Abstract
Multiple data are available on the self-assembly of mixtures of bilayer-forming amphiphiles, particularly phospholipids and micelle-forming amphiphiles, commonly denoted detergents. The structure of such mixed assemblies has been thoroughly investigated, described in phase diagrams, and theoretically rationalized in terms of the balance between the large spontaneous curvature of the curvophilic detergent and the curvophobic phospholipids. In this critical review, we discuss the mechanism of this process and try to explain the actual mechanism involved in solubilization. Interestingly, membrane solubilization by some detergents is relatively slow and the common attribute of these detergents is that their trans-bilayer movement, commonly denoted flip-flop, is very slow. Only detergents that can flip into the inner monolayer cause relatively rapid solubilization of detergent-saturated bilayers. This occurs via the following sequence of events: 1), relatively rapid penetration of detergent monomers into the outer monolayer; 2), trans-membrane equilibration of detergent monomers between the two monolayers; 3), saturation of the bilayer by detergents and consequent permeabilization of the membrane; and 4), transition of the whole bilayer to thread-like mixed micelles. When the detergent cannot flip to the inner monolayer, the outer monolayer becomes unstable due to mass imbalance between the monolayers and inclusion of the curvophilic detergent molecules in a flat surface. Consequently, the outer monolayer forms mixed micellar structures within the outer monolayer. Shedding of these micelles into the aqueous solution results in partial solubilization. The consequent leakage of detergent into the liposome results in trans-membrane equilibration of detergent and subsequent micellization through the rapid bilayer-saturation mechanism.
Introduction
Characterization of lipid-detergent mixtures
The self-assemblies formed in aqueous detergent-phospholipid mixtures have been extensively studied for different detergents and lipids as a function of their concentrations in the absence and presence of different lipids and at different temperatures, using various methods(1–3). The results of these studies have been presented in terms of phase diagrams, in which the total detergent concentrations Dt required for the onset and completion of mixed micelle formation (Dtsat and Dtsol, respectively) are described as a function of lipid concentration [L]. Notably, the phase boundaries, as detected by the dependence of Dt on [L], are apparently linear functions of the lipid concentration (Fig. 1).
Figure 1.
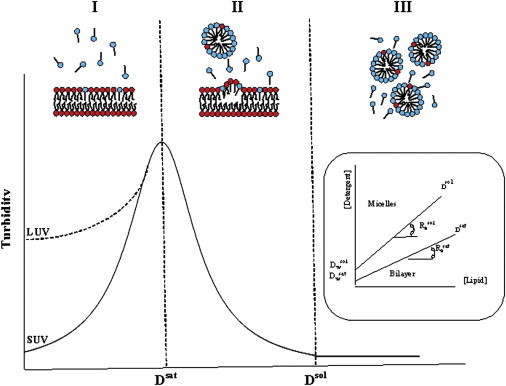
The three stages of bilayer solubilization by detergents (11). (Top) Schematic depiction of Stages I–III. (Red) Phospholipid headgroups. (Blue) Detergent headgroups. (Bottom) Solubilization of a vesicle preparation (small or large unilamellar vesicles) followed through changes in turbidity. Dsat and Dsol mark, respectively, the beginning and end of Stage II. (Inset) Partial phase diagram of a lipid-detergent-water system. Dsat and Dsol are plotted versus lipid concentration. The slopes of the resulting straight lines correspond to Resat and Resol. The lines define three regions, containing, respectively (from low to high detergent concentration), detergent-containing bilayers, a mixture of detergent-saturated bilayers and lipid-detergent mixed micelles, and lipid-detergent mixed micelles (5).
Accordingly, solubilization is commonly characterized by four terms (4,5), those of the effective (i.e., in the bilayer) detergents:
Resat, the lipid ratio required for the onset of solubilization;
Resol, a similar term for complete solubilization; and
Dwsat and Dwsol, which are the detergent concentrations obtained upon back-extrapolations (to [L = 0]) of the dependencies of Dtsat and Dtsol on [L].
Dwsat and Dwsol are actually lower than the critical micelle concentration (cmc) of the pure detergents, probably due to the finite size of the micelles (6).
Phase diagrams describe equilibrium states. In mixed systems of lipid and detergent in aqueous solutions, the phase boundaries describe the range of partial solubilization, namely the range of phospholipid and detergent concentrations where the energy associated with mixed micelles is of the same order of magnitude as that of the coexisting liposomes saturated with detergent (5). In several published investigations, the phase boundaries were independent of the procedure used to prepare the mixed system, assuring that the phase diagrams described equilibrium (e.g., Schnitzer et al. (7)). Unfortunately, in many publications the procedure used to study the phase diagrams was such that it cannot be guaranteed that all the experiments have been performed under equilibrium conditions (e.g., Kragh-Hansen et al. (8) and Stuart and Boekema (9)). Despite this shortcoming, we consider each steady-state mixture to be at equilibrium, but we are aware of the possibility that for certain compositions the structure of the lipid/detergent aggregates may be merely a reflection of kinetic traps, as described below.
In our recent review, we discussed the phase boundaries in terms of a balance between opposing driving forces, independent of the underlining mechanism (10). This review is devoted to mechanistic aspects of the various relevant processes, as studied by different methods.
The Three-Stage Model: Definitions and Ambiguities
Several mechanisms have been described for solubilization. The only general consensus is that it can be described in terms of a three-stage model (note that sometimes the terms “steps” or “phases” are used instead of “stages”), as proposed by Helenius and Simons (11).
The term “three-stage model” itself is quite ambiguous. It is often understood in thermodynamic terms to mean that, at equilibrium, in the range of Re values below Resat (Stage 1), the mixed assemblies are bilayers; that above Resol (Stage 3), they are micelles; and that within the range of Re values between Resat and Resol (Stage 2), bilayers and micelles coexist. However, the model is also often understood in kinetic or mechanistic terms, namely in terms of the sequence of processes that occur when a solubilizing concentration of detergent is added to lipid bilayers. In these terms, Stage 1 relates to interactions between detergent and lipids that do not yield micellar structures; Stage 2 is where detergent-saturated bilayers convert into mixed micelles; and Stage 3 is related to the reduction in the size of the mixed micelles as a result of their interaction with more detergent.
In terms of processes, the model is defined as a series of stages:
- Stage 1. Partitioning of detergent between lipid bilayers and the aqueous media, described by a partition coefficient K, defined as
where L is the lipid concentration and Db and Dw are the concentrations of detergent in membrane and in water, respectively. Hence,
Stage 2. A composition-induced disintegration of the bilayers to form long thread-like mixed micelles.
Stage 3. At yet higher detergent concentrations, solubilization is followed by an entropy-derived, relatively rapid series of mixing large detergent-phospholipid mixed micelles with pure detergent micelles, yielding smaller mixed micelles with a higher detergent/phospholipid ratio.
The first and third stages in this scheme are apparently trivial. Partitioning is commonly believed to be rapid, but bilayer saturation may be slowed down by the need for detergent repartitioning between the outer and inner monolayer of the bilayer (see below). Equilibration of mixed micellar systems (in Stage 3) is much faster than equilibration of micellar and lamellar systems. Stage 2 corresponds to the process of dissociation of detergent-containing bilayers, of a composition given by Resat, into lipid-containing micelles of a composition given by Resol. The detailed mechanism of this bilayer-micelles stage transformation (micellization) is not fully understood and is the main subject of this review.
