA extensively inhibited PC2hst channel function. Current decay after chelation with EGTA (symmetrical 10 μM Ca2+) was slower and incomplete, compared with the inhibition observed with BAPTA.
Surprisingly, similar experiments conducted with PC2iv showed no changes in channel activity. This discrepancy suggests that the PC2hst channel complex is somewhat different from the isolated protein. A difference may relate to a channel complex in the hST preparation that contains other channel subunits that are absent in the in vitro material and/or themselves regulated by Ca2+. TRP channel monomers other than PC2 have been reported to interact with PC2 (24). In such a case, the heterocomplex may respond to Ca2+ in a distinct manner not observed for the isolated protein. We previously have compared PC2 channel function from various preparations in membranes, including the hST, with isolated protein from PC2-overexpressing Sf9 cells (7) and renal epithelial cells (25,26) as well as the in vitro translated material (7,24,27). We thereby identified a number of intrinsic features of the channel that were confirmed throughout. All these PC2 preparations essentially show the same functional channel properties. TRPC1-PC2 heterocomplexes, for example, show distinct structural-functional properties, including overall and subconductance states, as well as pH and amiloride sensitivity. Thus, it is likely that the PC2hst channel may actually present distinct associated properties instead, which modified Ca2+ binding, but not its biophysical properties.
Changes in Ca2+ are already known to regulate PC2 (16,26), although these studies were conducted with channel preparations that may contain PC2-associated proteins as well. Ehrlich’s group recently found Ca2+-dependent conformational changes of the isolated carboxy terminus of PC2 (27), supporting the contention that the PC2 channel has Ca2+-interacting domains. It is presently unknown as to whether this phenomenon conveys any functional properties to the complete channel complex. Our contemporary observations suggest that the cis Ca2+ regulatory sites were not intrinsic to the channel, but instead related to associated proteins. The actual Ca2+-binding linkers implicated in this regulation remain to be identified. Nonetheless, we have preliminary evidence that the PC2iv channel can be made responsive to Ca2+ in a manner rather similar to the PC2hst material, simply by adding α-actinin (M. d. R. Cantero and H. F. Cantiello, unpublished). This is in agreement with previous studies (17,28), which demonstrated a direct structural-functional interaction between PC2 and the actin-associated protein α-actinin, which binds to, and modulates PC2hst channel function (28). Further, F-actin severing proteins, such as gelsolin, also regulate PC2hst in a Ca2+-dependent manner (17). Thus, cytoskeletal structures present in apical hST vesicles may provide a kinetic compartment, forming a Ca2+-dependent microdomain that controls PC2 channel function.
Number of regulatory Ca2+ binding sites associated with the PC2 channel
The reversal from inhibition by titration with Ca2+ after chelation followed a well-approximated Hill equation. The nH obtained in the presence of either chelator, was consistent with at least four Ca2+ regulatory sites, with apparent KD in the range of 1–5 nM. It is important to mention that although the KD and nH in Eq. 2 are mathematically possible, Hill assumptions are not physically possible except for the condition in which n = 1 (29). Thus, evaluation of the intrinsic dissociation constant for the first site, KS, was necessary to better understand the potential effect of Ca2+ at physiological levels. A strong positive cooperativity between the Ca2+ binding sites was observed by the nonlinear curves in the Scatchard and Hanes plots, and confirmed by the large shift in Rs to values much lower than 81 (4.31 and 3.44 for EGTA and BAPTA, respectively; see Results) (18,19). Although nH will be between the limits 1 < nH < n, it is also important to note that the more pronounced the positive cooperativity, the closer nH will approach n, which is the true number of binding sites, and when cooperativity is maximal, nH equals n. We further explored the decrease in the affinity constant for the first binding by expansion of the Hill equation, considering the interaction factors (Eq. 3). The summarized data in Table S2 clearly indicated that the first occupied site has an affinity of 105–111.6 nM, which is consistent with basal cytosolic Ca2+, and an increase in affinity between 10 and 100 times for the other sites, as expected (18,19).
