Abstract
Objective:
To assess the adherence to antiretroviral therapy (ART) in the human immunodeficiency virus (HIV)-infected population in India.
Design:
Systematic review and meta-analysis.
Materials and Methods:
The Medline and Cochrane library database were searched. Any prospective or retrospective study enrolling a minimum of 10 subjects with a primary objective of assessing ART adherence in the HIV population in India was included. Data were extracted on adherence definition, adherence estimates, study design, study population characteristics, recall period and assessment method. For metaanalysis, the pooled proportion was calculated as a back-transform of the weighted mean of the transformed proportions (calculated according to the Freeman-Tukey variant of the arcsine square root) using the random effects model.
Results:
There were seven cross-sectional studies and one retrospective study enrolling 1666 participants. Publication bias was significant (P = 0.003). Pooled results showed an ART adherence rate of 70% (95% confidence interval: 59–81%, I2 = 96.3%). Sensitivity analyses based on study design, adherence assessment method and study region did not influence adherence estimates. Fifty percent (4/8) of the studies reported cost of medication as the most common obstacle for ART adherence. Twenty-five percent (2/8) reported lack of access to medication as the reason for non-adherence and 12% (1/8) cited adverse events as the most prevalent reason for non-adherence. The overall methodological quality of the included studies was poor.
Conclusion:
Pooled results show that overall ART adherence in India is below the required levels to have an optimal treatment effect. The quality of studies is poor and cannot be used to guide policies to improve ART adherence.
Keywords: Anti-retroviral therapy, India, systematic review, treatment compliance
Introduction
India has one of the largest populations on the planet with a diverse set of social demographics. A seemingly small proportion (0.29%) of the population has been estimated to be living with human immunodeficiency virus (HIV) or acquired immune deficiency syndrome (AIDS).(1,2) However, the number is alarming and amounts to approximately 2.3 million people living with HIV/AIDS in India.(2) Combating the HIV/AIDS epidemic, to a large extent, has been possible due to advances in prevention and treatment of this disease. Advent of antiretroviral therapy (ART) has contributed to a significant change in the course of the disease, and the benefits of ART treatment are well documented.(3,4) Among several factors that can affect the ART outcome, adherence to the ART has been cited as a major factor associated with poor outcomes.(5,6,7) For ART to have maximum effect, greater than 95% adherence has been suggested.(4,8) Additionally, non-adherence to ART is a major cause of HIV drug resistance.(4,8) Especially in the Indian context, adherence to ART is very important due to the sheer number of HIV/AIDS cases, the socioeconomic status, diversity of the population and regions. That is, the socioeconomic challenges faced by patients contribute to non-adherence to ART in India. For example, most individuals living with HIV/AIDS in India are poorly educated and may not value the importance of ART, especially when their health status improves.(9) As a result, some interrupt ART, restarting when adequate funding becomes available.(10) In some seroconcordant couples, the partner clinically needing ART is known to share their medication with their HIV-negative partner or spouse.(9)
There have been several studies on ART adherence in India. However, results from these studies have shown varying estimates on ART adherence. While the study by Sarna et al.(11) demonstrated a high rate of 94% ART adherence, the study by Naik et al. showed a rate of 57%.(12) Therefore, the aim of this study is to systematically assess the rate of ART adherence across all studies conducted in India and the reasons for non-adherence (if any). We have performed a systematic review and metaanalysis to provide conclusive data on ART adherence in HIV/AIDS patients in India.
Materials and Methods
Literature search
We searched the Medline (Pubmed) and Cochrane library database until August 2009 using combinations of the following text and MeSH terms: “Medication Adherence,” “Patient Compliance,” “antiretroviral therapy,” “Antiretroviral Therapy, Highly Active,” “HIV,” “AIDS” and “India” for all years. No limits were applied on the basis of language, population or time period.
Inclusion criteria
Any prospective study or retrospective analysis assessing the rate of adherence to ART as a primary outcome in India was eligible for inclusion in the systematic review. Studies that were not related to HIV/AIDS or not related to adherence or focused on countries other than India were excluded.
Study selection and data extraction
Two reviewers (VA, AK) independently appraised the list of references and assessed the studies for their eligibility. Any disagreements were resolved by consensus. Data were extracted on definition of adherence, ART adherence estimates, study design, study population characteristics, recall period, study time frame and assessment method. We also extracted data on the methodological quality of the included studies.
For the purpose of metaanalysis, we first transformed the proportions into a quantity according to the Freeman-Tukey variant of the arcsine square root-transformed proportion.(13) The pooled proportion was calculated as a back-transform of the weighted mean of the transformed proportions using the random effects model. A formal statistical test for heterogeneity using the I2 test(14) was performed. We also explored heterogeneity and the robustness of the findings by conducting additional analyses according to study design, adherence assessment method and study region. The possibility of publication bias was also assessed using the Begg and Egger funnel plot method.(15,16) This method has its limitations but is widely used to assess publication bias.(17)
The metaanalysis was performed using STATA software.(18) The work was performed according to the PRISMA guidelines.(19) We evaluated the methodological quality of individual studies using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.(20) Individual studies were evaluated to assess whether each item on the STROBE checklist was met or not met.