Notably, in most of the commonly used solubilization protocols, the detergent concentration is sufficient to cause solubilization (at equilibrium), and the onset of micellization often occurs before equilibration of Stage 1. This of course adds a further complexity to the process. To avoid mistaken conclusions, one must be acquainted with the scope and limitations of the various methods. Hence, we first describe the most commonly used methods, and only then we discuss possible mechanisms of solubilization.
Methods used to Study the Mechanism of Solubilization
Investigations aimed at clarifying the dynamics of liposome solubilization require real-time continuous measurements of the self-assemblies present in lipid-detergent mixtures, namely the size, shape, and composition of the liposomes and mixed micelles, preferably without fractionation. The most straightforward method to provide information on the shape and size of lipid-detergent assemblies is cryo-transmission electron microscopy (cryo-TEM) (12–14). However, cryo-TEM cannot yield trustworthy quantitative results because, in the presence of large particles, the method is blind to small particles. The latter is also true for light scattering, both static and dynamic. Consequently, light scattering and other scattering techniques, though widely used for convenience reasons, yield questionable results on the onset of solubilization. Also note that light scattering methods may be perturbed by the increased turbidity of some detergents, e.g., Triton X-100, which above a certain temperature denoted the “cloud point”, form turbid dispersions (15–17).
In contrast, 31P-NMR spectroscopy is sensitive to small particles because only small particles (e.g., mixed micelles) yield relatively narrow (observable) signals (18–20), whereas the spectrum of nuclei of large vesicles is broadened almost beyond detection. Given the different sensitivities of light scattering and NMR, these methods are complementary. NMR can be used to monitor quantitatively the initial stage of solubilization (hence, Resat) whereas light scattering techniques can yield information of Resol.
Much information on the studied processes can be obtained using isothermal titration calorimetry (ITC) (21–26). If properly interpreted, this method can yield information on both the concentration at which stage transformation occurs and the heat associated with each process. ITC measurements do not yield structural information but the technique is quite helpful in gaining knowledge on the various rapid processes. Given the possible problems associated with the sensitivity of scattering techniques to large particles, ITC is beneficial in studying processes that are likely to be affected by such artifacts.
Fluorescence spectroscopy offers a whole set of techniques to be used in relation with membrane detergent solubilization. Release of entrapped water-soluble fluorescently labeled compounds from the liposomes to the external aqueous medium can be observed in real-time. Using fluorescently labeled compounds of different molecular weights (27–29) indicates the size of holes in the bilayer. Certain well-known fluorescent probes, e.g., diphenylhexatriene, provide information on the bilayer molecular order, and its perturbation by surfactants (30). Moreover, the trans-bilayer, or flip-flop, motion of amphiphiles, including surfactants, can also be assessed with particular fluorescence techniques (29,31).
In general, a combination of techniques is recommended. For instance, despite its shortcomings, cryo-TEM is a very helpful tool in the investigation of the mechanism of solubilization. The qualitative information on the type(s) of structures in lipid-detergent mixtures is essential for proper interpretation of the light scattering and NMR data. This is particularly important in mechanistic studies, because transition structures can be expected to contribute very little (if at all) to spectroscopic measurements. Yet, the mere existence of a transient structure provides clues on the mechanism of solubilization even when such structures are rarely observed. As an example, cryo-TEM of the structures present in dispersions made by adding the nonionic detergent octyl-glucoside to phospholipid vesicles revealed, before the formation of cylindrical mixed micelles, liposomes with pores of increasing sizes upon adding more octyl-glucoside (28). The latter observation accords with the fluorescence data on leakage of entrapped solutes of increased molecular weight (27,32,33). Although we cannot quantitate the formation of pores, the appearance of specific intermediate structures contributes much to clarification of the mechanism, as discussed below.
Another important methodological aspect of solubilization studies is the preparation of lipid-detergent mixtures. This issue is addressed in many of our previous publications (16,18). Of special importance is the need to avoid kinetic traps, particularly when the studied lipids are either in the gel state and/or not unilamellar. Kinetic traps are likely to occur when a relatively fast solubilization stage yields nonequilibrium, relatively stable mixed assemblies, as studied in the following systems.
Slow Solubilization and Kinetic Traps
Solubilization of gel phase bilayers
Detergent-phospholipid mixtures may be stable for long periods of time without being at equilibrium (7,34–36). As an example, when a detergent solution is added to preformed unilamellar vesicles with the lipid in the gel or crystalline states (e.g., DPPC below 40°C) (7,35), equilibration may be so slow that the mixture appears to be constant with respect to the state of aggregation of the mixed assemblies, despite not being at equilibrium. Kinetically trapped metastable aggregates may be stable for hours and sometimes for many days, as in the case of cholesterol-rich metastable micelles formed upon mixing cholesterol-rich phospholipid vesicles with bile salts (35,36). In the latter case, when a bile salt is added to PC-cholesterol liposomes, under certain conditions the added bile salt solubilizes the liposomes rapidly but subsequently the initially formed cholesterol-rich liposomes precipitate. Eventually, cholesterol crystallizes within the aggregates of vesicles, thus yielding cholesterol precipitates (37). Similar processes may be responsible for the formation of cholesterol gallstones (38).
Solubilization of multilamellar vesicles
Solubilization of multilamellar vesicles (MLVs) is a slow process. When MLVs are exposed to a detergent solution (34), only the outermost bilayer of the MLV is exposed to the added detergent. Relative to the latter bilayer, the detergent concentration may be sufficient to solubilize this bilayer, resulting in exposure of the next bilayer to the detergent. If the detergent concentration is sufficient to solubilize the entire lipid, the MLV bilayers may be peeled one after the other, but even if the total detergent concentration is sufficient to solubilize all the layers of lipid, the process will be much slower than the solubilization of unilamellar vesicles of identical composition (34). This is likely to result in serious errors if solubilization is so slow that at the time of measuring the turbidity of the dispersion, solubilization is not yet complete. In other words, evaluation of the detergent concentration required for complete solubilization will be overestimated.
In contrast, if the detergent concentration is sufficient to solubilize one or more but not all the lipid bilayers, the onset of solubilization will be apparent at a lower detergent concentration than needed for the onset of solubilization of unilamellar vesicles of identical composition and concentration. Under such conditions, the initially formed mixed micelles will reconvert into bilayers when more phospholipid becomes solubilized because the effective (interacting) detergent/lipid ratio will decrease. In this case, reaching a steady state may be very slow and when measured before complete equilibration, the onset of solubilization will be underestimated.
The Factors that Govern Solubilization
Understanding the mechanism of micellization requires information on at least three factors, namely:
-
1.
The lipid-detergent assemblies that exist at Resat;
-
2.
The product of the composition-induced phase transition, namely micelles of a composition given by Resol; and
-
3.
The examination of as many intermediate structures as needed to help in determining the mechanism.
An essential underlying assumption is that at any given time the detergent-lipid bilayer mixture is at a steady state. This may be the case for a slow titration experiment, as in ITC, but in general terms, Stage 2 (i.e., micellization) begins before the end of Stage 1, a fact that often complicates the interpretation of experimental data.