Differences between chelator responses
There were clear kinetic differences between the electrical responses of BAPTA and EGTA in symmetrical Ca2+ (10 μM). BAPTA was seven-times faster (3.6 vs. 0.4 min) than EGTA. This phenomenon could not be explained by differences in the forward rate constant (kon) for either chelator, because the BAPTA kon rate is a hundred times faster than that of EGTA (20). Moreover, EGTA produced a partial inhibition whereas BAPTA completely inhibited PC2hst channel function. Despite the fact that the experiments were conducted in a bilayer reconstitution system which may be envisioned as an entirely exchangeable infinite reservoir, the constraints of a kinetic compartment were in agreement with previous findings in cell models (30,31). The fast exocytotic response in melanotropic cells, a phenomenon coupled to voltage-gated Ca2+ channels, is very sensitive to mobile Ca2+ buffers, such that EGTA elicits a complete block of the response (31). Kits et al. (31) found that BAPTA blocked exocytosis only twice as effectively as EGTA. Using computer simulations, Kits et al. (31) demonstrated that these results could be explained neither by free diffusion nor by the binding rates of the mobile buffers, but instead that saturation of local Ca2+ buffers was involved. In their studies, a diffusional barrier for both Ca2+ and buffer molecules reduced diffusion 1000–10,000 times to generate similar local Ca2+ concentrations for specific concentrations of EGTA and BAPTA. Kits et al. concluded that when the effects of a fixed buffer are taken into consideration, the experimental results are not explained by diffusion or mobile buffer properties. This is qualitatively in agreement with our findings that show that the transport coefficient (h) obtained to correct the diffusional contribution of Ca2+ to both chelators was unable to explain the experimental differences for either chelator. Local buffer saturation may occur due to the existence of diffusional barriers formed by a kinetic constraint to Ca2+ binding. Thus, the influence of mobile Ca2+ buffers on Ca2+-dependent processes far from the site of Ca2+ entry may actually depend largely on submembranous compartmentalization (31).
Other studies (32,33) similarly showed that it is not the difference in binding rate but rather the difference in affinity constants that is relevant in the Ca2+ chelation by EGTA and BAPTA. In general, fixed buffers will slow down and prolong the occurrence of Ca2+-dependent processes because they contribute to initial chelating of ions but release Ca2+ when free levels drop, thus allowing for extended duration of the process (34). Naraghi and Neher (20) showed that fixed buffers do not affect the steady-state concentration of Ca2+ in the microdomain, but somewhat prolong the time course leading to the steady-state condition. Only very high levels of fixed buffer cause a drop in Ca2+ concentration very close to the membrane. Fixed buffers then will become dominant over mobile buffers, which results in equal effects of different concentrations of EGTA and BAPTA close to the membrane. This is particularly relevant in the context of our findings, which require kinetic constraints to slow down the response in the range of minutes, several orders-of-magnitude slower than either diffusion or binding rates.
Ca2+ transport through the PC2 channel
The differences in response between cis EGTA and BAPTA in the presence of 10 μM trans Ca2+ were twofold. On the one hand, there was a faster inhibition kinetics with BAPTA than EGTA; on the other hand, the inhibition with EGTA was incomplete, leaving a remainder channel function. This remaining activity proved to be efficient in reversing the inhibitory effect of the chelator by titration with trans Ca2+. Failure to recover channel function by addition of trans Ca2+ after complete inhibition by cis BAPTA suggested that the Ca2+ regulatory sites were only accessible from the cis side, and thus inaccessible once the channel closed. Titration after (cis) chelator inhibition therefore depended on both the chelator and the concentration of trans Ca2+ at the beginning of the experiment. This was confirmed by the second set of experiments in which cis Ca2+ chelation was conducted at different initial trans Ca2+ concentrations. In the presence of higher trans Ca2+ (1 mM), for example, cis EGTA only elicited a transient and lower inhibition. Similarly, cis BAPTA inhibition in higher initial trans Ca2+ (1 mM) was also transient, resembling the EGTA effect at lower trans Ca2+. This is consistent with a scenario whereby PC2-mediated Ca2+ fed through the channel into the cytoplasmic domain would help maintain channel function. This contribution could be quantified from the inverse relationship observed between t1/2 of the activating response, and the external Ca2+ concentration, such that the higher the Ca2+ gradient, the faster the saturation of the cis Ca2+ regulatory sites.