Results
Identification of studies
The flow chart [Figure 1] outlines the process of identification and selection of studies. The initial search yielded 25 citations, of which 17 were excluded for the reasons shown in Figure 1. Accordingly, eight studies met the inclusion criteria and were included in the systematic review.
Figure 1.
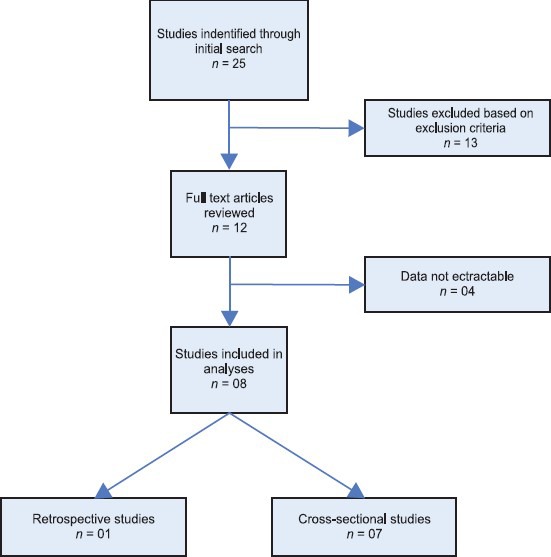
Flow chart
Characteristics of included studies
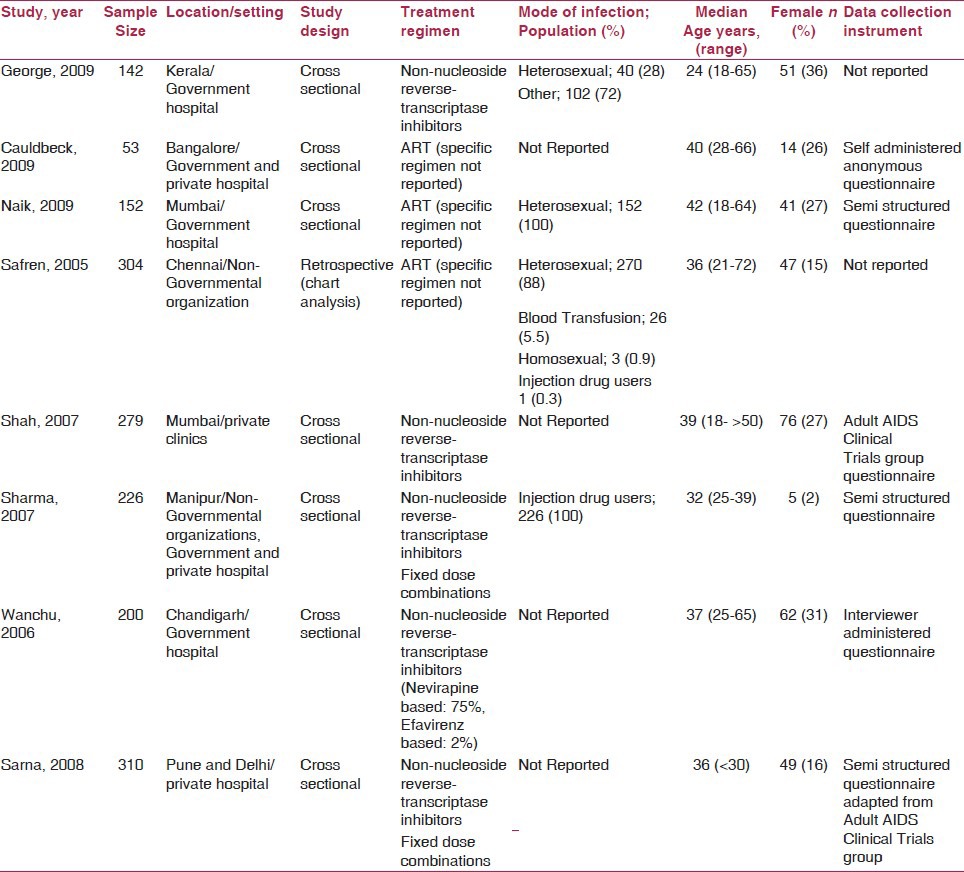
The eight included studies enrolled a total of 1666 participants (1322 male and 344 female participants). Characteristics of the included studies are summarized in Table 1. There were seven cross-sectional studies(11,12,21,22,23,24,25) and one retrospective chart analyses.(26) Three studies were conducted in southern India(21,24,26) and two each in western(12,25) and north India.(22,23) One study enrolled participants from western and northern India.(11) The average sample size was 208 (range: 53–310). The median age of participants in the included studies was 36 years (range: 24–42 years). The study by Shah et al.(25) used the Adult AIDS Clinical Trials group questionnaire for assessment of ART adherence while the study by Sarna et al.(11) used a semistructured questionnaire adapted from the Adult AIDS Clinical Trials group questionnaire.(27) The remaining studies (6/8) used unique data collection instruments designed by their respective study groups [Table 1].
Table 1.