Detergent-saturated vesicles (at Resat): chemical composition, and structural and physical properties
Partition of detergent from aqueous media to bilayers, including the inner monolayer
The first step of the bilayer/detergent interaction is the introduction of detergent molecules into the outer monolayer of the phospholipid bilayer. The partition coefficient that describes the detergent equilibrium between the bilayers and the aqueous solution is governed by two factors: namely,
-
1.
The hydrophobicity of the detergent; or, instead,
-
2.
Its hydrophilic/lipophilic balance and its spontaneous curvature (9,39).
The spontaneous curvature reflects the tendency of amphiphiles to be packed along a curved surface, as discussed in our recent review (10). Detergents are curvophilic, i.e., they have a positive spontaneous curvature, whereas phospholipids tend to self-assemble along a flat surface, hence their spontaneous curvature is about zero, or even slightly negative (25). The partition coefficient depends upon these factors but the rate of equilibration of the detergent concentration over the two monolayers may vary considerably. Notably, detergent accumulation in the outer monolayer induces flipping of additional detergent, probably by perturbing the interfacial region of the bilayer by inserted detergent molecules, which is likely to enhance the probability of formation of transient structural defects in the membrane (31). Flip-flop is often the rate-limiting factor of solubilization. In turn, trans-membrane flipping depends upon the size and polarity of the detergent headgroup (9). Specifically, flipping a molecule with a very large polar headgroup through the bilayer is not likely to happen (40,41). Hence, the system cannot reach equilibrium on a short timescale and solubilization commonly begins before equilibrium can be reached. The consequent mass imbalance may play a role in the mechanism of solubilization by these detergents, as described below.
Both the penetration of a detergent into the outer monolayer and its trans-membrane motion into the inner monolayer depend on the bilayer composition. As an example, in their study of the solubilization of liposomes of several different compositions by the nonionic detergent denoted a myristoyl sucrose, Toro et al. (42) found that the localization of the detergent in the lipid bilayer depends on the characteristics of the lipid polar headgroup, the latter influencing in this way the solubilization process. They also found that insertion of cholesterol molecules into the lipid bilayer protects it from solubilization.
Dissipative particle dynamics simulations (43), aimed at investigating the solubilization mechanism of vesicles by surfactants, focused on the effect of detergent hydrophobicity. In the authors’ interpretation, their results indicated that only surfactants with suitable hydrophobicity are able to solubilize vesicles. Hydrophilic detergents stay in the bulk solution, whereas surfactants with excessive hydrophobicity incorporate into bilayers, inducing vesicle size-growth (without solubilizing the membrane). Only surfactants with moderate hydrophobicity form perforated vesicles before the formation of mixed micelles, for reasons described below. The extent of perforation grows with increasing surfactant concentration, until eventually, total collapse of the vesicle is observed. Pore formation has been observed in many detergent-lipid systems before solubilization, as described below.
Detergent-induced vesicle size growth
Detergent-induced size growth of small unilamellar vesicles has been observed for many detergents and several different compositions of liposomes at subsolubilizing detergent concentrations (44–48). Vesicle size growth from a radius ri/rg is expected to result in an increase by a factor of (rg/ri)2 in the surface area of the vesicles, which means that the number of vesicles is expected to decrease by a factor of (ri/rg)2.
This process could have occurred via one (or more) of three mechanisms:
-
1.
Detergent-induced fusion, which implies mixing of the two entrapped aqueous compartments and of the bilayers of the fusing membranes;
-
2.
Breakdown-reassembly mechanisms, namely detergent-induced breakdown followed by reconstitution of larger vesicles; and
-
3.
Disproportionation of the liposomes, characterized by transfer of lipids from part of the vesicles (probably the smallest) to other (presumably larger) liposomes.
A major difference between the three possible mechanisms is the retention of the entrapped solute. Thus, entrapped high molecular weight solutes can be expected to remain entrapped in liposomes if size growth occurs via fusion (as commonly believed), whereas solubilization-reassembly is expected to be accompanied by loss of the solutes. The third possibility is expected to be accompanied by partial retention of solutes, i.e., only the solutes of those vesicles that became larger will remain entrapped, whereas those solute molecules that were entrapped in the diminishing vesicles will be found in the external solutions (45).
Detergent-induced size growth via vesicle fusion has been demonstrated for negatively charged liposomes in the presence of Ca2+ (49). Breakdown of vesicles made by sonication is followed by reassembly into larger liposomes, induced by Triton X-100, as proposed by Alonso et al. (46,47), and by Edwards and Almgren (13). The disproportionation mechanism was first proposed for bile salt-induced size growth (50,51). A study of liposomal size growth induced by subsolubilizing concentrations of the nonionic detergent octylglucoside revealed partial retention of high molecular weight, fluorescently labeled water-soluble entrapped dextran (45). The fraction of retained solute was consistent with the observed size growth, thus supporting the disproportionation mechanism (45). The similarity of detergent-induced size growth in the presence of surfactants as different as bile salts and octylglucoside (45–48) supports the idea that disproportionation may be a frequent mechanism for detergent-induced size growth.
Permeability of detergent-containing bilayers
Leakage of entrapped solutes from detergent-containing liposomes has been reported in several studies at subsolubilizing concentrations of different detergents (45–48,50). Part of this leakage can be attributed to the detergent-induced size growth but holes have been observed in LUVs in the absence of detergent-induced size growth. Dissipative particle dynamics simulations show that during the partition process (Stage 1), individual surfactant molecules incorporate themselves into the lipid bilayer in an independent manner (43). The compatibility of the hydrophobic tails between lipids and surfactants allows surfactants to penetrate through the membrane quite easily and surfactant molecules appear in the inner region of the bilayer. This detergent-induced penetration may be due to promotion of the trans-bilayer motion of detergent, or to penetration of detergent into the entrapped aqueous compartment and subsequent partitioning from the latter compartment and the inner monolayer.
In other words (52), upon increasing the detergent concentration (in the outer monolayers), the excess area that results from the binding of surfactants to the outer monolayer results in trans-bilayer pore formation, softening of the bilayer and enhancement of bilayer fluctuations, more frequently and/or likely when the surfactant concentration approaches Resat. Furthermore, as long as the detergent is inhomogeneously distributed, the vesicles have irregular, highly deformed forms before perforation of the vesicle takes place. Pore formation has been observed before solubilization in many investigations. With more holes developing by solubilizing surfactants, the vesicle ruptures into a few pieces of bilayers, which eventually disintegrate into small mixed micelles (52) upon surfactant addition. As an example, recent studies of the kinetics of release of liposomal content upon exposure of the liposomes to excess surfactant (53) revealed that, at low detergent concentrations, the release of the liposomal content is consistent with the formation of channels at a critical number of surfactant molecules. However, only at considerably higher concentrations does the kinetics accord with that expected for detergent-induced rupture of the liposomes. We think that the structure of such channels (or holes) is the key to understanding the detailed mechanism of solubilization, as discussed below.
The structure and physicochemical properties of mixed micelles of a composition given by Resol
Solubilization of lipids by bile salts has been studied in great detail, using many methods. These studies lead to a consensus on a discoidal structure of the mixed aggregates formed when the entire lipid is solubilized. According to that model, the mixed micelles were described as detergent-poor discoidal bilayers covered on their rim by a curved detergent-rich monolayer (54–57). Similarly, the data on the micelles formed upon titration of phospholipids by other detergents, particularly the results of dynamic light scattering and small angle x-ray scattering, were interpreted to mean that these micelles are oblate ellipsoids, with a gradient of detergent from the center to the perimeter following the long axis (15,18,57).
