Abstract
Objective:
To determine the prevalence of HCV co-infection and its correlation with demographic and risk factors among human immunodeficiency virus (HIV)-infected individuals attending Shiraz behavioral diseases consultation (SBDC) Center in southern Iran.
Materials and Methods:
In a cross-sectional study, 226 consecutive HIV-positive patients who referred to SBDC Center from April 2006 to March 2007 were interviewed face-to-face to record demographic data and risk factors of HIV transmission. A 10ml sample of venous blood was drawn from every subject and tested for HCV-antibodies by third generation enzyme linked immunosorbant (ELISA) and recombinant immunoblot assays (RIBA). All samples were also analyzed by qualitative reverse transcriptase polymerase chain reaction (RT-PCR) for detection of HCV-RNA.
Results:
The study population consisted of 214 men (94.7%) and 12 women (5.3%) with a mean age of 35.6 ± 7.9 years. The most prevalent risk factor was imprisonment (88.9%) followed by injecting drug use (79.2%). The prevalence of HCV infection was 88.5% by ELISA and 86.7% by RIBA, while HCV viremia was detected in 26.1% of the patients. HCV-antibody positivity was significantly associated with gender, age, marital status, occupation, injecting drug use, and history of imprisonment. It was inversely related to having an infected or high risk sexual partner. In the logistic regression model, the predictors of HCV-positivity were injecting drug use (OR = 24.9, P = 0.004) and imprisonment (OR = 21.4, P < 0.001).
Conclusions:
Prevalence of HCV infection among HIV-positive individuals in our region is very high and there is a need for stricter preventive actions against transmission of HCV among this group of patients.
Keywords: HIV, hepatitis C, iran, prevalence, risk factors
Introduction
Approximately 170 million people are infected with hepatitis C virus (HCV). One of the major health problems today is the viral hepatitis caused by HCV, because this virus has spread worldwide, it has different routes of transmission, and there is no efficient therapy.(1) In 2010, there were approximately 34 million people living with human immunodeficiency virus (HIV).(2) About 14,000 new HIV infections occur globally every day, 95% of which are in developing countries.(3)
It is estimated that among HIV-infected individuals, 4-12 million are co-infected with HCV. The clinical course of chronic HCV infection is accelerated by HIV co-infection which leads to an increased risk of cirrhosis, hepatocellular carcinoma, and decompensated liver disease.(4) As development of highly active antiretroviral therapy has significantly improved the prognosis of HIV, chronic liver diseases have become a prominent cause of morbidity and mortality in the HIV-HCV co-infected patients.(5)
The prevalence of HCV infection in HIV-positive patients varies from 4.0% to greater than 50.0% in different populations worldwide.(6) Depending on the route of transmission, this prevalence ranges from 3-15% in homosexual/bisexual men, to 80% in injecting drug users (IDUs), to 98% in hemophiliacs. The main determinant of HCV prevalence in HIV-infected cohorts is the proportion of various risk groups present in those cohorts.(7) The high prevalence of HIV/HCV co-infection is due to sharing the same risk factors for transmission, as both viruses are transmitted by blood products, intercourse, and vertical transmission.(8)
Due to differences in the risk factors of HIV/HCV co-infection in various geographical regions in the world, each region should provide its own statistics to create a base for planning the future preventive and therapeutic measures. The aim of this study was to determine the prevalence of HCV co-infection among HIV infected individuals attending Shiraz behavioral diseases consultation (SBDC) Center in Fars Province, southern Iran from April 2006 to March 2007, and to investigate any correlation that HCV co-infection may have with demographic factors and different risk factors.
Materials and Methods
Of 400 consecutive HIV-positive patients who referred to SBDC Center in Fars Province, southern Iran from April 2006 to March 2007, 226 subjects gave informed written consent to participate in this cross-sectional study (refusal rate: 43.5%). After finding out about SBDC center through their physicians or friends, individuals with a high risk for HIV infection voluntarily refer to this center, where consultations and work-ups are done at no cost. Only those with documented HIV infection (by serial ELISA and Western blot) were included in the study. Repeat visitors were excluded.