Study characteristics
Seventy-five percent (6/8) of the studies reported either median or range of duration of ART. However, only 50% (4/8) of the studies reported both median and range of ART duration.(11,12,25,26) The length of ART regimens ranged from 1 month to 72 months across studies [Table 2]. Seventy-five percent (6/8) of the studies reported the recall period.(11,21,22,24,25,26) The length of recall period varied from previous 4 days to lifetime recall [Table 2].
Table 2.
Treatment adherence data
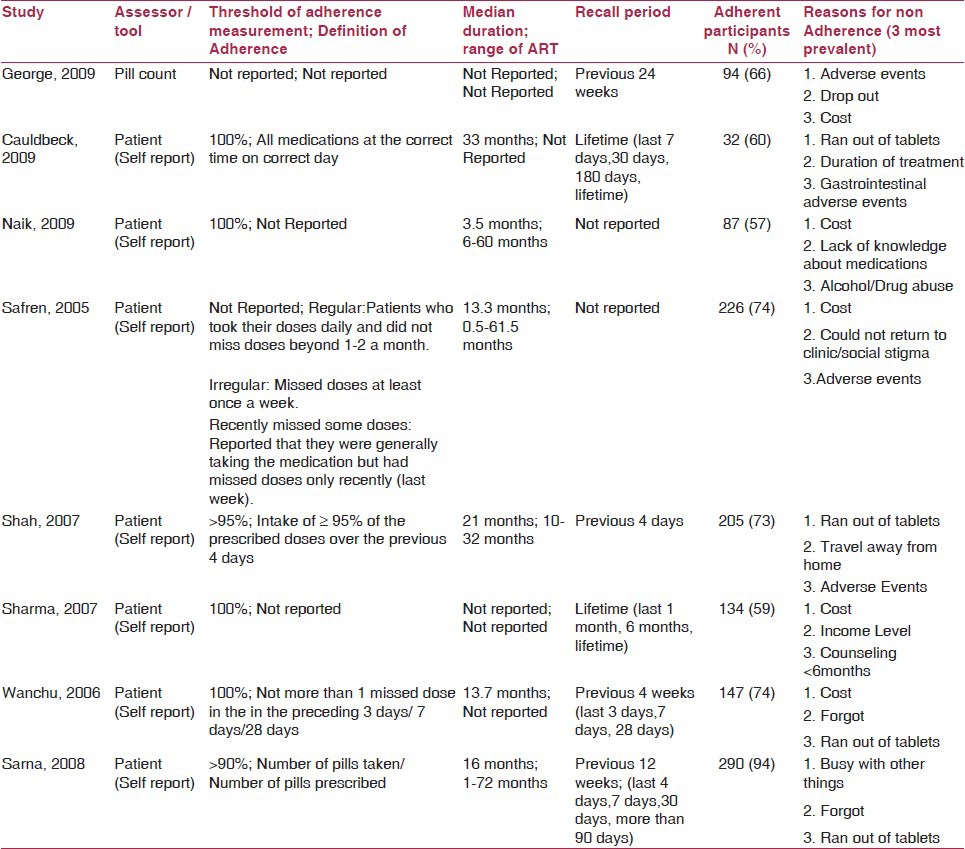
The definition of treatment adherence and associated cutoff values varied across the studies. Thirty-seven percent (3/8) of the studies(12,23,24) did not report the definition of treatment adherence. Sixty-two percent of studies (5/8) defined ART adherence. The study by Cauldbeck et al.(21) defined adherence as taking all medications as prescribed by the physician (all medications at a correct time on a correct day). The study by Wanchu and colleagues(22) specifically asked the participants if they forgot to take one or more dose(s) in the preceding 3 days or the last 1 and 4 weeks, and classified patients as being totally adherent (did not miss any dose) or non-adherent (missed at least one dose of medication in the preceding 4 weeks). The study by Sarna et al.(11) used a 4-day recall period as definition of adherence. In this study,(11) the mean 4-day adherence was calculated by dividing the number of pills actually taken by the number of pills needed to be taken for 4 days multiplied by 100, and then dichotomized as high adherence (>90%) and low adherence (<90%). The study by Shah and colleagues defined adherence as having taken ≥95% of the prescribed doses of medication over the past 4 days. Safren et al.(26) categorized adherence as regular versus irregular. Patients who took their prescribed doses daily and did not miss doses beyond one to two doses a month were categorized as “regular.” Patients who missed doses at least once a week were identified as “irregular.” Furthermore, patients who reported generally taking the medication but missed doses only recently (last week) were categorized as “recently missed some doses.” Adherence was measured across various time periods, ranging from previous 4 days to lifetime adherence [Table 2].
