Abstract
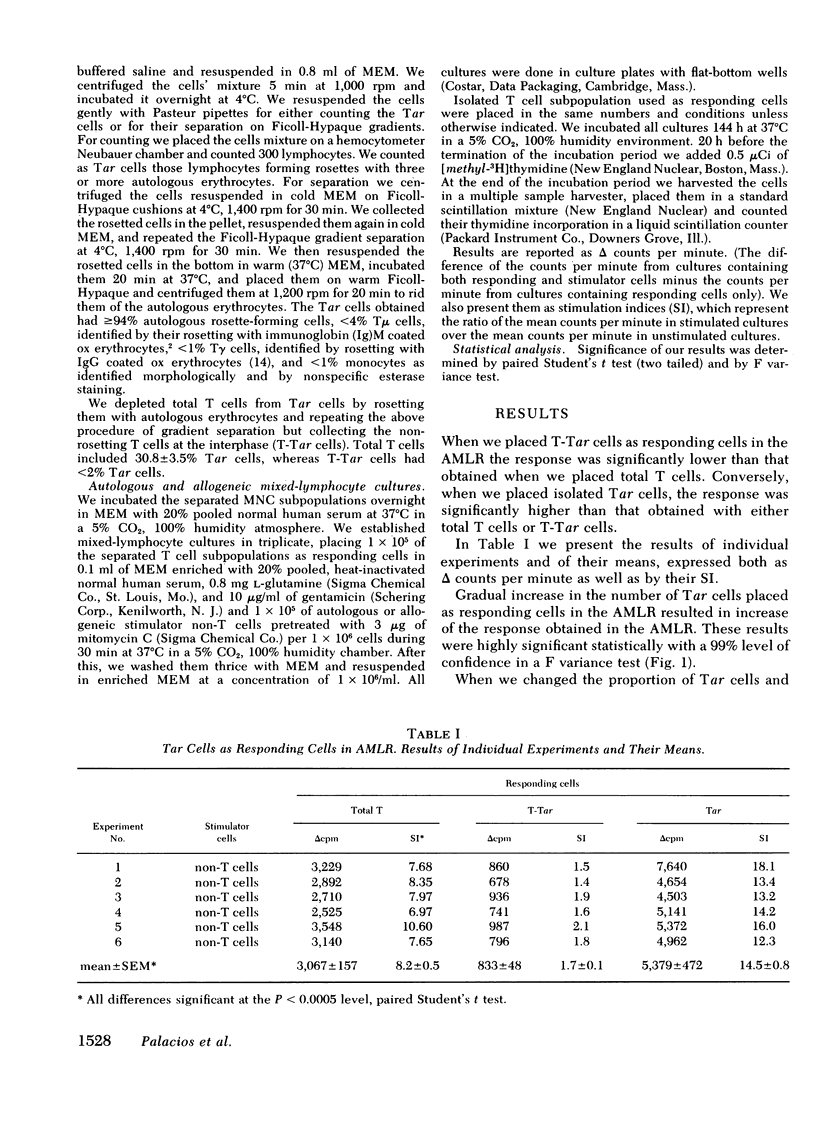
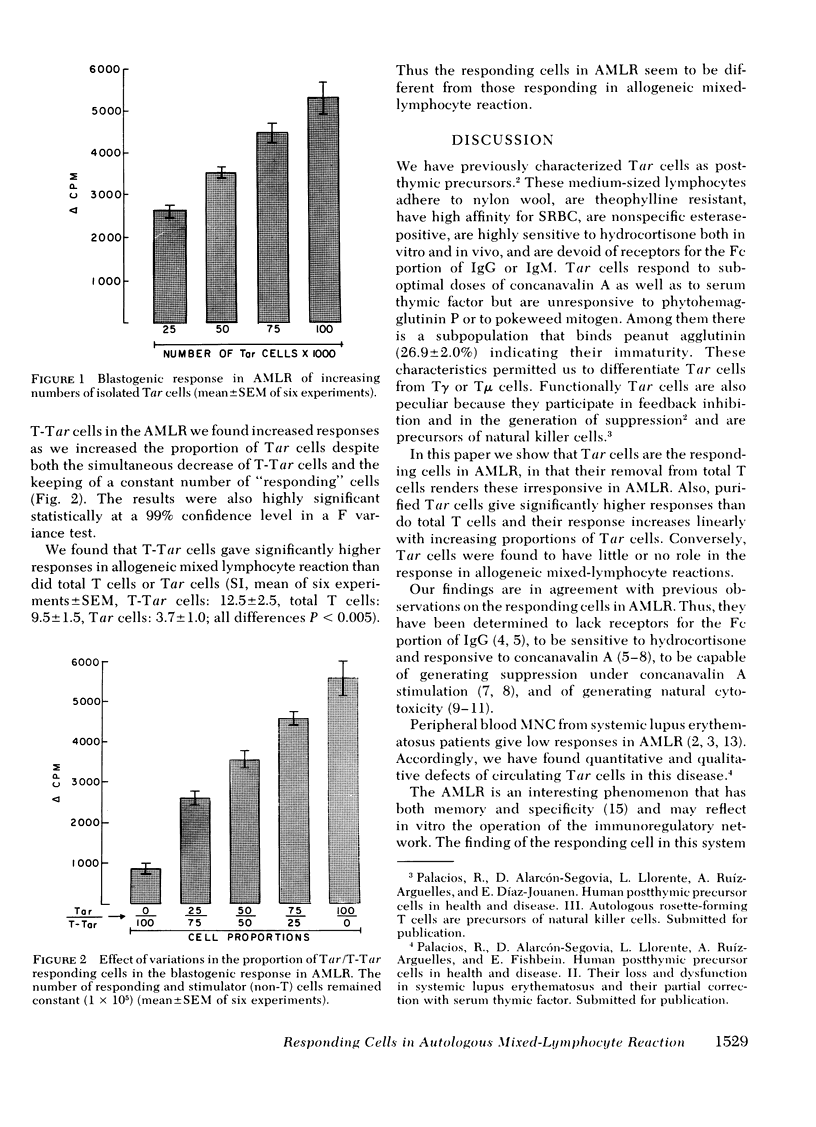
Autologous rosette-forming cells (Tar cells) have surface and functional characteristics of post-thymic precursors and among these characteristics there are some that have been identified in the responsive cell of the autologous mixed-lymphocyte reaction (AMLR). We therefore did AMLR with circulating mononuclear cells from normal subjects using as responding cells either total T cells, T cells depleted of Tar cells, or purified Tar cells. The response of Tar cells in AMLR was significantly greater than that of total T cells and these responded significantly more than Tar-depleted T cells. Conversely, Tar cells responded less than total T cells or T cells depleted of Tar cells in allogeneic mixed-lymphocyte reactions. Increasing numbers of Tar cells gave significantly greater AMLR responses both alone and when added to diminishing proportions of Tar-depleted T cells to keep the number of T cells constant in the system. Tar cells are the responding cells in AMLR but not in allogeneic mixed-lymphocyte reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon-Segovia D., Ruiz-Arguelles A., Llorente L. Antibody penetration into living cells. II. Anti-ribonucleoprotein IgG penetrates into Tgamma lymphocytes causing their deletion and the abrogation of suppressor function. J Immunol. 1979 May;122(5):1855–1862. [PubMed] [Google Scholar]
- Alarcón-Segovia D., Ruíz-Argüelles A. Decreased circulating thymus-derived cells with receptors for the Fc portion of immunoglobulin G in systemic lupus erythematosus. J Clin Invest. 1978 Dec;62(6):1390–1394. doi: 10.1172/JCI109260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Allison A. C., Ward P., Kight N. Identification of human mononuclear leucocyte populations by esterase staining. Clin Exp Immunol. 1977 Nov;30(2):289–298. [PMC free article] [PubMed] [Google Scholar]
- Ilfeld D. N., Krakauer R. S., Blaese R. M. Suppression of the human autologous mixed lymphocyte reaction by physiologic concentrations of hydrocortisone. J Immunol. 1977 Aug;119(2):428–434. [PubMed] [Google Scholar]