This approach yielded a reasonable, easily understood mechanism for solubilization and reconstitution of lipid bilayers. However, later studies with cryo-TEM showed long, thread-like objects rather than the expected disk-like structures (12,58,59). Subsequently, it was shown that the scattering data accorded with such flexible rods and theoretical studies explained “why cylinders, not disks” (25). Hence, the contemporary paradigm is that the largest mixed micelles are very long thread-like structures and that increasing the detergent concentration results in the reduced length of these micelles. In these structures almost all the lipid molecules are equivalent, with the exception of a small number of molecules on the two edges (caps) of the elongated micelles. The same is almost true for the detergent, except that the detergent concentration at the caps is somewhat higher.
Micellization of an LUV into one thread-like micelle
A theoretically derived, interesting implication of this model is that the solubilization of a LUV (100 nm in diameter) into a single, long thread-like micelle (if it really happens) would lead to a structure of the same order of magnitude (i.e., microns) as the longest micelles observed by cryo-TEM (see below).
Consider a LUV composed of phosphatidylcholine (PC) of a radius R = 50 nm. Its surface area will be S = 4 π R2 = 10,000 π nm2/monolayer. Assuming an average molecular area for PC SPC = 0.7 nm2, it follows that each bilayer is made of N = 20,000 π/0.7 molecules PC. Assuming a detergent molecular area SD = 0.5 nm2 and assuming that surface areas are additive, the surface area of a detergent-saturated LUV of a composition given by Resat = 1.0 will be
If Resol = 2.0, and the additivity of surface areas is maintained, then
A thread-like micelle can be viewed as a tube of a radius r = 1.5 nm, a length l, and a surface area St, given by the equation St = 2 π r l.
Hence,
Thread-like mixed micelles of similar length have been occasionally observed by cryo-TEM. This does not mean that solubilization of one LUV yields normally a single micelle, but suggests that solubilization may involve transformation of a detergent-saturated perforated liposome into a very long mixed micelle.
Intermediate structures observed in the coexistence range
As described above, the lipid bilayers can be considered supersaturated even below Resat, because they contain detergent-rich walls covering the edges of pores observed in cryo-TEM. When the bilayer contains more detergent, the size of the pores is larger so that larger molecules can leak out of the liposomes (45).
Cryo-TEM does not yield quantitative data, but the existence of structures other than those seen either at Resat or at Resol gives a clue to the mechanism of micellization of a supersaturated bilayer. Such intermediate structures have been observed by cryo-TEM, which is known to preserve the original microstructures of fluid systems, in studies of both mixtures of Triton X-100 (59) and octylglucoside with egg PC (28) below Resat. At somewhat higher detergent concentrations, in addition to liposomes and long thread-like micelles, the system contained some liposomes that appeared to have long objects (presumably mixed micelles) attached to them (28).
In conclusion, most solubilization procedures employ detergents that can bind to phospholipid bilayers and flip into the inner monolayer. In these cases, Stage 2 of solubilization is a composition-induced transition of large, perforated, detergent-supersaturated liposomes of a composition given by Resat into long, thread-like micelles of a composition given by Resol. The observed intermediate structures help understanding of the detailed mechanism as described below. By contrast, when a detergent molecule cannot flip to the inner monolayer, rapid solubilization via saturation of the bilayers is preceded by other processes that result in exposure of both monolayers to detergent, as described in the next section.
The Mechanism of Detergent-Induced Bilayer-to-Micelle Transition
Possible mechanisms
In relating to the mechanism(s) of solubilization, it is important to note that solubilization depends critically on the trans-membrane motion of detergent, commonly denoted flip-flop (8). Specifically, it has been shown that when the flipping of the detergent to the inner monolayer is rapid (e.g., detergents of Group A in Table 1), solubilization is rapid, whereas when the flipping of detergent is slow (e.g., Group B), solubilization is slow.
Table 1.
Selected slow- and fast-solubilizing detergents
| Group | Detergents | Reference |
|---|---|---|
| A, fast solubilizing | Chlorpromazine | (29) |
| C12E8 | (53,67) | |
| DDAO | (8) | |
| Triton X-100 | (8,34) | |
| B, slow solubilizing | Decylmaltoside | (9) |
| Dodecylmaltoside | (8,9,68) | |
| Lysophosphatidylcholine | (45,69) | |
| SDS | (8,21) |
Solubilization kinetics may be highly influenced by factors such as temperature, vesicle composition, and vesicle preparation procedure, among others. The data in this table have been collected mainly from publications in which various detergents have been compared under the same conditions.
Two detailed investigations that proposed specific mechanisms of solubilization postulated that the rapid and slow solubilization occur via different mechanisms (8,9). Both agreed that the fast solubilization occurs via saturation of the phospholipid bilayers. In one study (8), the mechanism of the rapid solubilization of bilayers was denoted the trans-bilayer mechanism and described as being an all-or-none process that results in solubilization of a whole liposome (or of none). In the other published mechanism, the same process was described as a fast solubilization of phospholipids that occurs via open vesicular intermediates (9). Both mechanisms are proposed for systems in which the rate of flip-flop is fast, and both are very similar if not identical.
By contrast, the mechanisms proposed for the slow solubilization differed considerably. Both studies agreed that the process is slow because the flip-flop is slow, and both agreed that the slow solubilization results in micellization of only a part of the phospholipid in a given liposome. Nonetheless, according to one mechanism (8), solubilization occurs via binding of micelles to the bilayers and subsequent “extraction of membrane components directly by detergent micelles” (9), whereas according to the other mechanism this process occurs via “micelles that pinch off from closed vesicles” (52).
Evaluation of the proposed mechanisms of slow solubilization
In our view, the evidence for binding of micelles to a bilayer is not convincing. The findings of premicellar aggregates much below the cmc (in a range similar to Dwsat), which could get stabilized by recruiting lipid from the membrane, is not consistent with the micelle extraction mechanism. Stabilized lipid-detergent premicelles are not very likely to be formed by extracting phospholipids by premicellar detergent because such premicelles are not likely to exist much below Dwsat before mixing the detergent with the stabilizing phospholipid liposomes.
In contrast, binding of monomers to the outer monolayer of the bilayer is probably sufficiently rapid to reach a near-equilibrium partitioning of detergent between the outer monolayer and the aqueous solution. The resulting detergent-containing outer monolayer is likely to be unstable due to two factors, namely:
-
1.
Mass imbalance between the outer and inner monolayers; and
-
2.
The presence of a curvophilic amphiphile (i.e., detergent) of a high positive spontaneous curvature in a nearly flat monolayer made of phospholipids, whose spontaneous radius of curvature is slightly negative.
Therefore, if a detergent molecule cannot flip to the inner monolayer, micellar structures are likely to be formed in the outer monolayer and pinch off from closed vesicles as mixed micelles (9). In other words, sufficiently high detergent concentration promotes detachment (shedding) of such mixed micelles from the surface of bilayers over a broad range of concentrations (52).