Sixty-three percent (5/8) of the studies reported treatment regimens. All these 63% (5/8) studies reported use of non-nucleoside reverse-transcriptase inhibitors (NNRTIs). However, only two studies reported the details of NNRTI dosage.(11,23) The quantity of pills consumed by study participants was reported only in 37% (3/8) of the studies.(11,21,22) The participants in the study by Caludbeck et al.(21) used two to more than 10 medication tablets per day, while patients in the study by Sarna et al.(11) consumed one to more than four tablets per day. Participants in the study by Wanchu et al.(22) consumed either two or three tablets per day. However, 99% (7/8) of the studies used the self-reporting method for assessment of treatment adherence, while only one study used the pill count method.(24) The study by George et al.(24) used the pill count method and followed three groups of patients for 6 months. Group 1 was given zidovudine, lamivudine and nevirapine, with a mean medication adherence of 90%; group 2 was given lamivudine, stavudine and nevirapine and achieved a mean medication adherence of 93%; and group 3 was given lamivudine, stavudine and efavirenz, and reported a mean medication adherence of 85%. In this study by George et al.,(24) the overall mean ART adherence showed regular improvement in the adherence rate over time among those who did not drop out of the study.
The impact of counseling on ART adherence was analyzed and reported in 37% (3/8) of the studies.(21,23,25) Specifically, Cauldbeck et al. reported that regular follow-up visits with the physician leads to improved ART adherence.(21) Shah et al. noted that pre-ART counseling had a positive impact on ART adherence.(25) However, Sharma et al. reported that counseling had no impact on ART adherence.(23)
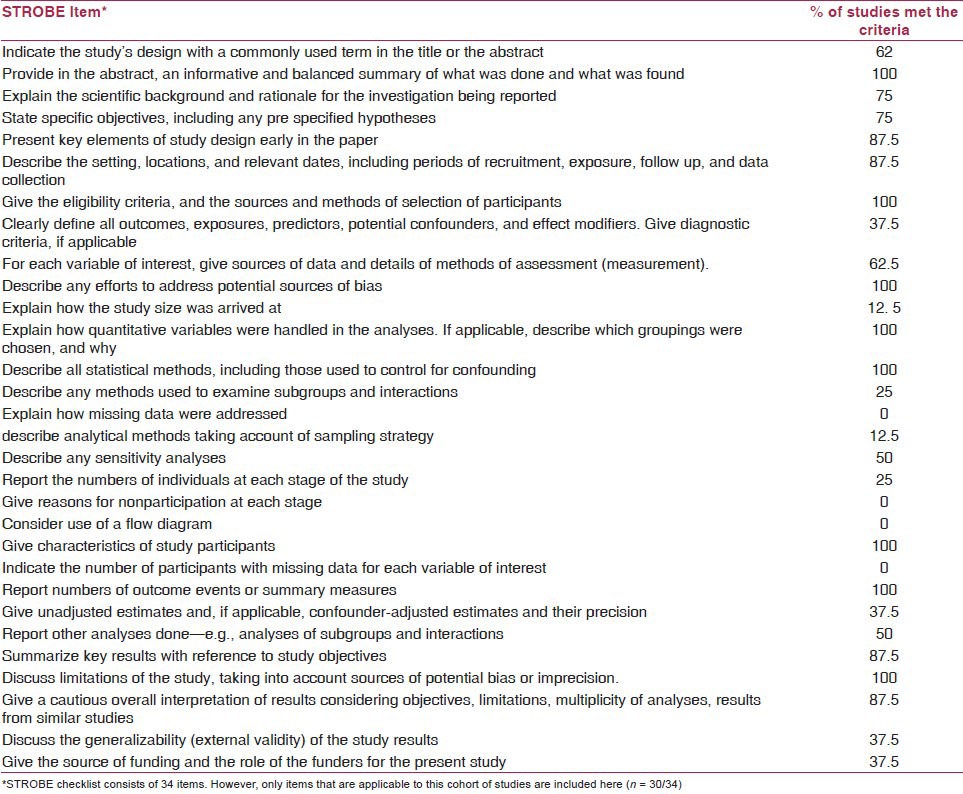
Methodological quality
The methodological quality varied across the included studies. The methodological quality of the included study is summarized in Table 3. Sixty-two percent (5/8) of the studies(11,12,21,23,24) indicated the study's design with a commonly used term in the title or the abstract. Only 37% (3/8)(11,26,28) of the studies clearly defined all outcomes, exposures, predictors, potential confounders and effect modifiers. Only one study(11) described the calculations for sample size estimation. Moreover, none of the studies explained the methods used to address missing data, number of participants with missing data for each variable of interest and analytical techniques used to account for the sampling strategies. Only 37% (3/8) of the studies(11,23,25) reported the unadjusted estimates and confounder-adjusted estimates and their precision (95% confidence interval) and made clear which confounders were adjusted for and why they were included. Only 25% (2/8) of the studies(24,26) reported the numbers of individuals at each stage of the study (e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up and analyzed). However, none of the studies employed a flow diagram depicting the follow-up of participants during the study period. All the included studies acknowledged and discussed the limitations of their study with potential sources of bias.
Table 3.
Methodological quality assessment (STROBE checklist)
Outcomes
ART adherence
The main results, ART adherence definition and period of assessment are presented in Table 2. Data on overall ART adherence were extractable from all (8/8) studies. As shown in Figure 2, the pooled proportion of ART adherence was 0.70 (95% CI: 0.59–0.81). However, there was a statistically significant heterogeneity for the outcome of overall ART adherence (I2 = 96.3%) among the included studies.
Figure 2.