All subjects were interviewed face-to-face and a form containing questions on demographic data and risk factors of HIV transmission was completed for each patient. The interviews were conducted, by two trained general physicians, in a designated room in SBDC Center where privacy could be provided for each subject. The risk factors under study included history of imprisonment, injecting drug use (IDU), high risk sexual behavior, recipient of blood/blood products, child of an infected mother, and having an infected or high risk sexual partner [Table 1].
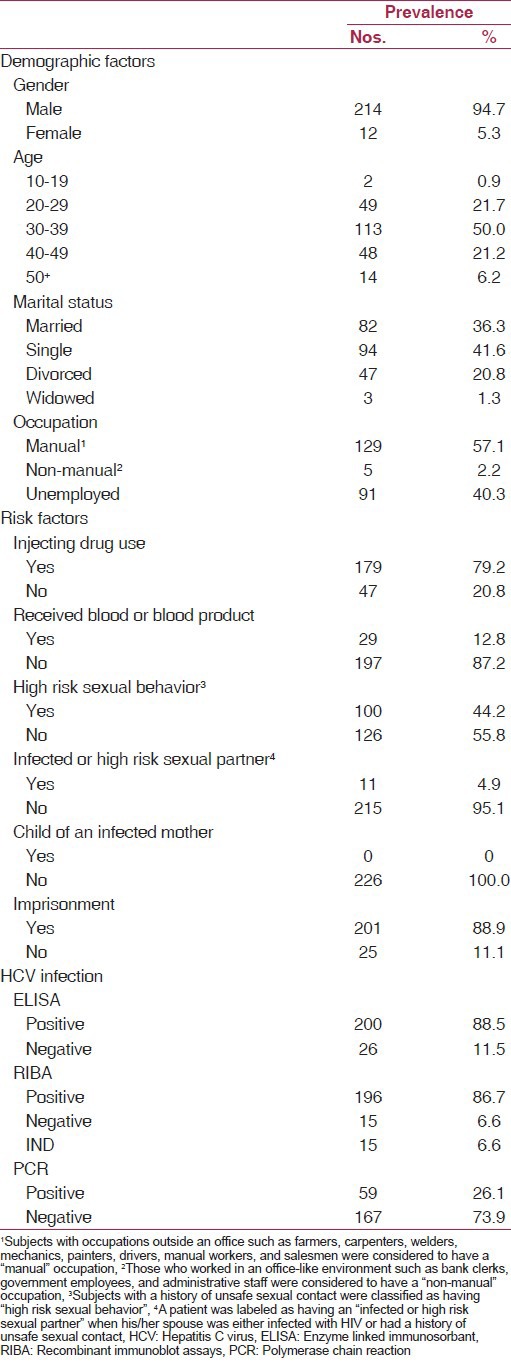
Table 1.
Distribution of demographic factors, risk factors, and HCV-positivity in the population under study
A 10 ml sample of venous blood was drawn from every subject; serum was extracted and immediately freezed at −20°C. All tubes were coded and transferred to a −70°C freezer in less than a week. The sera were tested for HCV-Ab by a third generation ELISA (HCV Ultra, China) according to the manufacturer's instructions. Then, a third generation RIBA for HCV antibodies (MP diagnostics, Singapore) was carried out on all samples, followed by a qualitative RT-PCR (Cinnagen Co, Iran) for detection of HCV-RNA.
All data were recorded in a computer database and were analyzed using the STATA software, version 10. Chi-square and Fisher's exact tests were used to evaluate the association of HCV prevalence with demographic and risk factors. Logistic regression analysis was performed to determine the predictors of HCV co-infection. A P < 0.05 was considered significant.
The study was approved by the ethical committee of Office of Vice-Chancellor for Research Affairs, Shiraz University of Medical Sciences, Shiraz, Iran.