A similar model, proposed by Mrówczyńska et al. (60), implies that when inserted into bilayers, the curvophilic detergent molecules affect bilayer bending, resulting in membrane perturbation. Specifically, when detergent penetrates into a bilayer, the membrane will become either invaginated or evaginated, yielding either endo- or exovesiculation, respectively. The altered shape of the bilayer is accompanied by curvature-dependent lateral segregation of membrane components. Based on these arguments, the authors hypothesized that solubilization occurs via membrane-curvature-dependent segregation of membrane components (8). This mechanism can be regarded as consistent with formation of membrane domains that cannot be solubilized by Triton X-100 (detergent-resistant membranes). In relation to the slow solubilization, this hypothesis implies that accumulation of detergent in the outer monolayer results in the following course of events:
-
1.
Monomeric detergent intercalates into the nearly flat membrane areas, which results in expansion of the outer monolayer; and
-
2.
When the detergent does not flip into the inner monolayer, it accumulates in the outer monolayer—thereby striving to bend the membrane outwards, creating small membrane invaginations (or mixed micelles).
Notably, if the detergent can flip into the inner monolayer and its distribution is in favor of the inner monolayer, it will also create small membrane invaginations or vesicle-like domains (buds).
Mixed micelles formed from detergent-rich parts of the outer monolayer can exist below Dwsat and may extract membrane components directly into the mixed micelles (8). Hence, as long as trans-membrane equilibration is very slow and permeation of detergent through the bilayer does not happen, detergent-rich lateral curved domains are likely to be formed in the outer monolayer and undergo shedding or pinched-off micelles, namely in micellization of only a part of the membrane. Assuming that both the shedding and (mixed) micellar solubilization contribute to the slow solubilization observed when trans-membrane equilibration is very slow, the question is which of the latter two possibilities contributes more to the solubilization. Unfortunately, we do not know, as of this writing, how to evaluate the relative contribution of the two mechanisms.
The bilayer saturation mechanism for relatively rapid solubilization
The rapid solubilization observed when the detergent can flip from the outer to the inner monolayers, denoted “fast solubilization” of phospholipids that occurs via open vesicular intermediates (9) or a trans-bilayer attack, after flip-flop of detergent molecules across the lipid bilayer (8), has been investigated in greater detail. The main attribute of this mechanism is that solubilization via this mechanism occurs after the whole bilayer becomes saturated (or supersaturated) and the whole detergent-rich membrane becomes solubilized via an all-or-none solubilization mechanism (8), as discussed above. Notably, this mechanism may follow partial solubilization by either of the alternative mechanisms because trans-bilayer equilibration can be expected if the bilayer becomes leaky to detergent, due to detergent addition to the outer monolayer. Thus, both partial solubilization mechanisms pave the way for exposure of the inner monolayer to detergent and by that to the rapid solubilization via a fast bilayer saturation mechanism. This is particularly clear when rapid solubilization is apparently preceded by a lag of varying periods of time during which the solubilization is much slower.
During the lag time, detergent molecules saturate the two monolayers either by flipping into the inner monolayer or via leaking through the detergent-induced holes. Eventually disintegration (micellization) of the whole membrane occurs (29,60). In the bilayer/micelle coexistence range, cryo-TEM reveals mostly lamellar and thread-like micelles as well as some defective bilayer intermediates, such as perforated vesicles (8). In many systems, the bilayers become destabilized at a certain content of surfactant in the membrane, and then disintegrate, forming mixed micelles, or a hexagonal phase, or other intermediate structures. In some systems, the perforated membranes go over into thread-like micelles via lace-like structures and other intermediates, in the form of disks, micelles, or bilayer fragments, particularly in systems containing a large fraction of cholesterol in the bilayer. However disk-like micelles are not the main structures, because they exist as transients in the transformation from mixed micelles to vesicles (8).
The available information on the temporal order of these observations and on the dependence of temporal order on the physico-chemical properties and concentrations of the lipid and detergent is quite limited. It is known, however, that solubilization is preceded by leakage of entrapped solutes, as indicated by investigations of permeability at subsolubilizing detergent concentrations (61,62). When solubilization is preceded by a lag, the leakage is slow, assuming that detergent equilibration over the two monolayers is a prerequisite to pore formation and solubilization and thus it appears that solubilization occurs only after the bilayer is perforated (29). Not much is known about the time dependence of the detergent flip-flop rate, but the difference between the concentrations of the detergent in the two monolayers is likely to vanish when the membrane becomes permeable to the detergent.
Under Resat conditions, detergent-lipid mixtures usually contain large vesicles, even if the original vesicles were small (due to detergent-induced size growth), and the membrane is perforated, with holes covered by curved detergent-rich walls as described in Fig. 2. These structures probably reflect equilibration after an entropy-driven, relatively rapid introduction of the curvophilic detergent into the bilayers, via a subsequent size growth (if the original vesicles were small). Eventually, the vesicles become perforated, to accommodate the curvophilic detergent molecules that reside along the curved detergent-rich walls of the holes.
Figure 2.
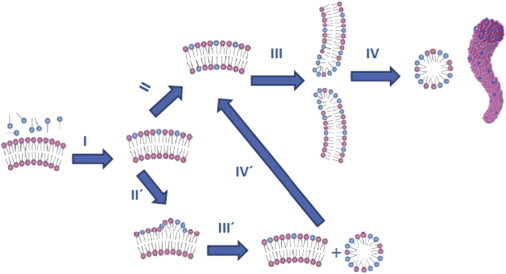
Proposed mechanism(s) of bilayer micellization in a vesicle: (I) Detergent monomers become inserted into the vesicle outer monolayer. (II) Detergent equilibrates between the inner and outer monolayer through rapid flip-flop. (III) Pores are formed in the bilayer. (IV) Lipid-detergent mixed micelles are formed. Both a cross section and an overall view of an elongated micelle are shown. When rapid flip-flop is not possible, then an alternative pathway occurs: (II′) Insertion of multiple curvophilic detergent molecules cause a large increase in the curvature of the outer bilayer. (III′) Lipid-detergent mixed micelles are shed from the bilayer, and this process leads to (IV′) detergent trans-bilayer movements. The system can then undergo processes III and IV.
Micellization of supersaturated liposomes
Transformation of detergent-saturated liposomes of a composition given by Resat into mixed micelles of a composition given by Resol requires that, within the range of Resat to Resol, added detergent will be unevenly distributed. Specifically, if at Resat each liposome is made of L molecules of PC and L·Resat molecules of detergent, addition of detergent at a concentration sufficient for partial solubilization yields a mixture of liposomes whose composition remains unaltered. However, other liposomes absorb sufficient detergent to be decomposed to mixed micelles, containing L·Resol detergent molecules, where Resol is much higher than Resat. Our interpretation of this uneven distribution is that detergent binding to bilayers is cooperative, perhaps because it initially accumulates at lattice defects, and further detergent molecules mix better with lipid-detergent microdomains than with pure lipids (17).