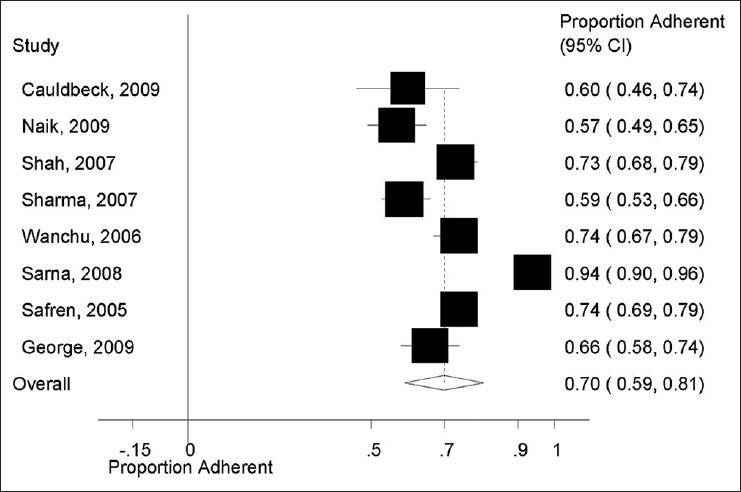
Overall proportion of antiretroviral therapy adherence
Additional analyses
To assess the robustness of our findings and explore the reasons behind heterogeneity, we performed additional sensitivity analyses for the outcome of ART adherence. As shown in Figure 3, there was no difference in estimates of ART adherence according to study design. The pooled proportion of ART adherence in studies employing a cross-sectional design was 0.72 (95% CI: 0.61, 0.83) compared with 0.59 (95% CI: 0.53, 0.66) in the retrospective study. However, the issue of heterogeneity within subgroups could not be assessed due to the limited number of prospective and retrospective studies (i.e., there were seven cross-sectional studies compared with only one retrospective study). Similarly, as shown in Figure 4, subgroup analyses according to ART adherence assessment method also showed no difference. The pooled proportion of ART adherence was 0.71 (95% CI: 0.59, 0.82) in studies using the self-assessment method for the measurement of ART adherence versus 0.66 (95% CI: 0.58, 0.74) in studies using the pill count method. For similar reasons as in the case, study design assessment of heterogeneity within subgroups was not possible due to only one study using the pill count method for the assessment of ART adherence. Additional analysis according to the study regions (South India vs. North India vs. Western India) did not show any difference in the rates of ART adherence (data not shown).
Figure 3.
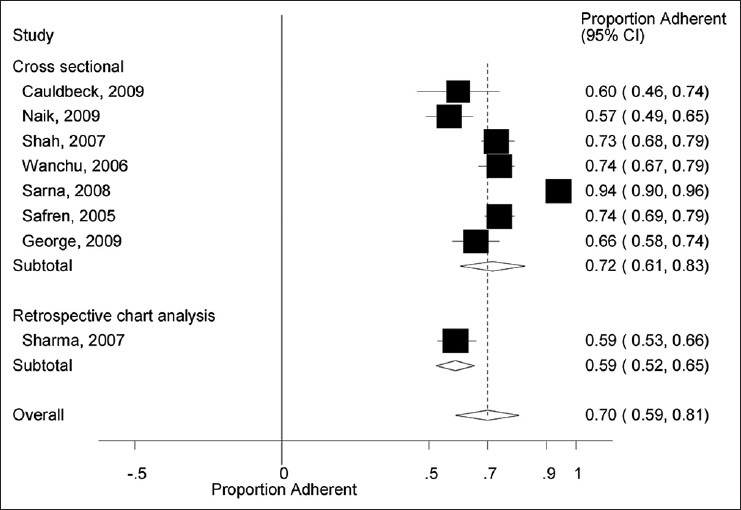
Antiretroviral therapy adherence by study design
Figure 4.
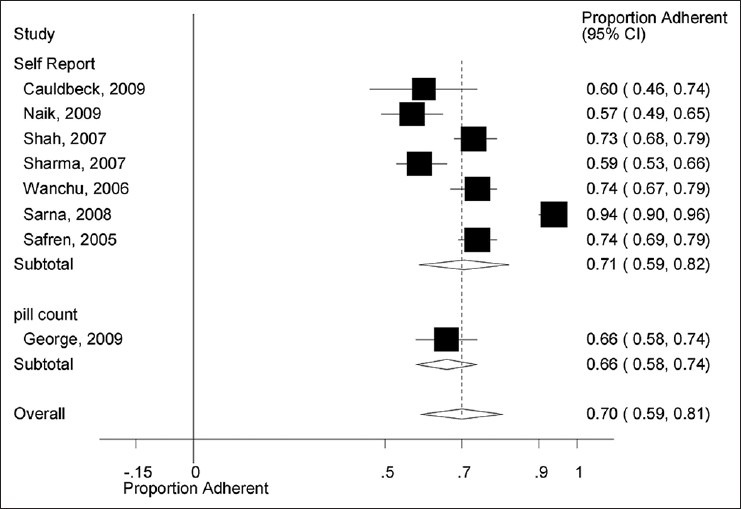
Antiretroviral therapy adherence by method of assessment
Reasons for non-adherence
The reasons for non-adherence to ART across the included studies are summarized in Table 2. Fifty percent (4/8) of the studies(12,22,23,26) reported the cost of medication as the most common obstacle for treatment adherence. Patients reported not having access to sufficient amount of mediation in two studies as the reason for non-adherence to ART.(21,25) Only one study reported adverse events as the most prevalent reason for non-adherence.(24) Similarly, only one study reported the patients being busy with other activities that lead to treatment non-adherence.(11)
Publication bias
The assessment for publication bias in the included studies using the Begg and Egger funnel plot for the outcomes of ART adherence showed a skewed pattern, indicating a publication bias (P value = 0.003) [Figure 5].