Results
The study population consisted of 226 HIV-infected patients with the age range 10-57 years and a mean age of 35.6 ± 7.9 years. The majority of the subjects (214) were male (94.7%) while there were only 12 females (5.3%). Distribution of demographic factors, risk factors and HCV positivity among the study population is demonstrated in Table 1. The most prevalent risk factor was a history of imprisonment (201, 88.9%) followed by injecting drug use (179, 79.2%), while none of the patients were “child of an infected mother”.
ELISA analysis for HCV antibodies was reactive in 200 subjects (88.5%) and negative in 26 cases (11.5%). Similarly, 196 patients (86.7%) were anti-HCV positive by RIBA analysis, while 15 patients (6.6%) had negative results. In the remaining 15 cases (6.6%), RIBA was indeterminate. RT-PCR analysis for detection of HCV-RNA showed that 59 subjects (26.1%) had HCV viremia whereas 167 patients (73.9%) were clear [Table 1].
Table 2 demonstrates the relationship of HCV seropositivity determined by RIBA in HIV-infected patients with demographic factors as well as with risk factors. HCV infection had a significant association with gender (P < 0.001), age (P = 0.010), marital status (P = 0.014), occupation (P = 0.003), IDU (P < 0.001), history of imprisonment (P < 0.001), and having an infected or high risk sexual partner (P < 0.001). The prevalence of HCV infection was higher in men (96.5%), 40-49 age group (97.7%), injecting drug users (99.4%), and in those with manual occupations (96.7%) and a history of imprisonment (98.4%). It was lower in married subjects (85.1%) and in those with an infected or high risk sexual partner (22.2%).
Table 2.
Prevalence of HCV co-infection in HIV-positive patients according to demographic and risk factors
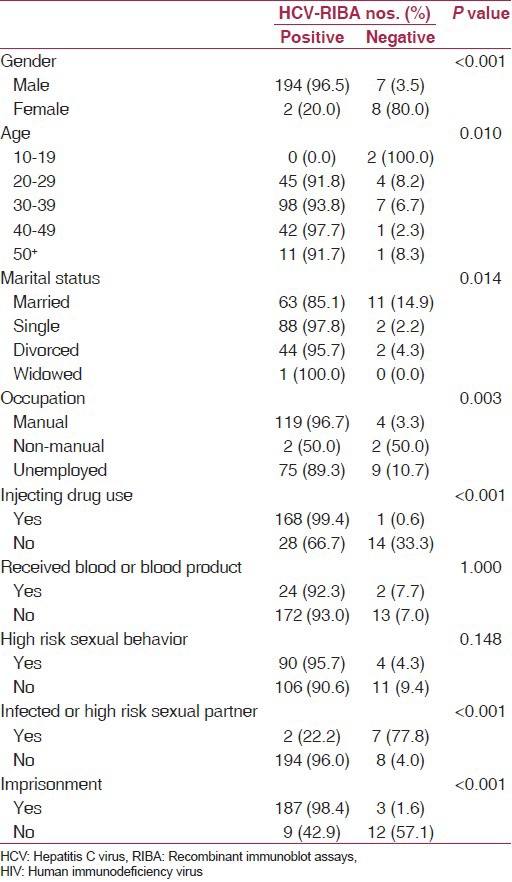
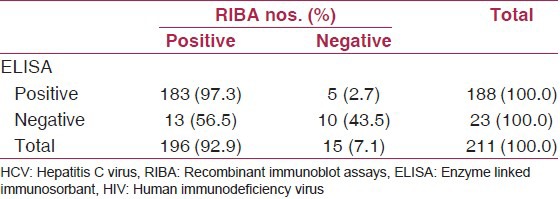
Table 3 gives a comparison of ELISA and RIBA tests for HCV-antibody detection in HIV-infected patients. A total of 15 patients who had undetermined (IND) result in RIBA test were excluded from this analysis. Assuming RIBA to be the gold standard test for detection of HCV-antibody, ELISA test gave a false positive result in 2.7% of the cases and a false negative result in 56.5% of the cases. Moreover, sensitivity of ELISA was 93.4% (95% CI = 88.9-96.4) and its specificity was 66.7% (95% CI = 38.4-88.2).
