Abstract
Literature review for indocyanine green angiography and evaluate the role of indocyanine green angiogram (ICGA) in patients with posterior uveitis seen at a tertiary referral eye care centre. Detailed review of the literature on ICGA was performed. Retrospective review of medical records of patients with posterior uveitis and dual fundus and ICGA was done after institutional board approval. Eighteen patients (26 eyes) had serpiginous choroiditis out of which 12 patients had active choroiditis and six patients had healed choroiditis, six patients (12 eyes) had ampiginous choroiditis, six patients (12 eyes) had acute multifocal posterior placoid pigment epitheliopathy, eight patients (10 eyes) had multifocal choroiditis, four patients (eight eyes) had presumed ocular histoplasmosis syndrome, four patients (eight eyes) had presumed tuberculous choroiditis, two patients (four eyes) had multiple evanescent white dot syndrome and two patients (four eyes) had Vogt Koyanagi Harada (VKH) syndrome. The most characteristic feature noted on ICGA was the presence of different patterns of hypofluorescent dark spots, which were present at different stages of the angiogram. ICGA provides the clinician with a powerful adjunctive tool in choroidal inflammatory disorders. It is not meant to replace already proven modalities such as the fluorescein angiography, but it can provide additional information that is useful in establishing a more definitive diagnosis in inflammatory chorioretinal diseases associated with multiple spots. It still needs to be determined if ICGA can prove to be a follow up parameter to evaluate disease progression.
Keywords: Ampiginous choroiditis, fluorescein angiography/fluorescein angiogram, indocyanine green angiogram, multifocal choroiditis, posterior uveitis, serpiginous choroiditis
The choroid is composed of vascular elements and is involved in a majority of chorioretinal inflammatory disorders. Intraocular inflammation is associated with vascular alterations and very often include disturbances of the retinal and/or choroidal circulations.[1,2]
The fluorescein angiography (FA) technique is an important diagnostic tool in diseases of the posterior segment of the eye. Fluorescein angiograms provide information regarding the localization and extent of posterior segment inflammatory diseases.[3,4]
FA findings are helpful in illustrating the inflammatory process and anatomic changes within the retina and its vessels, generally FA patterns are not diagnostic or pathognomonic for any particular intraocular inflammatory disease.[5,6,7] However, the major drawback of FA is the inability to image the choroidal pathology.[5,6,7] This is especially difficult during the stages where the lesions involve the deeper choroidal layers because absorption and emission of photonic fluorescin energy in the blue green wavelength is impaired by the pigmented layers of the fundus.[5,6,7] This problem can be overcome by the use of indocyanine green angiography (ICGA).[5,6,7]
Systematic analysis to reproduce the quality of images is possible with the digitalization of images and the infrared cameras. The major application for which ICGA is useful is the disorders involving, choriocapillaris and the choroid. The combination of FA with ICGA could be of help in reaching a decision regarding the most accurate differential diagnosis in cases of inflammatory process within the fundus.[8,9,10,11,12,13,14,15,16,17]
Objective
The main objective of the present study was to evaluate the role of ICGA in posterior uveitis and its correlation with FFA and also to analyze and speculate the pathophysiologic mechanisms in chorioretinal inflammatory disorders based on literature review.
Review of Literature
History of indocyanine green in ophthalmology
Indocyanine green (ICG) dye was developed in the Kodak Research Laboratories in 1955 and has been used diagnostically in humans since 1956.[10] ICG was used in other areas of medicine long before it was introduced in ophthalmology.[10] Clinically ICG has been used to measure hepatic blood flow, function and cardiac output.[10] It was first used as a fluorescing agent for imaging the ocular circulation in 1969 by Kogure,[18] but problems with the dye and imaging technology available at the time limited its use. ICG was first studied in depth by Flower, beginning in 1970.[19] The success of these early experiments was limited by the photographic media used to record the angiographic images. Improvement in technology related to imaging and ever increasing clinical experience has made ICG an important diagnostic tool in the evaluation of choroidal and chorioretinal inflammatory disorders.[18,19,20,21,22,23,24,25]
Physical properties of indocyanine green dye
ICG has a fluorescent efficiency which is twenty five times less than that of sodium fluorescein.[24] The infrared film used at the time for ICG angiography was insufficient to yield acceptable quality angiograms.[24] In 1980s the major technologic advancement related to excitatory and emission barriers greatly improved their effective utilisation. These filters, which selectively limit the passage of light to the appropriate infrared wavelengths, decreased the overlap transmission to 0.5%. Introduction of videoangiography (ICG-V system) by Hayashi et al. and its application by Destro et al.[26,27,28,29] utilized the ICG molecule which absorbs and emits photonic fluorescing energy in the near-infrared wavelength range. Which penetrates, to some extent, melanin pigments, hemorrhage, macular xanthophyll pigment and other obstacles such as turbid fluids. These characteristics allow imaging of the normal and disturbed choroidal and retinal circulations.[30,31]