Figure 5.
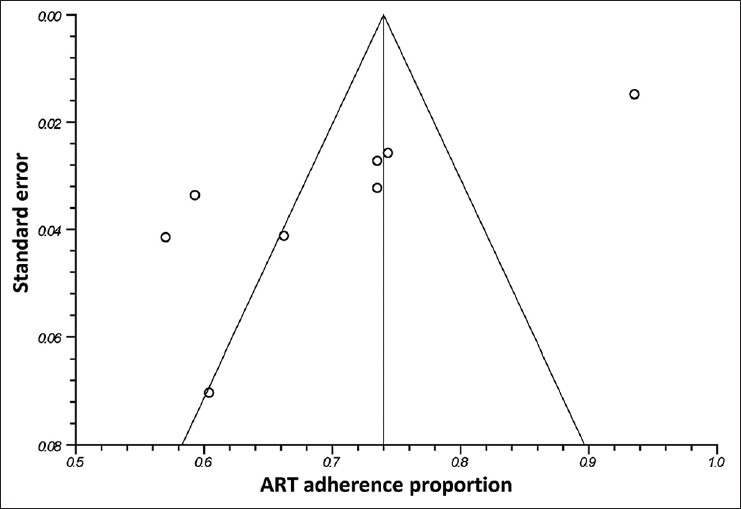
Funnel plot for publication bias
Discussion
This is the first systematic review and meta-analysis addressing the issue of ART adherence in India, which has one of the largest populations of HIV/AIDS patients in the world.(1) The results from this systematic review and metaanalysis show that overall adherence to ART in India is around 70%, which may be inadequate for the effective control of viremia. These results also show that achieving the desirable ART adherence of more than 75–80% with NNRTI regimen is often challenging in the Indian context. In addition to the well-recognized factors of cost, complexity and adverse events, lifestyle factors and issues in the patient–provider relationship may adversely influence adherence in Indian patients.(29) As shown in the results, the study by George et al.(24) cited adverse events as the major reason for non-adherence. However, this study had three ART regimens groups (zidovudine, lamivudine, nevirapine vs. lamivudine, stavudine, nevirapine vs. lamivudine, stavudine, efavirenz), and the rate of adverse events was similar across all treatment groups, excluding association of any particular ART regimen with increased risk for non-adherence due to adverse events. Cost was noted as the most common reason, followed by adverse events for non-adherence in this systematic review. Nevertheless, the study by Sarna et al. contradicted the finding of cost as a reason for non-adherence. The patients enrolled in the study by Sarna et al. treated at a private facility reported an adherence rate of 94%, despite the fact that this is expensive in the Indian context. A subgroup of patients in this study receiving HIV treatment at no cost had poor adherence to ART. These findings differ from a previously reported systematic review/metaanalysis of ART intervention programs in a resource-poor setting.(30) The results from this systematic review/metaanalysis showed that provision of medications free of charge was associated with a higher probability of achieving higher adherence.(30) Similarly, ART adherence studies by Kumarasamy et al. and Wanchu et al. conducted in India also showed the association of high cost of ART as a barrier to ART adherence.(10,22) In addition, none of the studies mentioning cost as a barrier to ART adherence explain in detail what constitutes cost. There are multiple costs, including the cost of the medication, the cost of travel and access to physicians or cost of diagnostic tests as a barrier to ART adherence. Moreover, where the Government of India is providing ART for over 0.3 million individuals in approximately 239 ART centers,(2) only 50% (4/8) studies included in this review discussed ART cost in the context of subsidized ART compared with ART offered at private clinics.(11,22,23,24)
The lowest ART treatment adherence rate was reported by Sharma et al.(23) with 59% adherence over 16 months. The low ART adherence as reported in this study can be explained by two factors: this study enrolled only injecting drug users, and used the self-reported assessment to measure ART adherence. Previous studies assessing adherence in injecting drug users have shown to have higher rates of non-adherence compared with the general HIV/AIDS population.(31,32,33) Additionally, studies have shown the self-reported assessment method to be unreliable in assessing ART adherence.(34,35,36) The variability of the study methodology, specifically variations in assessment of adherence, may explain the observed heterogeneity in the included studies. Overall, our findings are in line with a previous systematic review and metaanalysis on ART adherence enrolling patients in Sub Saharan Africa [ART adherence: 77% (95% CI 68–85%)].(37) There are patients in both (Indian and Sub Saharan African) settings that have suboptimal adherence and that factors beyond poverty, such as forgetfulness, severity of adverse events and the level of complexity of the drug regimen, play an important role.(37)
The results from this systematic review also show that the quality of reporting of the included studies is poor. For example, several key elements of a study were either not reported or reported in a fashion that cannot be used for informed decision-making. Only few of the studies (12.5%) reported analytical methods taking account of sampling strategy or report the numbers of individuals at each stage of the study (25%), which is a key to interpret the findings and assess the generalizability of the results. The results from this systematic review also show a lack of sociocultural acclimatization of the tools used to assess ART adherence. For example, only 37% (3/8) of the studies(11,12,21) reported any efforts to adapt the questionnaire used for data collection to the study population. However, none of the included studies reported