Table 3.
Comparison of ELISA and RIBA tests for HCV-antibody detection in HIV-infected patients
Logistic regression analysis was performed with HCV-positivity based on RIBA, as the dependent variable, and nine demographic and risk factors, as independent variables. It showed that HCV infection was significantly associated with two factors, injecting drug use (OR = 24.9, P = 0.004) and imprisonment (OR = 21.4, P < 0.001). In other words, in the logistic regression model, injecting drug use and a history of imprisonment were the only predictors of HCV co-infection among HIV-positive patients [Table 4].
Table 4.
Logistic regression model for HCV co-infection based on RIBA test in HIV-positive patients

Discussion
The prevalence of HCV co-infection among HIV-positive patients under study, as determined by ELISA method, was 88.5%. This is consistent with the findings of Rahimi-Movaghar and colleagues in Tehran who reported an HCV prevalence of 80.6% in HIV-infected patients.(9) As in our study, they used ELISA for HCV diagnosis, but all their subjects were injecting drug users.
When sera of our HIV-infected patients were tested for HCV-antibodies by RIBA analysis, the prevalence of HCV infection was 86.7%. This is higher than the rates found by other studies from different parts of the world which reported anti-HCV positivity of 8 to 50% based on RIBA.(10,11,12,13) Since percutaneous exposure is a very efficient route for HCV transmission,(4) the high positivity rate for RIBA in our study could be accounted for by the large number of injecting drug users (80%) among our subjects.
Using RT-PCR to detect HCV-RNA, we found an HCV viremia of 26.1% among our subjects. This is similar to the findings of Kim and colleagues in the USA who reported an HCV prevalence of 25% by PCR test. In their study of HIV-positive subjects in New York, they first used ELISA to determine HCV serology and then confirmed the positive results by PCR analysis.(14) The observed difference between positivity rates for RIBA and PCR in our study could be due to permanent clearance of hepatitis C virus in some patients and fluctuation of viremia in those with chronic HCV infection.
We observed that there was a highly significant association between male gender and HCV co-infection among HIV-positive subjects. This is in keeping with other studies in USA,(14) Brazil(15) and Gambia.(16) Men are often more likely to engage in high risk behavior in comparison to women and are therefore more prone to common routes of HCV transmission; this is probably a contributing factor to why they have a higher rate of HCV co-infection. However, the low rate of HCV positivity among female participants in our study may not be very reliable, because there were very few HIV positive females in the study.
We found that the rate of HCV positivity increased with increasing age and was highest in the 40-49 age group. This is similar to Tedaldi et al., study in the United States.(17) Likewise in Tanzania, the prevalence of HCV infection among HIV-positive patients increased with age with the oldest age group (50+ years) having the highest proportion.(18)
Prevalence of HCV co-infection was highest in single and widowed groups and lowest in married patients. This is as we expected since married people are less likely to practice high risk sexual behavior and are therefore less prone to transmission of HCV via the sexual route. In their study of an HIV-positive population, Nagu et al., demonstrated that divorced and widowed subjects had the highest prevalence of HCV co-infection whereas currently married patients had the lowest prevalence.(18)
Similarly, prevalence of HCV co-infection was significantly higher in patients with manual occupations compared to those with non-manual jobs. This is parallel to the findings of previous studies in populations other than HIV-positive subjects. In their community-based study of over 6000 subjects in Taiwan, Wang and colleagues demonstrated that manual occupation was a risk factor for HCV seropositivity.(19) Moreover, occupation as a laborer or agriculture worker was correlated with HCV infection in a group of blood donors in Thailand.(20)
We observed a significant association between IDU and prevalence of HCV co-infection. Many other studies in HIV-positive populations from different regions of the world have consistently declared that the risk of HCV co-infection is higher in injecting drug users. Intravenous drug use was the most frequent risk factor for HCV acquisition in patients with HIV infection in Brazil.(21) Badridze et al., also reported that risk of HCV co-infection among injecting drug users was more than three-folds the risk in non-IDUs.(22) The reason for the strong association between IDU and HCV co-infection in HIV-positive patients is that percutaneous exposure is a very efficient route for HCV transmission.