In some cases, the detergent-saturated vesicles existing at Resat arise from the rapid trans-bilayer (flip-flop) movement of detergent molecules from the outer to the inner vesicle lipid monolayer. This would be the case in particular with detergents (denoted as Group A in Table 1), whose polar moiety is relatively small and trans-bilayer motion is relatively fast. In this case, solubilization would consist of dissociation of a whole supersaturated liposome into one or more thread-like micelles. In conclusion, bilayers undergo complete solubilization by detergent when the whole bilayer (including the inner monolayer) is saturated with detergent to the extent that the bilayer is perforated with supersaturating levels of detergent.
We consider that no available data is inconsistent with this mechanism. This includes the experiments that were considered evidence for both the “mechanism based on the direct formation of mixed micelles” (63) and the mechanism based on “destruction of vesicles via small particles containing large amounts of detergents” (64).
Dependence of the rate of solubilization on the detergent and membrane physico-chemical properties
Incorporation of detergent into the membranes depends on both the detergent and the membrane. Under certain conditions, it may be virtually restricted to the outer monolayer because the flip-flop of detergent molecules to the inner monolayer is slow (21,40). Eventually, bilayer perforation will abolish the gradient of detergent concentration between the two monolayers, but this occurs simultaneously with Stage 2.
The data in Table 1 can be interpreted in terms of the hypothesis that when solubilization is preceded by a lag, it occurs only after the above-mentioned gradient decays. Notably, detergents that belong to this group, herein denoted Group B, probably penetrate only into the outer monolayer because their headgroups are large and polar, so that they can approach the inner monolayer only after the membrane becomes perforated. This is also consistent with the finding that even detergents of this group can readily solubilize bilayers, as long as they are loosely packed (e.g., at higher temperatures) (21,65).
Thus, the mechanism responsible for the slow onset of solubilization by Group B detergents is due to the gradient of detergent concentration between the two monolayers and the consequent mass imbalance that causes shedding (escape) of lipid-detergent mixed micelles from the outer monolayers. Interaction between bilayers and pure detergent micelles is perhaps less likely than penetration of detergent monomers into the outer monolayer. As long as the detergent has no access to the inner monolayer, some individual mixed micelles (presumably spherical) can be formed; but when the inner monolayer becomes available to the detergent, due to increased membrane permeability, the mechanism becomes similar to the one followed by Group A detergents, and extensive solubilization ensues.
It has been proposed (8,9) that each member of the two groups of detergents, the fast and slow solubilizers (respectively, Groups A and B in Table 1), would follow a different solubilization mechanism. Group A acts in a trans-bilayer mechanism, as a consequence of rapid flip-flop, causing vesicle disintegration, whereas Group B-forming detergent micelles would gradually extract phospholipids from the membrane outer monolayer. However, the available data (29,53,66–69) show, for different Group B detergents, varying lipid compositions and vesicle concentrations, that those detergents (cholate, deoxycholate, sodium dodecyl sulphate (SDS)) cause complete or almost complete bilayer solubilization at concentrations well below their cmc, so that phospholipid extraction from monolayers by micelles is not likely.
The difference between the two groups of detergents is very clear from the recent optical microscopy observations of the solubilization of the lipid bilayers of giant unilamellar vesicles by two representative detergents, Triton X-100 (Group A) and SDS (Group B) (70). In the presence of Triton X-100, GUVs initially showed an increase in their surface area, due to insertion of detergent molecules with rapid equilibration between the two leaflets of the bilayer. Then, above a solubility threshold, several holes opened, giving the bilayer a lace fabric appearance, and the bilayer gradually vanished. On the other hand, SDS caused only an increase in the membrane spontaneous curvature that can be attributed to incorporation of SDS into the outer layer. The resultant stress in the membrane can either cause opening of transient macropores with substantial decrease in vesicle size or complete vesicle bursting of the vesicles.
As a further test for our interpretation, a detailed investigation of the temporal order of events would be required: change of detergent flip-flop rates, leakage of dyes through the bilayer, and solubilization. Thus far, such information is missing.
In conclusion, Stage 2 of the solubilization of lipid bilayers by detergent, namely the composition-induced transition of bilayers to micelles, can be understood in terms of a model based on the structure of bilayers saturated with membranes. Formation of such bilayers is entropy-driven but to compensate for the introduction of a curvophilic detergent molecule into a flat bilayer, the bilayer becomes perforated and eventually micellized. This sequence of events can occur only if the detergent concentration on the two monolayers is equal. Detergents with large hydrophilic headgroups cannot flip to the inner monolayer. Instead, they form lipid-detergent mixed micelles and, through escape of these aggregates, make the bilayer permeable to detergent molecules, thus in fact enabling equilibration of detergent and subsequently, solubilization of the whole liposome.
Acknowledgments
We thank Misha Kozlov for very helpful discussions.
The authors acknowledge financial help from the Spanish Ministry of Economy (grant No. BFU 2012-36241), Bizkaia Xede, and from The Lady Davis Fund.
References
- 1.Goñi F.M., Alonso A. Detergents in biomembrane studies. Biochim. Biophys. Acta. 2000;1508:51–68. doi: 10.1016/s0304-4157(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenberg D., Goñi F.M., Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Heerklotz H. Interactions of surfactants with lipid membranes. Q. Rev. Biophys. 2008;41:205–264. doi: 10.1017/S0033583508004721. [DOI] [PubMed] [Google Scholar]
- 4.Schurtenberger P., Mazer N.A., Kanzig W. Micelle to vesicle transition in aqueous solutions of bile salts and lecithin. J. Phys. Chem. 1985;89:1042–1059. [Google Scholar]
- 5.Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim. Biophys. Acta. 1985;821:470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- 6.Roth Y., Opatowski E., Kozlov M.M. Phase behavior of dilute aqueous solutions of lipid-surfactant mixtures: effects of finite size of micelles. Langmuir. 2000;16:2052–2061. [Google Scholar]