ICG dye is selectively taken up by hepatocytes and is then excreted via the hepatobiliary system. Its excretion into bile is mediated by adenosine tri-phosphate dependent transport process. The compound is not metabolized and does not undergo enterohepatic recirculation.[32,33,34,35]
Advantages of indocyanine green dye for chorioretinal inflammatory diseases
Being more protein bound the is reduces amount of leakage through the fenestrations of the choriocapilllaries. This enables a clear view of the choroidal circulation. FA does not normally demonstrate the choroidal circulation because the unbound fluorescein molecule is very small and rapidly leaks from the choriocapillaris and obscures the underlying choroidal vessels. With ICGA, the dye leaks unimpaired in the choroid but slowly from the fenestrated choriocapillaris. During recirculation, ICG remains in the choroidal tissue as the ICG – protein complex is only slowly reabsorbed into the circulation. Gradual impregnation of the choroid with time causes intermediate and late choroidal background fluorescence. This choroidal impregnation by ICG fluorescence is disturbed in chorioretinal inflammatory disorders causing areas of altered fluorescence, hence making it a very useful adjunctive tool in chorioretinal inflammatory diseases.[25,30,32]
The early ICGA phase is useful in determining the choroidal filling pattern of the posterior pole through posterior ciliary arteries, the choroidal arteriovenous and the retinal arteriovenous circulation.[5,6] Second advantage of ICGA is its fluorescence in the near infrared spectrum. The retinal pigment epithelium and choroids absorb approximately 59-75% of light at 500 nm but only 21-38% of light at 800 nm. Therefore, light at wavelengths in the near infrared are able to penetrate the pigmented layers of the fundus much better than in the visible spectrum that is utilized by fluorescein angiography. Similarly, near infrared light is able to penetrate lipid deposits, serous exudation, and hemorrhage better than the fluorescein angiography.[30,32]
Normal digital videoangiography
The phases of the ICGA can be divided into early, intermediate and late phases although the utility of this is limited.[36,37,38,39,40] The early phase of the ICGA has been characterized by the first appearance of ICG dye in the choroidal circulation. Large choroidal arteries and veins can be observed as well as the retinal vasculature. This occurs 1 min after injection of ICG. Between 5 and 15 min after injection the middle phase is entered with the choroidal veins becoming less distinct and a diffuse homogenous choroidal fluorescence is observed. The hyperfluorescence of the retinal blood vessels is diminished. Hyperfluorescent lesions begin to be contrasted from the fading background fluorescence. In the late phase, which occurs approximately 15 min after injection, no retinal or choroidal vasculature detail is observed. The optic disc is dark and the large choroidal vessels become hyperfluorescent. Choroidal abnormalities stand out in contrast to the markedly diminished background fluorescence during this phase.[16,17,21,36,37,38,39,40,41,42]
Continuous videoangiography obtained from the scanning laser ophthalmoscopy yields information regarding the flow of ICG through the retinal and choroidal circulation.[42] These techniques allow for the observation of the early filling of the large choroidal vessels and choroidal new vessels. Late images obtained by continuous imaging (e.g., videoangiography) were less revealing of the blood flow through the choroidal circulation.[16,17,21,36,37,38,39,40,41,42]
Indocyanine green angiography findings in retinal diseases
The use of ICGA has been studied and reported in retinal disorders.[19] Certain clinical conditions where ICGA is of utmost importance diagnostically as well as therapeutically are in cases with choroidal neovascularization (CNV) associated with polypoidal choroidal vasculopathy and age related macular degeneration.[11,25,26,27,28,43,44,45,46,47,48,49] Destro and Puliafito reported that ICG videoangiography was particularly useful in studying occult CNV with overlying hemorrhage and recurrent CNV.[27] In an expanded series Yannuzi and associates reported the results of an ICGA study of 100 consecutive patients with occult CNV by fluorescein angiography.[50] Various non inflammatory choroidal disorder which can be assessed with accuracy on ICGA are idiopathic polypoidal choroidal vasculopathy,[51] choroidal rupture,[52] macroaneurysms,[53] angioid streaks,[54] lacquer cracks associated with high myopia,[54] drusen,[55,56] intraocular tumors arising from the choroid,[57] heredomacular degeneration,[58] choroidal hemangioma,[59] pigment epithelial detachments,[60] central serous chorioretinopathy,[61] and choroidal osteoma.[62]
Characteristic indocyanine green angiogram features of chorioretinal inflammatory disorders
Choroidal vasculitis of the large vessels may be observed during the hyper-acute stage of Vogt-Koyanagi–Harada disease.[63,64] The optimal time for analysis of background choroidal ICG fluorescence is around 5-15 min (intermediate phase) and beyond 18-22 min (late phase). The more heavily pigmented the fundus, the earlier the late frames have to be taken.[5,6,11,12,16]