Surprisingly, HCV-antibody positivity was inversely related to “having an infected or high risk sexual partner” which is in disagreement with a number of other studies. Heterosexual contact with a high-risk partner had a significant correlation with HCV infection in a group of HIV patients in the USA.(23) Furthermore, Mendes-Correa et al., found that being a sexual partner of an HIV seropositive person was the second largest risk factor for acquisition of HCV.(21) The difference between our finding and that of other authors probably lies within the Iranian culture where AIDS is a social taboo. Besides, high risk practices such as IDU and prostitution are illegal in Iran. It is possible that our subjects were not totally honest in reporting their own risk factors and those of their spouses.
The present study revealed that prevalence of HCV co-infection was significantly higher in patients who had a history of imprisonment. Other studies have also shown that imprisonment is a risk factor for HCV infection in HIV-positive patients as well as in other populations.(24,25,26) Imprisonment is a constellation of other risk factors for transmission of HCV because of the very large rate of high risk behaviors practiced by inmates such as IV drug abuse, unsafe sex, sharing of needles and blades, tattooing, etc., In addition, most of these high risk behaviors contribute to percutaneous transmission of HCV which is a very effective route of transmission.
Comparing ELISA test results in detection of HCV-antibody to those of RIBA showed that ELISA had a false positive result in 2.7% of cases and a false negative result in 56.5% of the case. The very low rate of false positive result is because the subjects in our study were high risk for acquiring hepatitis C. In fact, the predictive value of ELISA depends on the prevalence of HCV infection in the population under study.(27) Furthermore, we found that sensitivity of ELISA was 93.4% while its specificity was 66.7%. Similarly, other authors stated that the sensitivity of third generation ELISA for detection of HCV-antibody in a high-prevalence population was as high as 97%.(28)
According to logistic regression model, IDU and imprisonment appeared to be predictors of HCV co-infection in HIV-positive subjects. The risk of HCV infection in injecting drug users was 24.9 fold the risk in those with no history of IDU. Likewise, the patients with a history of imprisonment were 21.4 times more likely to have HCV infection. These findings are supported by other authors.(14,24) In Mohsen et al., study, a history of IDU was identified as an independent risk factor for HCV infection in multivariate logistic regression analysis (OR = 107.2, 95% CI = 38.5-298.4).(7) Furthermore, a study of a group of injecting drug users in Iran showed that in a multiple regression model, history of prior imprisonment was an independent predictor of HCV infection (adjusted OR = 4.35, 95% CI = 1.88-10.08).(25)
In summary, HCV co-infection is highly prevalent among HIV-positive individuals in our region, and its two predictors are injecting drug use and imprisonment. Therefore, there is a need for stricter preventive actions against transmission of HCV among patients with HIV, such as provision of free disposable syringes to IV drug abusers, designing programs for cessation of IV drug addiction, and establishment of educational centers in prisons.
Acknowledgments
The authors would like to thank the Office of Vice Chancellor for Research Affairs of Shiraz University of Medical Sciences for financial support, and Dr. Ebrahim Ghaderi at the Department of Epidemiology for data analysis. Thanks also to Mr. Saeed Amirizadehfard and Ms. Maryam Nejabat for performing the laboratory tests.
Footnotes
Source of Support: The funding for this project was provided by the office of Vice Chancellor for Research Affairs, Shiraz University of Medical Sciences, Shiraz, Iran
Conflict of Interest: None declared.