use of any qualitative techniques to pilot test data collection strategies to ensure cultural relevance and validity. The lack of these formative evaluations could have unfavorable ramifications in a country like India with diverse social and economic settings. That is, the instrument validated for assessment of ART adherence in one study population in India may not prove to be useful to assess treatment adherence in other populations. In general, these studies investigated numerous socioeconomic factors correlated to ART adherence. They largely maintain the claim that in India, HIV-infected adults’ socioeconomic context with the treatment-related factors (e.g., adverse events) play a key role in ART adherence. However, none of the included studies linked these socioeconomic and treatment-related variables to a culturally appropriate model for ART adherence. Therefore, the information from these studies limits the end users ability to use this information in any useful way to improve ART adherence. Possibly due to the issues mentioned above, the adherence rate did not differ across the geographical regions in India. Another area of concern is the use of varying ART duration (range: 1–72 months) and recall periods (range: 4 days to lifetime). Moreover, studies using multiple recall periods did not report adherence estimates for each period, limiting our ability to compare adherence estimates and recommend an optimum recall period.
This systematic review has limitations. We performed searches of only standardized databases (Medline and Cochrane library) and, therefore, might have missed either unpublished studies or articles reported in the grey literature. Different interpretations of what constitutes adherence versus non-adherence makes the generalizability of the results limited. We were not able to conduct sensitivity analyses to evaluate possible relationships between different regimens and patient characteristics and adherence (e.g., presumed higher adherence among patients with lower pill burden vs. higher pill burden), because this information was rarely available among the identified studies. Similarly, we were unable to analyze the impact of counseling on ART adherence in a metaanalysis as the data were scarcely reported. Finally, it is possible that most of the studies in this systematic review might be overestimating adherence levels, due mainly to the fact that seven out of eight included studies relied only on self-reported adherence–a measure known to overestimate patients’ true adherence levels.
In the future, researchers should standardize adherence definitions and use a standardized recall period, such as the 7-day recall, which is shown to have has better sensitivity.(35) Our efforts to increase access to ART must be combined with efforts to design new and effective strategies of improving adherence in a resource-limited setting. The report found the overall adherence to be poor in comparison with documented effective adherence for HIV/AIDS treatment. There is abundant variation in the definition of adherence and what constitutes “optimal adherence” across the studies with poor reporting regarding data collection methods. These facts prohibit extrapolating the results to guide policies to improve ART adherence.
This first systematic review and metaanalysis assessing the adherence to ART in the HIV-infected population in India highlights lower overall adherence to ART in India. However, the quality of included studies is poor and needs improvement in analysis and reporting, such as impact of counseling on ART adherence. Similarly, future researchers should explore the association of various ART cost attributes and ART adherence in detail. In conclusion, this systematic review shows an urgent need to improve the quality of reporting, such as standardized adherence definition, threshold, period of measurement, etc. to be established, where data can be easily extracted, interpreted and used for informing programs and policies to improve ART adherence.
Acknowledgments
We are grateful to Ms. Sue Felber at the Moffitt cancer center for her assistance in performing a literature search. This study was supported in part by the Fogarty International Center/USNIH: Grant # 1D43TW006793-01A2-AITRP. The study sponsor had no role in study design, collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Source of Support: Nil
Conflict of Interest: All authors [RM, VA, PE, BD, SP, AP, EN, SM, AK] declare that they have no non-financial interests that may be relevant to the submitted work. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.UNAIDS World Health Organization. AIDS epidemic update. Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) 2009 [serial on the Internet] [Last cited in 2009]. Available from: http://www. data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf .
- 2.Department of AIDS Control Annual Report 2009-10. India: National Aids Control Organization; 2010. National Aids Control Organization, India. [Google Scholar]
- 3.Rajasekaran S, Jeyaseelan L, Ravichandran N, Gomathi C, Thara F, Chandrasekar C. Efficacy of antiretroviral therapy program in children in India: Prognostic factors and survival analysis. J Trop Pediatr. 2009;55:225–32. doi: 10.1093/tropej/fmm073. [DOI] [PubMed] [Google Scholar]
- 4.Boyd MA. Improvements in antiretroviral therapy outcomes over calendar time. Curr Opin HIV AIDS. 2009;4:194–9. doi: 10.1097/COH.0b013e328329fc8d. [DOI] [PubMed] [Google Scholar]
- 5.Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: A meta-analysis. Clin Infect Dis. 2009;48:484–8. doi: 10.1086/596482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: A research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41:285–97. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 7.Haubrich RH, Little SJ, Currier JS, Forthal DN, Kemper CA, Beall GN, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS. 1999;13:1099–107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- 8.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 9.Solomon S, Solomon SS, Ganesh AK. AIDS in India. Postgrad Med J. 2006;82:545–7. doi: 10.1136/pgmj.2006.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumarasamy N, Safren SA, Raminani SR, Pickard R, James R, Krishnan AK, et al. Barriers and facilitators to antiretroviral medication adherence among patients with HIV in Chennai, India: A qualitative study. AIDS Patient Care STDS. 2005;19:526–37. doi: 10.1089/apc.2005.19.526. [DOI] [PubMed] [Google Scholar]
- 11.Sarna A, Pujari S, Sengar AK, Garg R, Gupta I, Dam J. Adherence to antiretroviral therapy and its determinants amongst HIV patients in India. Indian J Med Res. 2008;127:28–36. [PubMed] [Google Scholar]
- 12.Naik E, Casanas B, Pazare A, Wabale G, Sinnott J, Salihu H. Cost of treatment: The single biggest obstacle to HIV/AIDS treatment adherence in lower-middle class patients in Mumbai, India. Indian J Sex Transm Dis. 2009;30:23–7. doi: 10.4103/2589-0557.55476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuart A, Ord J. 6th ed. London: Edward Arnold; 1994. Kendall's advanced theory of statistics. [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–62. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 18.College Station, TX: StataCorp LP; 2006. StataCorp. Stata Statistical Software, Release 9. [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology. 2007;18:805–35. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 21.Cauldbeck MB, O’Connor C, O’Connor MB, Saunders JA, Rao B, Mallesh VG, et al. Adherence to anti-retroviral therapy among HIV patients in Bangalore, India. AIDS Res Ther. 2009;6:7. doi: 10.1186/1742-6405-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanchu A, Kaur R, Bambery P, Singh S. Adherence to generic reverse transcriptase inhibitor-based antiretroviral medication at a Tertiary Center in North India. AIDS Behav. 2007;11:99–102. doi: 10.1007/s10461-006-9101-y. [DOI] [PubMed] [Google Scholar]
- 23.Sharma M, Singh RR, Laishram P, Kumar B, Nanao H, Sharma C, et al. Access, adherence, quality and impact of ARV provision to current and ex-injecting drug users in Manipur (India): An initial assessment. Int J Drug Policy. 2007;18:319–25. doi: 10.1016/j.drugpo.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.George C, Yesoda A, Jayakumar B, Lal L. A prospective study evaluating clinical outcomes and costs of three NNRTI-based HAART regimens in Kerala, India. J Clin Pharm Ther. 2009;34:33–40. doi: 10.1111/j.1365-2710.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- 25.Shah B, Walshe L, Saple DG, Mehta SH, Ramnani JP, Kharkar RD, et al. Adherence to antiretroviral therapy and virologic suppression among HIV-infected persons receiving care in private clinics in Mumbai, India. Clin Infect Dis. 2007;44:1235–44. doi: 10.1086/513429. [DOI] [PubMed] [Google Scholar]
- 26.Safren SA, Kumarasamy N, James R, Raminani S, Solomon S, Mayer KH. ART adherence, demographic variables and CD4 outcome among HIV-positive patients on antiretroviral therapy in Chennai, India. AIDS Care. 2005;17:853–62. doi: 10.1080/09540120500038439. [DOI] [PubMed] [Google Scholar]
- 27.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee and Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 28.Shah CA. Adherence to high activity antiretrovial therapy (HAART) in pediatric patients infected with HIV: Issues and interventions. Indian J Pediatr. 2007;74:55–60. doi: 10.1007/s12098-007-0028-8. [DOI] [PubMed] [Google Scholar]
- 29.WHO Adherence Meeting Report. Geneva: WHO; 2001. World Health Organization, Adherence to Long-Term Therapies - Evidence for Action. [Google Scholar]
- 30.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: A meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–24. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 31.Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11:1665, 70. doi: 10.1097/00002030-199714000-00002. [Editorial] [DOI] [PubMed] [Google Scholar]
- 32.Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’shaughnessy MV, Montaner JS, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547, 9. doi: 10.1001/jama.280.6.547. [Report] [DOI] [PubMed] [Google Scholar]
- 33.Sherer R. Adherence and antiretroviral therapy in injection drug users. JAMA. 1998;280:567–8. doi: 10.1001/jama.280.6.567. [DOI] [PubMed] [Google Scholar]
- 34.Wagner G. Utility of self-reported antiretroviral adherence: Comment on Simoni et al. (2006) AIDS Behav. 2006;10:247–8. doi: 10.1007/s10461-006-9122-6. [DOI] [PubMed] [Google Scholar]
- 35.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–45. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straka RJ, Fish JT, Benson SR, Suh JT. Patient self-reporting of compliance does not correspond with electronic monitoring: An evaluation using isosorbide dinitrate as a model drug. Pharmacotherapy. 1997;17:126–32. [PubMed] [Google Scholar]
- 37.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis. JAMA. 2006;296:679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]