From the cis-to-trans Ca2+ response ratio, we obtained a flux coefficient (tCa) of 2 × 10−5 s, reflecting the average time it took for Ca2+ to pass through the pore (trans→cis), then access and activate the cis-located Ca2+ regulatory binding sites. The tCa also provided a tool for estimating the Ca2+ flux (JCa) through the PC2hst channel pore at physiologically relevant Ca2+ concentrations. To turn this value into either a meaningful JCa or current (ICa), we created a geometrical model of the channel pore, envisioned as a cylinder of area π × r2, and volume π × r2 × L. We assumed an inner radius of the channel to be at least 5 nm, based on its cationic permeability properties (33). Other dimensions included a maximum diameter of 20 nm and total length of 10 nm, based on a 1:5 total ratio, and 10 times the protruding section of the channel (L), respectively, as we recently determined by atomic force microscopy (35). Thus, the internal volume (Vpore) that a given ion should travel would be between 0.079 and 1.25 × 10−17 cm3. The theoretical Ca2+ permeability of the pore could then be estimated by
| (8) |
where PCa is the Ca2+ permeability coefficient in this concentration range. The calculated PCa was between 0.39 and 6.28 × 10−13 cm3/s. Thus, JCa through PC2hst would then be obtained as postulated by Läuger (36) such that
| (9) |
where a = RTV/zF and V is the applied holding potential. The value obtained, JCa, in the range of 9.32 × 10−20 mol/s and 1.48 × 10−18 mol/s, would be consistent with a PC2hst Ca2+ conductance of ∼0.15–2.38 pS at 1 mM external Ca2+. These results were in agreement with a boundary condition obtained by calculation of the maximal Ca2+ permeability , as estimated by Läuger (36),
| (10) |
where ro is a hemispherical surface parameter that represents an effective capture radius. Considering an ro = 0.1 nm (36) and the Ca2+ diffusion coefficient of 660 μm2/s (20), the and would be 4.15 × 10−13 cm3/s and 3.92 × 10−15 mol s−1, respectively, giving a maximal conductance of 6.30 pS. This value represents a very high Ca2+ transport rate compared to most Ca2+-permeable channels under the imposed electrochemical conditions (37). In addition, we validated the Ca2+ transport data by direct electrodiffusional experiments (see the Supporting Material). We obtained current-to-voltage (I/V) relationships of PC2hst single channel currents under biionic conditions with a cis→trans K+ gradient and a high trans Ca2+ concentration (90 mM), such that Ca2+ currents from trans to cis could be determined (see Fig. S2). The I/V data obtained were well fitted with both the Goldman-Hodgkin-Katz equation and an absolute rate theory model containing two internal Ca2+ binding sites within the pore of the channel (38). The KD values calculated were used to obtain a Michaelis-Menten-type gPC2 versus Ca2+trans curves. A Ca2+ single channel conductance in the range of 0.12–1.33 pS was obtained for 1 mM trans Ca2+, in close agreement with 0.15–2.38 pS, obtained from the Ca2+ transfer data.
Size of the Ca2+ microdomain
From the range obtained under our experimental conditions, and the average time it took for Ca2+ to attain a given activation response, say an increase in channel activity from 20 to 80%, it could be estimated that the delivery of ∼5 × 10−17 moles of Ca2+ to the cis side would be required. This estimation suggests a local volume associated with the kinetic differences between EGTA and BAPTA of ∼8–10 nL, which reflects a large microdomain associated with the PC2hst channel. Considering the geometry and expected diffusional properties of the cis chamber, it is expected that this microdomain is most likely associated with the cytoskeletal network linked to the channel in the hST apical vesicles (as seen in Montalbetti et al. (17)).
Phenomenological model
The results in this report were best followed by a phenomenological model (Eq. 7) describing the kinetic changes in PC2hst channel activity as a function of Ca2+, and entails, as indicated above, the presence of a local pool of Ca2+ (microdomain) buffering Ca2+ regulatory sites associated with the cytoplasmic side of the PC2hst channel, whose saturation would depend on two contributing processes: the high affinity binding sites of an associated protein and the rate of Ca2+ delivered through the channel. The first contribution is evidenced as Ca2+ is depleted by addition of the chelating agent. The other (compensating) contribution lies in the Ca2+ entry through the channel pore. This is described as a linear parameter related to the electrochemical Ca2+ gradient. The parameters obtained using this model (see Table S3) could be interpreted as the contribution of both Ca2+ input and output to and from the microdomain near the channel, respectively.