References
- 1.Czepiel J, Biesiada G, Mach T. Viral hepatitis C. Pol Arch Med Wewn. 2008;118:734–40. [PubMed] [Google Scholar]
- 2.World Health Organization. Media Center. HIV/AIDS. Fact sheet N°360. [Last accessed on 2011 Nov]. Available from: http://www.who.int/mediacentre/factsheets/fs360/en/index.html .
- 3.Fauci AS. Twenty-five years of HIV/AIDS. Science. 2006;313:409. doi: 10.1126/science.1131993. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Robinson M, Zhang FJ. Human immunodeficiency virus and hepatitis C virus co-infection: Epidemiology, natural history and the situation in China. Chin Med J (Engl) 2009;122:93–7. [PubMed] [Google Scholar]
- 5.Soriano V, Garcia-Samaniego J, Rodriguez-Rosado R, Gonzalez J, Pedreira J. Hepatitis C and HIV infection: Biological, clinical, and therapeutic implications. J Hepatol. 1999;31(Suppl 1):119–23. doi: 10.1016/s0168-8278(99)80387-0. [DOI] [PubMed] [Google Scholar]
- 6.Reiche EM, Bonametti AM, Morimoto HK, Morimoto AA, Wiechemann SL, Matsuo T, et al. Epidemiological, immunological and virological characteristics, and disease progression of HIV-1/HCV-co-infected patients from a southern Brazilian population. Int J Mol Med. 2008;21:387–95. [PubMed] [Google Scholar]
- 7.Mohsen AH, Murad S, Easterbrook PJ. Prevalence of hepatitis C in an ethnically diverse HIV-1-infected cohort in south London. HIV Med. 2005;6:206–15. doi: 10.1111/j.1468-1293.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 8.Verucchi G, Calza L, Manfredi R, Chiodo F. Human immunodeficiency virus and hepatitis C virus coinfection: Epidemiology, natural history, therapeutic options and clinical management. Infection. 2004;32:33–46. doi: 10.1007/s15010-004-3063-7. [DOI] [PubMed] [Google Scholar]
- 9.Rahimi-Movaghar A, Razaghi EM, Sahimi-Izadian E, Amin-Esmaeili M. HIV, hepatitis C virus, and hepatitis B virus co-infections among injecting drug users in Tehran, Iran. Int J Infect Dis. 2010;14:e28–33. doi: 10.1016/j.ijid.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Quan CM, Krajden M, Grigoriew GA, Salit IE. Hepatitis C virus infection in patients infected with the human immunodeficiency virus. Clin Infect Dis. 1993;17:117–9. doi: 10.1093/clinids/17.1.117. [DOI] [PubMed] [Google Scholar]
- 11.Bryan JP, Sjogren MH, Malone JL, MacArthy P, Kao TC, Wagner K, et al. Recombinant immunoblot assays for hepatitis C in human immunodeficiency virus type 1-infected US Navy personnel. J Infect Dis. 1993;167:715–9. doi: 10.1093/infdis/167.3.715. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi PH, Flynn N, McCurdy SA, Kuramoto IK, Holland PV, Zeldis JB. Prevalence of hepatitis C virus antibodies among patients infected with human immunodeficiency virus. J Med Virol. 1991;33:177–80. doi: 10.1002/jmv.1890330307. [DOI] [PubMed] [Google Scholar]
- 13.van der Poel CL, Reesink HW, Mauser-Bunschoten EP, Kaufmann RH, Leentvaar-Kuypers A, Chamuleau RA, et al. Prevalence of anti-HCV antibodies confirmed by recombinant immunoblot in different population subsets in The Netherlands. Vox Sang. 1991;61:30–6. doi: 10.1111/j.1423-0410.1991.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Psevdos G, Suh J, Sharp VL. Co-infection of hepatitis B and hepatitis C virus in human immunodeficiency virus-infected patients in New York City, United States. World J Gastroenterol. 2008;14:6689–93. doi: 10.3748/wjg.14.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho FH, Coelho MR, Vilella Tde A, Silva JL, Melo HR. HIV/HCV coinfection at an university hospital in Recife, Brazil. Rev Saude Publica. 2009;43:133, 9. doi: 10.1590/s0034-89102009000100017. [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 16.Mboto CI, Fielder M, Davies-Russell A, Jewell AP. Prevalence of HIV-1, HIV-2, hepatitis C and co-infection in The Gambia. West Afr J Med. 2009;28:16–9. doi: 10.4314/wajm.v28i1.48418. [DOI] [PubMed] [Google Scholar]