- Innes J. B., Kuntz M. M., Kim Y. T., Weksler M. E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J Clin Invest. 1979 Dec;64(6):1608–1613. doi: 10.1172/JCI109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Targan S. In vitro induction of cytotoxic effector cells with spontaneous killer cell specificity. J Exp Med. 1978 Jun 1;147(6):1621–1636. doi: 10.1084/jem.147.6.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Fauci A. S. Autologous and allogeneic intercellular interactions: modulation by adherent cells, irradiation, and in vitro and in vivo corticosteroids. J Immunol. 1979 Nov;123(5):2270–2277. [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. The cellular basis of the impaired autologous mixed lymphocyte reaction in patients with systemic lupus erythematosus. J Clin Invest. 1979 Jan;63(1):151–153. doi: 10.1172/JCI109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Specificity and suppressor function of human T cells responsive to autologous non-T cells. J Immunol. 1979 Aug;123(2):584–589. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3464–3468. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonari K. Cytotoxic T cells generated in the autologous mixed lymphocyte reaction. I. Primary autologous mixed lymphocyte reaction. J Immunol. 1980 Mar;124(3):1111–1121. [PubMed] [Google Scholar]
- Vande Stouwe R. A., Kunkel H. G., Halper J. P., Weksler M. E. Autologous mixed lymphocyte culture reactions and generation of cytotoxic T cells. J Exp Med. 1977 Dec 1;146(6):1809–1814. doi: 10.1084/jem.146.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler M. E., Kozak R. Lymphocyte transformation induced by autologous cells. V. Generation of immunologic memory and specificity during the autologous mixed lymphocyte reaction. J Exp Med. 1977 Dec 1;146(6):1833–1838. doi: 10.1084/jem.146.6.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolos J. A., Davey F. R. T-cell subpopulations in the autologous and allogeneic mixed lymphocyte reaction. Cell Immunol. 1979 Dec;48(2):415–419. doi: 10.1016/0008-8749(79)90136-9. [DOI] [PubMed] [Google Scholar]