Competition between Ca2+ influx through the channel, and Ca2+ chelation by either mobile buffer, determines the degree of depletion (or not) of the microdomain, and therefore the effected system, PC2hst function. Thus, in the presence of BAPTA and 10 μM cis Ca2+, the contribution of the exponential term is larger, compared to that obtained in the presence of EGTA. This indicates a greater depletion of the microdomain in the presence of BAPTA. In the presence of BAPTA and 1 mM trans Ca2+, however, the absence of channel inhibition would reflect the larger contribution of Ca2+ entry through the channel, from the trans side. Moreover, in the presence of either chelator and 1 mM trans Ca2+, the linear term of Eq. 3 representing Ca2+ influx through the channel is greater than in the presence of lower concentrations (0.6 nM and 10 μM). Thus, Ca2+ transport (trans → cis) through the channel largely contributes to maintain the channel open (Fig. 6 c).
Conclusions
The data in this report indicate that a local cytoplasmic Ca2+ binding region associated with PC2 (CBR, Fig. 6 c) regulates its channel activity in the hST preparation. This Ca2+ regulatory region is absent in the isolated protein, and represents a Ca2+ binding protein(s) that associate with, and confer Ca2+-dependence to, the channel. Ca2+ feed through the channel would replete and control this Ca2+ microdomain. The data suggest a high intrinsic Ca2+ permeability by PC2.
Acknowledgments
The authors thank Dr. Patricia Bonazzola for unconditional support and encouragement, and acknowledge Dr. Fernando Marengo (Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires), for providing BAPTA.
M.d.R.C. and H.F.C. are members of the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina.
Supporting Material
References
- 1.Givens M.H., Macy I.C. The chemical composition of the human fetus. J. Biol. Chem. 1933;102:7–17. [Google Scholar]
- 2.Salle B.L., Senterre J., Putet G. Vitamin D metabolism in preterm infants. Biol. Neonate. 1987;52(Suppl 1):119–130. doi: 10.1159/000242749. [DOI] [PubMed] [Google Scholar]
- 3.Brunette M.G. Calcium transport through the placenta. Can. J. Physiol. Pharmacol. 1988;66:1261–1269. doi: 10.1139/y88-207. [DOI] [PubMed] [Google Scholar]
- 4.Belkacemi L., Bédard I., Lafond J. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium. 2005;37:1–8. doi: 10.1016/j.ceca.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Hoenderop J.G.J., Nilius B., Bindels R.J.M. Calcium absorption across epithelia. Physiol. Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 6.Hoenderop J.G., Vennekens R., Nilius B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J. Physiol. 2001;537:747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Perrett S., Kim K., Cantiello H.F. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl. Acad. Sci. USA. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochizuki T., Wu G., Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 9.Cantiello H.F. Regulation of calcium signaling by polycystin-2 (Review) Am. J. Physiol. Renal Physiol. 2004;286:F1012–F1029. doi: 10.1152/ajprenal.00181.2003. [DOI] [PubMed] [Google Scholar]
- 10.Tsiokas L. Function and regulation of TRPP2 at the plasma membrane. Am. J. Physiol. Renal Physiol. 2009;297:F1–F9. doi: 10.1152/ajprenal.90277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J. Polycystins and primary cilia: primers for cell cycle progression (Review) Annu. Rev. Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 12.Imredy J.P., Yue D.T. Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 13.Li Q., Liu Y., Chen X.Z. The calcium-binding EF-hand in polycystin-L is not a domain for channel activation and ensuing inactivation. FEBS Lett. 2002;516:270–278. doi: 10.1016/s0014-5793(02)02513-9. [DOI] [PubMed] [Google Scholar]
- 14.Nilius B., Weidema F., Bindels R.J. The carboxyl terminus of the epithelial Ca2+ channel ECaC1 is involved in Ca2+-dependent inactivation. Pflugers Arch. 2003;445:584–588. doi: 10.1007/s00424-002-0923-9. [DOI] [PubMed] [Google Scholar]
- 15.Vassilev P.M., Guo L., Zhou J. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem. Biophys. Res. Commun. 2001;282:341–350. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y., Anyatonwu G., Somlo S. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J. Biol. Chem. 2004;279:19987–19995. doi: 10.1074/jbc.M312031200. [DOI] [PubMed] [Google Scholar]