- 17.Tedaldi EM, Hullsiek KH, Malvestutto CD, Arduino RC, Fisher EJ, Gaglio PJ, et al. Prevalence and characteristics of hepatitis C virus coinfection in a human immunodeficiency virus clinical trials group: The Terry Beirn Community Programs for Clinical Research on AIDS. Clin Infect Dis. 2003;36:1313–7. doi: 10.1086/374841. [DOI] [PubMed] [Google Scholar]
- 18.Nagu TJ, Bakari M, Matee M. Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dares Salaam, Tanzania. BMC Public Health. 2008;8:416. doi: 10.1186/1471-2458-8-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CS, Chang TT, Yao WJ, Chou P. Comparison of hepatitis B virus and hepatitis C virus prevalence and risk factors in a community-based study. Am J Trop Med Hyg. 2002;66:389–93. doi: 10.4269/ajtmh.2002.66.389. [DOI] [PubMed] [Google Scholar]
- 20.Luksamijarulkul P, Thammata N, Sujirarat D, Tiloklurs M. Hepatitis C virus infection among Thai blood donors: Antibody prevalence, risk factors and development of risk screening form. Southeast Asian J Trop Med Public Health. 2004;35:147–54. [PubMed] [Google Scholar]
- 21.Mendes-Correa MC, Barone AA, Guastini C. Hepatitis C virus seroprevalence and risk factors among patients with HIV infection. Rev Inst Med Trop Sao Paulo. 2001;43:15–9. doi: 10.1590/s0036-46652001000100003. [DOI] [PubMed] [Google Scholar]
- 22.Badridze N, Chkhartishvili N, Abutidze A, Gatserelia L, Sharvadze L. Prevalence of hepatitis B and C among HIV positive patients in Georgia and its associated risk factors. Georgian Med News. 2008:54–60. [PubMed] [Google Scholar]
- 23.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 24.Bollepalli S, Mathieson K, Bay C, Hillier A, Post J, Van Thiel DH, et al. Prevalence of risk factors for hepatitis C virus in HIV-infected and HIV/hepatitis C virus-coinfected patients. Sex Transm Dis. 2007;34:367–70. doi: 10.1097/01.olq.0000240295.35457.b1. [DOI] [PubMed] [Google Scholar]
- 25.Kheirandish P, Seyed Alinaghi S, Jahani M, Shirzad H, Seyed Ahmadian M, Majidi A, et al. Prevalence and correlates of hepatitis C infection among male injection drug users in detention, Tehran, Iran. J Urban Health. 2009;86:902–8. doi: 10.1007/s11524-009-9393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez CM, Suarez E, Torres EA, Roman K, Colon V. Seroprevalence of hepatitis C virus and associated risk behaviours: A population-based study in San Juan, Puerto Rico. Int J Epidemiol. 2005;34:593–9. doi: 10.1093/ije/dyi059. [DOI] [PubMed] [Google Scholar]
- 27.Mandell GL, Bennett JE, Dolin R. Philadelphia: Elsevier Churchill Livingstone; 2005. Mandell, Douglas, and Bennett's principle and practice of infectious diseases. [Google Scholar]
- 28.Richter SS. Laboratory assays for diagnosis and management of hepatitis C virus infection. J Clin Microbiol. 2002;40:4407–12. doi: 10.1128/JCM.40.12.4407-4412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