ICG hyperfluorescence is caused by leakage and never by window defects, as the pigment epithelium does not practically interfere with ICG fluorescence. Slowly increasing hyperfluorescence is generally associated with choriocapillaris leakage. Extensive leakage originates from large choroidal vessels that are normally impermeable to the protein-ICG complex. It is seen in cases with birdshot chorioretinopathy,[65,66,67] VKH disease[63,64,68] Behcet's disease,[69,70,71] toxoplasmic retinochoroiditis,[72,73] tuberculous retinochoroiditis[74] and in sarcoid posterior uveitis.[75,76,77] Choroidal localized hyperfluorescence can also result from the formation of neovascular membranes in cases of chronic inflammation.[8,9,16] However, the most characteristic feature on ICGA in inflammation of the choroid is the formation of hypofluorescent spots appearing as dark dots.[3,4,8,9,16,20,22,37,78,79] Hypofluorescent areas have to be analysed during the different angiographic phases and on subsequent ICGAs, and have to be correlated with fundus findings, infrared photographs and fluorescein angiographic findings.[3,4,8,9,16,20,22,37,78,79]
Yang et al. also reported about the role of ICGA in various choroidal inflammatory disorders.[68] Howe et al. found that ICG angiography detects the birdshot lesions more readily than FA and may benefit in assessing disease activity. It reveals multiple hyperfluorescent lesions resembling holes in the fluorescence of the choriocapillaris. These lesions correspond to the clinically seen creamy lesions.[66]
The acute lesions of serpiginous choroiditis show blockage of fluorescence on ICGA. However, after resolution, the choroidal ICG fluorescence in that area reappears.[80,81] Giovannini et al. reported on the ICGA findings in 17 patients with serpiginous choroiditis.[80] They described the presence of occult satellite choroidal lesions without clinical or FA counterparts, possibly representing occult lesions.[80,81]
Slakter et al. reported about the ICGA in a series of 14 patients with multifocal choroiditis (MC).[82] Fourteen (50%) of the 28 eyes were found to have large hypofluorescent spots in the posterior pole that were otherwise not seen clinically or on FA. In seven eyes exhibiting enlarged blind spots on visual field testing, ICGA showed confluent hypofluorescence surrounding the optic nerve. ICGA was found useful in evaluating the natural course in two patients with MC, as well as response to oral steroids, the patients were noted to have decreased symptoms and less vitritis on examination.[82] ICGA showed reduction in the size and number of the hypofluorescent spots in three patients, with complete resolution of these angiographic lesions noted in the fourth patient.[82] Vadala et al. have also published about the similar ICGA findings in MC.[83]
Acute multifocal posterior placoid pigment epitheliopathy (APMPPE) lesions are hypofluorescent by ICGA in both the early and late phases of the study.[84,85,86,87] The ICG choroidal fluorescence in APMPPE may be due to a partial choroidal vascular occlusion secondary to occlusive vasculitis.[84,85]
Multiple evanescent white dot syndrome (MEWDS) has a characteristic appearance on ICGA in the acute phase of the disease.[88] Unlike the subtle lesions seen clinically or the indistinct punctate hyperfluorescence seen with the FA, a pattern of hypofluorescent spots throughout the posterior pole and peripheral retina is seen with the ICGA. These hypofluorescent spots appear approximately 10 min after the dye injection in the mid ICG phase and persist throughout the study. These spots appear larger than the white dots seen clinically, varying in diameter from less than 50 microns to 500 microns. Many more lesions can easily be identified on ICG angiography than on fundus examination or FA. During the convalescent phase, there is resolution of the hypofluorescent spots seen on ICGA with return of visual function and normalization of clinical examination.[88,89,90]
ICGA of POHS also showed characteristic blocked hypofluorescent lesions.[91] ICGA of Best's disease was described by Bischoff et al.[9] ICGA findings in posterior scleritis was described by Auer and Herbort.[92] Caccavale and Mignemi reported FA and ICGA in a case of post-streptococcal syndrome with erythema nodosum and posterior uveitis.[93] Arevalo et al. reported on ICGA features of multifocal Cryptococcus Neoformans choroiditis in a patient with acquired immunodeficiency syndrome.[94]
A relatively new variant of serpiginous choroiditis also known as ampiginous choroiditis has been described. It starts as APMPPE and later on develops into a clinical picture of serpiginous choroiditis which is more florid, commonly involving macula.[95,96,97,98] It was labelled as ‘relentless placoid Chorioretinitis’ by Jones et al. It was also described by Blumenkranz et al. in the year 1982[98] but no further qualifications had come for that disease entity till Gupta et al.[97] and Jones et al.[95] described it.
Digital video angiography as reported in literature is thus valuable in the diagnosis and monitoring of patients with choroiditis. ICGA is a useful adjunctive diagnostic technique to FA for studying chorioretinal inflammatory disorders.
Materials and Methods
Patients seen at the uveitis clinic with posterior uveitis and choroiditis diagnosed clinically, were subjected to both FA and ICGA. Patients were explained regarding the procedure and its relevance to the disease process. Patients’ profile, clinical findings, color fundus photograph, FA and ICGA were recorded in a precoded proforma [Annexure 1].
Annexure 1.

Proforma for indocyanine green angiography in posterior uveitis
Indocyanine green digital angiographic system
A Topcon fundus camera with a continuous 300-watt halogen bulb was used. The barrier and excitation filters were modified for ICG peak absorption (805 nm) and fluorescence (835 nm). Near infrared reflective coating was applied to the lenses. An infrared video camera system designed by ophthalmic imaging systems was adapted to the fundus with an interfacing adapter. The images were then displayed on a high resolution screen and stored on the hard disc in a computer.