- 7.Schnitzer E., Lichtenberg D., Kozlov M.M. Temperature-dependence of the solubilization of dipalmitoylphosphatidylcholine (DPPC) by the non-ionic surfactant Triton X-100, kinetic and structural aspects. Chem. Phys. Lipids. 2003;126:55–76. doi: 10.1016/s0009-3084(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 8.Kragh-Hansen U., le Maire M., Møller J.V. The mechanism of detergent solubilization of liposomes and protein-containing membranes. Biophys. J. 1998;75:2932–2946. doi: 10.1016/S0006-3495(98)77735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart M.C., Boekema E.J. Two distinct mechanisms of vesicle-to-micelle and micelle-to-vesicle transition are mediated by the packing parameter of phospholipid-detergent systems. Biochim. Biophys. Acta. 2007;1768:2681–2689. doi: 10.1016/j.bbamem.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenberg D., Ahyayauch H., Goñi F.M. Detergent solubilization of lipid bilayers: a balance of driving forces. Trends Biochem. Sci. 2013;38:85–93. doi: 10.1016/j.tibs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Helenius A., Simons K. Solubilization of membranes by detergents. Biochim. Biophys. Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 12.Almgren M. Mixed micelles and other structures in the solubilization of bilayer lipid membranes by surfactants. Biochim. Biophys. Acta. 2000;1508:146–163. doi: 10.1016/s0005-2736(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 13.Edwards K., Almgren M. Solubilization of lecithin vesicles by C12E8-structural transitions and temperature effects. J. Colloid Interface Sci. 1991;147:1–21. [Google Scholar]
- 14.Walter P., Vinson P.K., Talmon Y. Cryo-TEM reveals structural transitions of EggPC and sodium cholate mixtures. Biophys. J. 1990;57:A476. [Google Scholar]
- 15.Yedgar S., Lichtenberg D., Gatt S. Enzymatic-hydrolysis of sphingomyelin in the presence of Triton X-100- Effects of temperature and cloud point. Isr. J. Med. Sci. 1979;15:75–76. [Google Scholar]
- 16.Lichtenberg D., Yedgar S., Gatt S. Studies on the molecular packing of mixed dispersions of Triton X-100 and sphingomyelin and its dependence on temperature and cloud point. Biochemistry. 1979;18:2574–2582. doi: 10.1021/bi00579a022. [DOI] [PubMed] [Google Scholar]
- 17.Ahyayauch H., Collado M.I., Goñi F.M. Lipid bilayers in the gel phase become saturated by triton X-100 at lower surfactant concentrations than those in the fluid phase. Biophys. J. 2012;102:2510–2516. doi: 10.1016/j.bpj.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtenberg D., Zilberman Y., Zamir S. Structural and kinetic studies on the solubilization of lecithin by sodium deoxycholate. Biochemistry. 1979;18:3517–3525. doi: 10.1021/bi00583a013. [DOI] [PubMed] [Google Scholar]
- 19.London E., Feigenson G.W. Phosphorus NMR analysis of phospholipids in detergents. J. Lipid Res. 1979;20:408–412. [PubMed] [Google Scholar]
- 20.Ahyayauch H., Collado M.I., Lichtenberg D. Cholesterol reverts Triton X-100 preferential solubilization of sphingomyelin over phosphatidylcholine: a 31P-NMR study. FEBS Lett. 2009;583:2859–2864. doi: 10.1016/j.febslet.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 21.Keller S., Heerklotz H., Blume A. Thermodynamics of lipid membrane solubilization by sodium dodecyl sulfate. Biophys. J. 2006;90:4509–4521. doi: 10.1529/biophysj.105.077867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnulphi C., Sot J., Goñi F.M. Triton X-100 partitioning into sphingomyelin bilayers at subsolubilizing detergent concentrations: effect of lipid phase and a comparison with dipalmitoylphosphatidylcholine. Biophys. J. 2007;93:3504–3514. doi: 10.1529/biophysj.107.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opatowski E., Lichtenberg D., Kozlov M.M. The heat of transfer of lipid and surfactant from vesicles into micelles in mixtures of phospholipid and surfactant. Biophys. J. 1997;73:1458–1467. doi: 10.1016/S0006-3495(97)78178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opatowski E., Kozlov M.M., Lichtenberg D. Partitioning of octyl glucoside between octyl glucoside/phosphatidylcholine mixed aggregates and aqueous media as studied by isothermal titration calorimetry. Biophys. J. 1997;73:1448–1457. doi: 10.1016/S0006-3495(97)78177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlov M.M., Lichtenberg D., Andelman D. Shape of phospholipid/surfactant mixed micelles: cylinders or disks? Theoretical analysis. J. Phys. Chem. B. 1997;101:6600–6606. [Google Scholar]
- 26.Heerklotz H., Seelig J. Titration calorimetry of surfactant-membrane partitioning and membrane solubilization. Biochim. Biophys. Acta. 2000;1508:69–85. doi: 10.1016/s0304-4157(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz J., Goñi F.M., Alonso A. Surfactant-induced release of liposomal contents. A survey of methods and results. Biochim. Biophys. Acta. 1988;937:127–134. doi: 10.1016/0005-2736(88)90234-9. [DOI] [PubMed] [Google Scholar]
- 28.Vinson P.K., Talmon Y., Walter A. Vesicle-micelle transition of phosphatidylcholine and octyl glucoside elucidated by cryo-transmission electron microscopy. Biophys. J. 1989;56:669–681. doi: 10.1016/S0006-3495(89)82714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahyayauch H., Bennouna M., Goñi F.M. Detergent effects on membranes at subsolubilizing concentrations: transmembrane lipid motion, bilayer permeabilization, and vesicle lysis/reassembly are independent phenomena. Langmuir. 2010;26:7307–7313. doi: 10.1021/la904194a. [DOI] [PubMed] [Google Scholar]
- 30.Nazari M., Kurdi M., Heerklotz H. Classifying surfactants with respect to their effect on lipid membrane order. Biophys. J. 2012;102:498–506. doi: 10.1016/j.bpj.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantaler E., Kamp D., Haest C.W. Acceleration of phospholipid flip-flop in the erythrocyte membrane by detergents differing in polar head group and alkyl chain length. Biochim. Biophys. Acta. 2000;1509:397–408. doi: 10.1016/s0005-2736(00)00322-9. [DOI] [PubMed] [Google Scholar]
- 32.de la Maza A., Parra J.L. Structural phase transitions involved in the interaction of phospholipid bilayers with octyl glucoside. Eur. J. Biochem. 1994;226:1029–1038. doi: 10.1111/j.1432-1033.1994.t01-1-01029.x. [DOI] [PubMed] [Google Scholar]
- 33.Ladokhin A.S., White S.H. ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochim. Biophys. Acta. 2001;1514:253–260. doi: 10.1016/s0005-2736(01)00382-0. [DOI] [PubMed] [Google Scholar]
- 34.Alonso A., Urbaneja M.A., Gómez-Fernández J.C. Kinetic studies on the interaction of phosphatidylcholine liposomes with Triton X-100. Biochim. Biophys. Acta. 1987;902:237–246. doi: 10.1016/0005-2736(87)90301-4. [DOI] [PubMed] [Google Scholar]
- 35.Schnitzer E., Kozlov M.M., Lichtenberg D. The effect of cholesterol on the solubilization of phosphatidylcholine bilayers by the non-ionic surfactant Triton X-100. Chem. Phys. Lipids. 2005;135:69–82. doi: 10.1016/j.chemphyslip.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Fudim-Levin E., Bor A., Lichtenberg D. Cholesterol precipitation from cholesterol-supersaturated bile models. Biochim. Biophys. Acta. 1995;1259:23–28. doi: 10.1016/0005-2760(95)00119-w. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenberg D., Ragimova S., Halpern Z. Stability of mixed micellar bile models supersaturated with cholesterol. Biophys. J. 1988;54:1013–1025. doi: 10.1016/S0006-3495(88)83039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichtenberg D., Ragimova S., Halpern Z. Stability of mixed micellar systems made by solubilizing phosphatidylcholine-cholesterol vesicles by bile salts. Hepatology. 1990;12:149S–154S. [PubMed] [Google Scholar]