- 17.Montalbetti N., Li Q., Cantiello H.F. Cytoskeletal regulation of calcium-permeable cation channels in the human syncytiotrophoblast: role of gelsolin. J. Physiol. 2005;566:309–325. doi: 10.1113/jphysiol.2005.087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segel I.H. Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems. Wiley-Interscience; New York: 1975. Enzyme kinetics. [Google Scholar]
- 19.Bisswanger H. Principles and Methods. 2nd Ed. Wiley-VCH, Weinheim; Germany: 2008. Enzyme kinetics. [Google Scholar]
- 20.Naraghi M., Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klingauf J., Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys. J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau R., Hamel A., Lafond J. Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta. Biol. Reprod. 2002;67:1473–1479. doi: 10.1095/biolreprod.102.005397. [DOI] [PubMed] [Google Scholar]
- 23.Bernucci L., Henríquez M., Riquelme G. Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta. 2006;27:1082–1095. doi: 10.1016/j.placenta.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P., Luo Y., Cantiello H.F. The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum. Mol. Genet. 2009;18:1238–1251. doi: 10.1093/hmg/ddp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., Dai X.Q., Chen X.-Z. A modified mammalian tandem affinity purification procedure to prepare functional polycystin-2 channel. FEBS Lett. 2004;576:231–236. doi: 10.1016/j.febslet.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Chen X.Z., Segal Y., Zhou J. Transport function of the naturally occurring pathogenic polycystin-2 mutant, R742X. Biochem. Biophys. Res. Commun. 2001;282:1251–1256. doi: 10.1006/bbrc.2001.4720. [DOI] [PubMed] [Google Scholar]
- 27.Ćelić A.S., Petri E.T., Boggon T.J. Calcium-induced conformational changes in C-terminal tail of polycystin-2 are necessary for channel gating. J. Biol. Chem. 2012;287:17232–17240. doi: 10.1074/jbc.M112.354613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q., Montalbetti N., Chen X.Z. Alpha-actinin associates with polycystin-2 and regulates its channel activity. Hum. Mol. Genet. 2005;14:1587–1603. doi: 10.1093/hmg/ddi167. [DOI] [PubMed] [Google Scholar]
- 29.Weiss J.N. The Hill equation revisited: uses and misuses. FASEB J. 1997;11:835–841. [PubMed] [Google Scholar]
- 30.Mansvelder H.D., Kits K.S. The relation of exocytosis and rapid endocytosis to calcium entry evoked by short repetitive depolarizing pulses in rat melanotropic cells. J. Neurosci. 1998;18:81–92. doi: 10.1523/JNEUROSCI.18-01-00081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kits K.S., de Vlieger T.A., Mansvelder H.D. Diffusion barriers limit the effect of mobile calcium buffers on exocytosis of large dense cored vesicles. Biophys. J. 1999;76:1693–1705. doi: 10.1016/S0006-3495(99)77328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seward E.P., Nowycky M.C. Kinetics of stimulus-coupled secretion in dialyzed bovine chromaffin cells in response to trains of depolarizing pulses. J. Neurosci. 1996;16:553–562. doi: 10.1523/JNEUROSCI.16-02-00553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowycky M.C., Pinter M.J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys. J. 1993;64:77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys. J. 1990;57:313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anyatonwu G.I., Ehrlich B.E. Organic cation permeation through the channel formed by polycystin-2. J. Biol. Chem. 2005;280:29488–29493. doi: 10.1074/jbc.M504359200. [DOI] [PubMed] [Google Scholar]
- 36.Läuger P. Diffusion-limited ion flow through pores. Biochim. Biophys. Acta. 1976;455:493–509. doi: 10.1016/0005-2736(76)90320-5. [DOI] [PubMed] [Google Scholar]
- 37.Demuro A., Parker I. Imaging single-channel calcium microdomains. Cell Calcium. 2006;40:413–422. doi: 10.1016/j.ceca.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantero M.R., Cantiello H.F. Effect of lithium on the electrical properties of polycystin-2 (TRPP2) Eur. Biophys. J. 2011;40:1029–1042. doi: 10.1007/s00249-011-0715-2. [DOI] [PubMed] [Google Scholar]
- 39.Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 1978;72:409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