Indocyanine green
ICG (Cardiogreen, Hynson, Wescott and Dunning, Inc, Baltimore, MD) was prepared in solution. One to two milligrams of ICG per kilogram of weight was injected into a peripheral vein. This infusion was immediately followed by a flush of sterile saline.
Fluorescein angiography
Digital FA was also performed by injecting sodium fluorescein 10% (3-5 ml) into a peripheral vein.
Detailed analysis of all these patients was done comparing the fundus photographs, FA and ICGA. The standard ICGA protocol was followed [Annexure 2].
Annexure 2.

Indocyanine green angiography Protocol
Results
The particulars of the patient including diagnosis and ICGA features are listed in Table 1.
Table 1.
Diagnosis of Patients who underwent FFA+ indocyanine green angiogram and characteristic indocyanine green angiogram findings
The most characteristic feature noted in ICGA of chorioretinal inflammatory disorders was the presence of hypofluorescent dark spots, which were present at different stages of angiogram. Different patterns were noted based on the fluorescence of hypoflourescence dots at different stages of the angiogram. Patterns of FA and ICGA fluorescence observed in different posterior uveitic entities:
Eyes with serpiginous choroidopathy (Case 1, 2, 4, 5, 7, 14, 15, 23, 24, 26, 27, 29, 30, 32, 39, 40, 48, 49 [18 cases, 26 eyes]) – Figs. 1 and 2: The area of choroiditis was mainly present around the posterior pole and in the mid periphery with multiple areas of white to yellowish geographic lesions radiating in a snail track manner towards the macula. It was clinically noted to be inactive in six out of the eighteen cases seen with serpiginous choroiditis. The lesions in the healed inactive stage demonstrate patterns compatible with pigmented scars and choriocapillaris atrophy. FA showed areas of staining around the edge of the lesion in late phases in inactive diseases but showed leakage in cases with active disease, the area of activity was noted in six out of nine cases. During the early phases of FA the large choroidal vessels was easily observed within the affected area. This is because of the absence of choriocapillaris and pigment epithelium following their atrophy as part of the disease process. During the late arteriovenous phase of FA, there was staining of the lesion borders, but no dye leakage in healed serpiginous choroidopathy. During the late phases of FA, pooling of the dye intensifies within the scarred areas. The hyperpigmented spots, however shows blocking of the fluorescence throughout the angiogram, with only mild staining at the edge of the lesion.
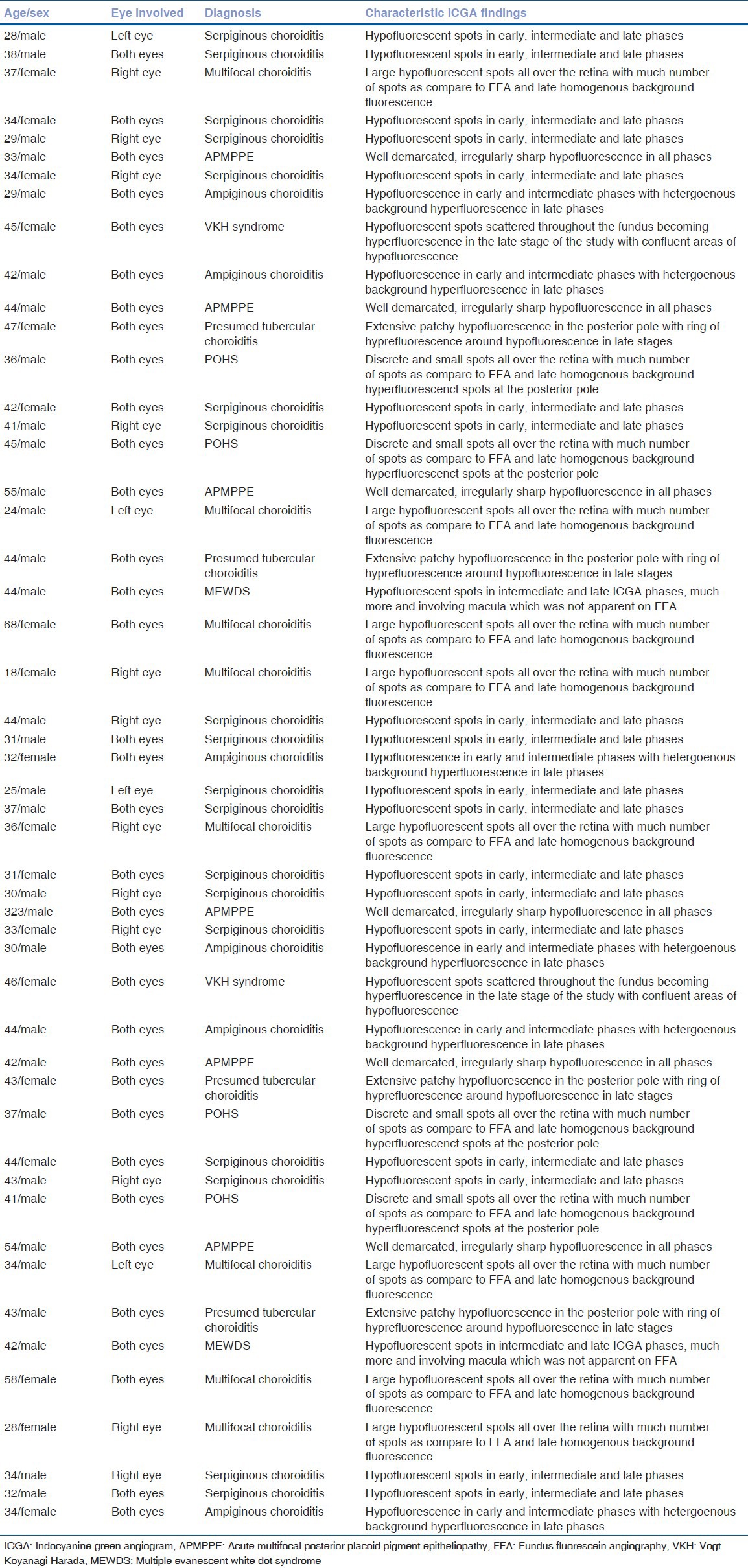
Figure 1.