- 39.le Maire M., Møller J.V., Champeil P. Binding of a nonionic detergent to membranes: flip-flop rate and location on the bilayer. Biochemistry. 1987;26:4803–4810. doi: 10.1021/bi00389a030. [DOI] [PubMed] [Google Scholar]
- 40.Cócera M., Lopez O., de la Maza A. Influence of the level of ceramides on the permeability of stratum corneum lipid liposomes caused by a C12-betaine/sodium dodecyl sulfate mixture. Int. J. Pharm. 1999;183:165–173. doi: 10.1016/s0378-5173(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 41.Heerklotz H. Membrane stress and permeabilization induced by asymmetric incorporation of compounds. Biophys. J. 2001;81:184–195. doi: 10.1016/S0006-3495(01)75690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toro C., Sanchez S.A., Gunther G. Solubilization of lipid bilayers by myristyl sucrose ester: effect of cholesterol and phospholipid head group size. Chem. Phys. Lipids. 2009;157:104–112. doi: 10.1016/j.chemphyslip.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin C.-M., Chang G.-P., Sheng Y.-J. Solubilization mechanism of vesicles by surfactants: effect of hydrophobicity. J. Chem. Phys. 2011;135:045102. doi: 10.1063/1.3615540. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenberg D., Opatowski E., Kozlov M.M. Phase boundaries in mixtures of membrane-forming amphiphiles and micelle-forming amphiphiles. Biochim. Biophys. Acta. 2000;1508:1–19. doi: 10.1016/s0304-4157(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 45.Almog S., Litman B.J., Lichtenberg D. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry. 1990;29:4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- 46.Alonso A., Villena A., Goñi F.M. Lysis and reassembly of sonicated lecithin vesicles in the presence of Triton X-100. FEBS Lett. 1981;123:200–204. doi: 10.1016/0014-5793(81)80287-6. [DOI] [PubMed] [Google Scholar]
- 47.Alonso A., Sáez R., Goñi F.M. Increase in size of sonicated phospholipid vesicles in the presence of detergents. J. Membr. Biol. 1982;67:55–62. doi: 10.1007/BF01868647. [DOI] [PubMed] [Google Scholar]
- 48.Lasch J. Interaction of detergents with lipid vesicles. Biochim. Biophys. Acta. 1995;1241:269–292. doi: 10.1016/0304-4157(95)00010-o. [DOI] [PubMed] [Google Scholar]
- 49.Almog S., Lichtenberg D. Effect of calcium on kinetic and structural aspects of dilution-induced micellar to lamellar phase transformation in phosphatidylcholine-cholate mixtures. Biochemistry. 1988;27:873–880. doi: 10.1021/bi00403a006. [DOI] [PubMed] [Google Scholar]
- 50.Long M.A., Kaler E.W., Lee S.P. Structural characterization of the micelle-vesicle transition in lecithin-bile salt solutions. Biophys. J. 1994;67:1733–1742. doi: 10.1016/S0006-3495(94)80647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson N.W., Kaler E.W. Size disproportionation in vesicular dispersions. J. Colloid Interface Sci. 1987;116:444–457. [Google Scholar]
- 52.Elsayed M.M.A., Cevc G. The vesicle-to-micelle transformation of phospholipid-cholate mixed aggregates: a state of the art analysis including membrane curvature effects. Biochim. Biophys. Acta. 2011;1808:140–153. doi: 10.1016/j.bbamem.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Velluto D., Gasbarri C., Fontana A. Use of simple kinetic and reaction-order measurements for the evaluation of the mechanism of surfactant-liposome interactions. J. Phys. Chem. B. 2011;115:8130–8137. doi: 10.1021/jp2026187. [DOI] [PubMed] [Google Scholar]
- 54.Fromherz P., Rocker C., Ruppel D. From discoid micelles to spherical vesicles. The concept of edge activity. Faraday Discuss. Chem. Soc. 1986;81:39–48. [Google Scholar]
- 55.Lasic D.D. A molecular model for vesicle formation. Biochim. Biophys. Acta. 1982;692:501–502. doi: 10.1016/0005-2736(82)90404-7. [DOI] [PubMed] [Google Scholar]
- 56.Lasic D.D. The mechanism of vesicle formation. Biochem. J. 1988;256:1–11. doi: 10.1042/bj2560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lichtenberg D., Robson R.J., Dennis E.A. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim. Biophys. Acta. 1983;737:285–304. doi: 10.1016/0304-4157(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 58.Walter A., Vinson P.K., Talmon Y. Intermediate structures in the cholate-phosphatidylcholine vesicle-micelle transition. Biophys. J. 1991;60:1315–1325. doi: 10.1016/S0006-3495(91)82169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards K., Almgren M., Brown W. Effects of Triton X-100 on sonicated lecithin vesicles. Langmuir. 1989;5:473–478. [Google Scholar]
- 60.Mrówczyńska L., Salzer U., Hägerstrand H. Curvature factor and membrane solubilization, with particular reference to membrane rafts. Cell Biol. Int. 2011;35:991–995. doi: 10.1042/CBI20100786. [DOI] [PubMed] [Google Scholar]
- 61.Ahyayauch H., Requero M.A., Goñi F.M. Surfactant effects of chlorpromazine and imipramine on lipid bilayers containing sphingomyelin and cholesterol. J. Colloid Interface Sci. 2002;256:284–289. doi: 10.1006/jcis.2002.8690. [DOI] [PubMed] [Google Scholar]
- 62.Urbaneja M.A., Goñi F.M., Alonso A. Structural changes induced by Triton X-100 on sonicated phosphatidylcholine liposomes. Eur. J. Biochem. 1988;173:585–588. doi: 10.1111/j.1432-1033.1988.tb14039.x. [DOI] [PubMed] [Google Scholar]
- 63.López O., Cócera M., de la Maza A. Solubilization of liposomes by sodium dodecyl sulfate: new mechanism based on the direct formation of mixed micelles. Arch. Biochem. Biophys. 1999;367:153–160. doi: 10.1006/abbi.1999.1267. [DOI] [PubMed] [Google Scholar]
- 64.Viriyaroj A., Kashiwagi H., Ueno M. Process of destruction of large unilamellar vesicles by a zwitterionic detergent, CHAPS: partition behavior between membrane and water phases. Chem. Pharm. Bull. (Tokyo) 2005;53:1140–1146. doi: 10.1248/cpb.53.1140. [DOI] [PubMed] [Google Scholar]
- 65.Tan A., Ziegler A., Seelig J. Thermodynamics of sodium dodecyl sulfate partitioning into lipid membranes. Biophys. J. 2002;83:1547–1556. doi: 10.1016/S0006-3495(02)73924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vecino A.J., Segura R.L., Alkorta I. Reconstitution in liposome bilayers enhances nucleotide binding affinity and ATP-specificity of TrwB conjugative coupling protein. Biochim. Biophys. Acta. 2010;1798:2160–2169. doi: 10.1016/j.bbamem.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Sáez-Cirión A., Alonso A., Rivas E.A. Equilibrium and kinetic studies of the solubilization of phospholipid-cholesterol bilayers by C12E8. The influence of the lipid phase structure. Langmuir. 2000;16:1960–1968. [Google Scholar]
- 68.de Foresta B., le Maire M., Lee A.G. Membrane solubilization by detergent: use of brominated phospholipids to evaluate the detergent-induced changes in Ca2+-ATPase/lipid interaction. Biochemistry. 1989;28:2558–2567. doi: 10.1021/bi00432a032. [DOI] [PubMed] [Google Scholar]
- 69.Arouri A., Mouritsen O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res. 2013;52:130–140. doi: 10.1016/j.plipres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Sudbrack T.P., Archilha N.L., Riske K.A. The solubilization of lipid bilayers by detergents was studied with optical microscopy of GUVs. J. Phys. Chem. B. 2011;115:269–277. doi: 10.1021/jp108653e. [DOI] [PubMed] [Google Scholar]