Active Serpiginous choroiditis (a) Color fundus photograph of the right eye showing presence of yellowish white subretinal confluent lesions radiating away from the disc sparing the fovea, (b) Transit phase FFA showing presence of confluent hypofluorescent lesions radiating away from the disc not involving the fovea with staining around these lesions, (c) Early phase ICGA showing presence of numerous confluent hypofluorescent lesions around the disc and surrounding the fovea, (d) Persistence of the hypofluorescent lesions with involvement of fovea with areas of loss of choriocapillaris on ICGA
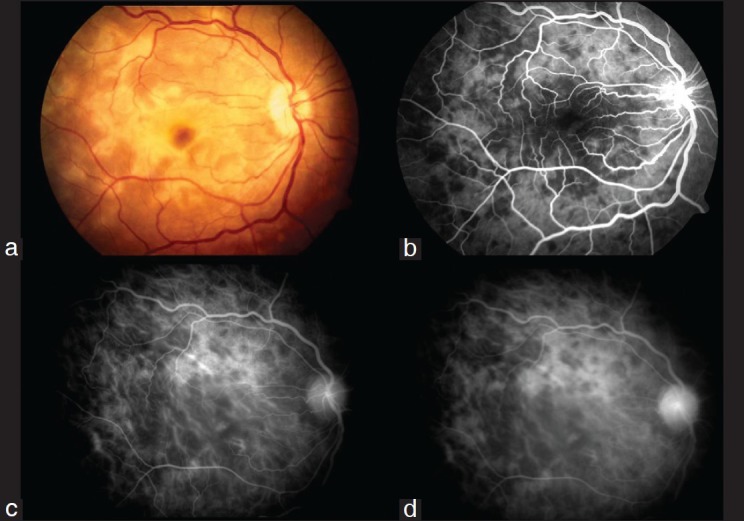
Figure 2.
Healed Serpiginous Choroiditis (a) Color fundus photograph of the right eye showing presence of peripapillary confluent pigmented chorioretinal atrophic lesions radiating away from the disc sparing the fovea, (b) Transit phase FFA showing presence of confluent hypofluorescent lesions radiating away from the disc not involving the fovea; there is presence of staining around these radiating lesions, (c) Early phase indocyanine green angiogram showing more number of hypofluorescent lesions as more as compared to clinical photograph and FFA, (d) Later phases of indocyanine green angiography demonstrates persistence of the hypofluorescent lesions with areas of loss of choriocapillaris
FA in patients with suspected active serpiginous choroiditis (six out of eighteen cases) showed hypofluorescent lesions in the early stages, with progressively increasing hyperflourescence along the borders of the lesions, which can be due to leakage of dye from the surrounding choriocapillaris. During the late phases of the angiogram there were fuzzy areas of hyperflourescence along the borders of the lesions which indicate areas of active inflammation. In cases with inactive or healed serpiginous choroiditis there was presence of mottled hyperflourescence with some staining of the lesion probably caused by pigmentary clumping. There was presence of late staining of the scar as well, which could be because of the fibrous scar tissue associated with the inflammatory process.
ICGA in these patients showed presence of persistent areas of hypofluorescence in all the phases of the angiogram which could be due to homogenous blockage of deeper choroidal fluorescence throughout this region. The active cases also showed presence of hyperfluorescent halos along the margin of the lesions in the late phase. In general, in the active stage of the disease ICGA demonstrates a larger area of involvement along with absence or presence of macular involvement than FA or on fundus evaluation. In the healed serpiginous choroiditis cases there was an heterogenous area of hypofluorescence in all the phases of the disease.
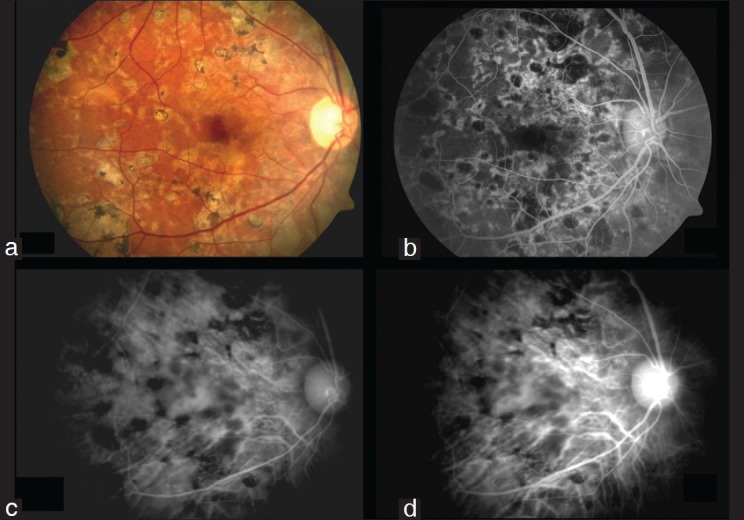
Eyes with ampiginous choroiditis (Case 8, 10, 25, 33, 35, 50 [six cases, twelve eyes]) – Fig. 3: This is a rare variant of serpiginous choroiditis. On fundus evaluation there were presence of multiple placoid lesions at the posterior pole (resembling APMPPE) along with coalescing placoid lesions forming a serpiginous pattern at the posterior pole involving the macula in four out of six cases. FA revealed early hypoflourescence followed by late hyperfluorescence of both placoid lesions and serpiginous lesions. On ICGA, the findings were similar to those seen in cases with active serpiginous choroiditis wherein there were hypofluorescent spots in the early and intermediate phase which became hyperflourescent in the late AV phases with a heterogenous background fluorescence.
Figure 3.
Ampiginous choroiditis
Eyes with APMPPE (Case 6, 11, 17, 31, 36, 42 [6 cases, 12 eyes]) – Fig. 4: Fluorescein angiogram of acute lesions shows early hypofluorescence followed in the later phases by staining of lesions. The early hypofluorescence could be interpreted as either blockage of fluorescence by inflammatory material or choriocapillaris non-perfusion. The ICGA shows marked choroidal hypofluorescence in all three phases of the angiogram. The early phases also show large choroidal vessels perfusing beneath the areas of hypofluorescence, which seem to be at the level of the choriocapillaris. The hypofluorescence persists in the late phases and appears as a well demarcated irregularly shaped area.
Figure 4.
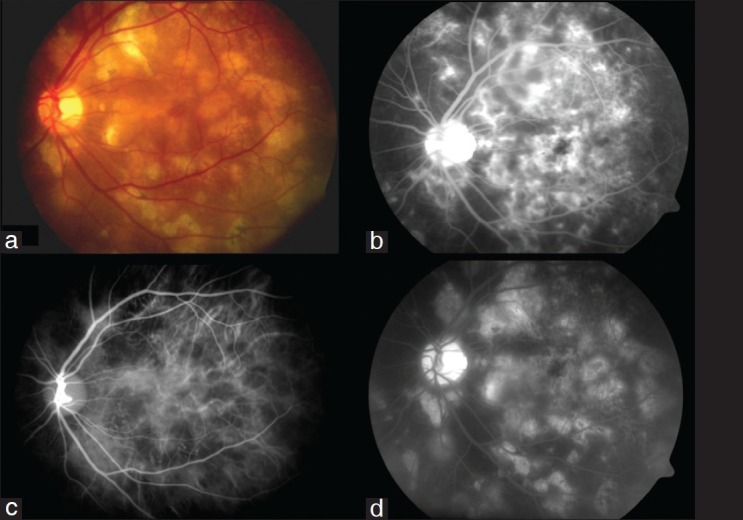
Acute multifocal posterior placoid pigment epitheliopathy
Eyes with MC (Case 3, 18, 21, 22, 28, 43, 46, 47 [8 cases, 10 eyes]): On FFA there were hypofluorescent lesions in the early stages, which in the later stages showed hyperflourescence. On ICGA there were corresponding larger hypofluorescent spots all over the posterior pole, which were much more in number than seen on fundus examination and FFA. These hypofluorescent spots persisted in all stages of the angiogram. There was no change in size of spots, but there was presence of homogenous background fluorescence in the later stages.
Eyes with POHS (Case 13, 16, 38, 41 [4 cases, 8 eyes]): FFA showed focal spots of hyperflourescent lesions in mid and late arteriovenous phases which were small and dispersed all over the posterior pole. On ICGA there were more discrete spots as compared to those seen on clinical examination and FFA. These spots were hypofluorescent in the intermediate and late phases of the angiogram but were very discrete and small as compared to the spots seen in cases with MC.
Eyes with MEWDS (Case 20, 40 [two cases with bilateral involvement]) – Fig. 5: FFA showed multiple hyperfluorescent spots in the mid and late arteriovenous phase which were not seen on fundus evaluation whereas ICGA showed multiple hypofluorescent spots in the intermediate and late phases which were well defined and much more in number as compared to those of fluorescein angiogram. It was found to be involving the macular area on ICGA which was not apparent clinically and on FFA indicating the need for urgent intervention.
Figure 5.
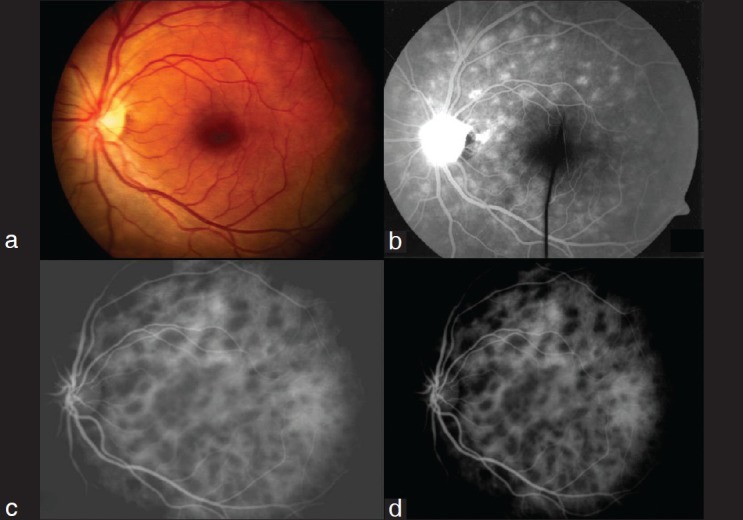
Multiple evanescent white dot syndrome (MEWDS)
Eyes with VKH (Case 9, 34 [two cases with bilateral involvement]): FFA showed multiple areas of hypofluorescence in the early stage of the angiogram which most likely represent blockage by foci of cellular infiltration in the superficial choroid. In mid phases there was presence of pin point areas of hyperflourescence which shows leakage in the late stages. On ICGA there was presence of hypofluorescent spots scattered throughout the fundus; which became faintly hyperfluorescent in the late stage of the study with confluent areas of hypofluorescence. The hypofluorescent spots correlate with the yellow white lesions seen clinically. Presumably they represent blockage of the underlying ICG fluorescence by inflammatory infiltration of the choroid and by overlying retinal pigment epithelium (RPE) change. In addition, inflammatory material may produce filling defects of inner choroidal circulation leading to hypofluorescence relative to the surrounding areas.
Eyes with presumed tubercular choroiditis (Case 12, 19, 37, 44 [four cases, eight eyes]) – Fig. 6: Fundus examination revealed areas of active chorioretinitis patches with vitritis. FA revealed area of progressively increasing hyperflourescence, indicating presence of active lesion, whereas ICGA showed extensive patchy hypofluorescene in the posterior pole along with the areas of hypoflourescence corresponding to the hyperfluorescent lesion of fluorescein angiogram. Areas of hypoflourescence remained hypoflourescence throughout with a ring of hyperflourescence around the hypofluorescent lesion in late ICGA films.
Figure 6.
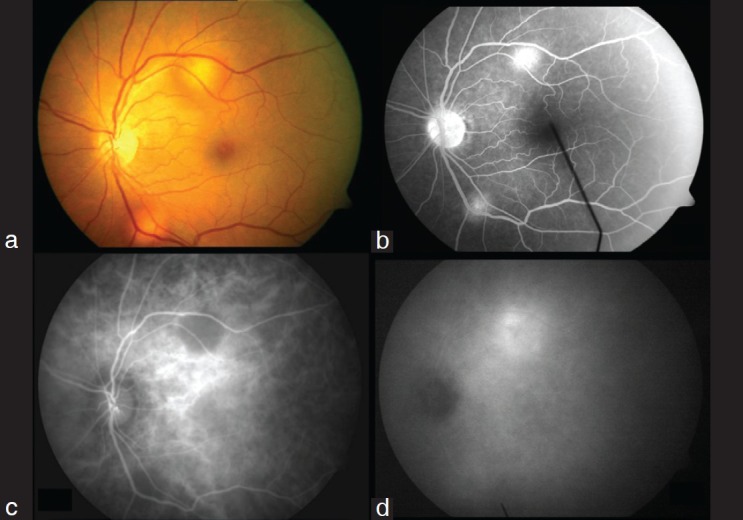
Presumed tubercular choroiditis
Discussion
Direct examination of the retina using slit-lamp biomicroscopy and indirect ophthalmoscopy remains an indispensable clinical technique for the diagnosis and management of posterior segment diseases. The simplest technique to evaluate the retina and other posterior pole structures is the use of the direct ophthalmoscope. The resolution and magnification are adequate, but the lack of stereopsis, narrow field of view, and image degradation with even mild media opacities limit the use of this technique. Binocular indirect ophthalmoscopy and slit lamp biomicroscopy of the retina requires considerable experience but it provides relatively wide field stereoscopic images with good depth of focus.[18]
FFA introduced in 1960s, has become one of the most widely used ancillary techniques to image the retina and has provided insight into the anatomy, physiology, and pathology of the retina. Used with direct observation many retinal diseases are now more effectively diagnosed and treated. However digital FA systems has several inherent disadvantages and limitations in not able to exactly delineate subretinal diseases and in cases with overlying hemorrhage and pigment proliferations not able to visualize the underlying retinal and choroidal structures precisely.[18] ICGA has hitherto provided the opportunity to examine the choroid all disorders.[18]
When analyzing ICGA in posterior segment inflammatory disorders, crucial differences with FA have to be kept in mind to correctly analyze images obtained. During the initial circulation, ICGA is comparable to fluorescein but the difference occurs during recirculation time, when ICGA is progressively leaking out from the fenestrated choroidal capillaries, gradually impregnating the whole choroidal thickness. The process can be affected if by a decrease in the physiologic extrusion of the ICG from the choriocapillaris or by the impairment of the filling of the choroidal tissue by the ICG molecule because of space occupying lesions. Secondly, impregnation of the choroidal space can be enhanced by increased leakage from the choriocapillaris or from the large choroidal vessels.[18]
ICGA demonstrates better definition of the changes within the choroidal circulation that occur in the active and healed stages of serpiginous choroidopathy. 63-101 in the active phase of the disease, ICGA is characterized by generalized hypofluorescence through all phases of the study. This may be caused by a combination of both choroidal perfusion abnormalities and blockage of fluorescence by inflammatory exudative material or edema of the RPE and outer retina. 63-101 its hypothesized based on the fact that mid sized choroidal arteries are not seen at the edge of active lesions which is possible because of edema or exudation. However the persistence of choroidal arterial hyperfluorescence into the venous phase of the study suggests a relative retention of the dye in these choroidal arteries as compared to the remaining choroidal arterial circulation. This may occur as a result of increased resistance to flow in the area of inflammation, resulting from partial closure of the vessels themselves or increased resistance from external compression and infiltration of the perivascular space with the inflammatory material. With ICG angiography delayed choroidal filling has been noted in areas that appear normal on clinical and fluorescein angiographic examination. The location of these areas of delayed filling was related to the location of the active lesion in specific individuals. These territories of delayed choroidal perfusion may represent either abnormalities in the choroidal arteries or increased intravascular resistance in the tributary areas at the level of choriocapillaris, which might be the result of vascular occlusion.[68,79,80,81]
ICGA however can be useful in staging the disease in coordination with FFA. Two different patterns of fluorescence was seen in our study. In the first there was more homogenous hypofluorescence in all stages of the angiogram with well delineation of the margins of the lesions with no visualization of the underlying choriocapillaris; this pattern was indicative of the active disease. Secondly, in healed choroiditis there was stippled heterogenous hyperfluorescence and small choroidal vessels seen as well. In contrast to this, FA demonstrates hyperfluorescence in both the active and healed stages of the disease and can't delineate whether any disease activity is present or not which is otherwise possible with the ICG angiogram. As a result ICG angiogram may help to distinguish between an actively inflamed lesion and whether involving the macula or not, which warrant urgent intervention from an otherwise healed lesion which requires only the observation and no therapeutic intervention.[68,79,80,81]
MC is typified by the presence of inflammatory lesions in the posterior pole and periphery. Clinically active gray or yellow lesions were characteristic of the acute phase of the disease, whereas atrophic spots developed in the chronic from of the disease. FA did demonstrate presence of early hypoflourescence followed by late hyperflourescence but did not reveal any further characteristic of the spots. Indocyanine green angiographic evaluation demonstrated presence of multiple large hypofluorescent spots, which were present in clusters around the posterior pole and were found to be deep to the retinal pigment epithelium, perhaps in the mid choroid. They have been shown to be more numerous and involve a much wider area than was appreciated on clinical or fluorescein angiographic evaluation. The hypofluorescent spots appear to be indicative of acute or subacute disease. Larger or smaller hypofluorescent spots or both were visualized in all patients with active disease, as exemplified by the presence of vitritis in these patients. These hypofluorescent spots noted in MC may be the result of either perfusion abnormalities or blocked fluorescence. It may represent focal collections of inflammatory cells or post inflammatory debris obscuring the underlying choroidal fluorescence patterns. This would place the inflammatory lesions at the level of the choriocapillaris. Alternatively, inflammatory cells or debris or both could accumulate in the middle layers of the choroid resulting in a space occupying defect, preventing extravasation of ICG dye and thus reducing concentration of ICG dye in the inflamed areas causing an area of hypoflourescence in the mid and late phases of the ICGA.[79,82,83]
As a diagnostic adjunct, ICGA hase proved to be useful for identifying features that would assist in differentiating MC from other inflammatory conditions with a similar clinical appearance. In particular, similarities between MC and POHS make the distinction between these entities difficult at times. This is especially true during periods of relative inactivity in patients with MC where vitreous cells are lacking. Patients with MC have hypofluorescent spots at the posterior pole during periods of relative activity, whereas patients with POHS do not show this spots, on the contrary it shows hyperfluorescent lesions at the posterior pole, which is never seen in patients with MC.[79,82,83,99]
No doubt, the principal means for the diagnosis of patients with MC will remain a clinical one. Indocyanine green angiography offers the opportunity to identify another areas of activity and help in understanding the pathology of this inflammatory condition.
ICG angiography can serve as a complimentary tool in the diagnosis of an inflammatory chorioretinopathy like MEWDS.[88,89,90] The appearance of the hypofluorescent spots on ICG angiography is unique and revealing. These spots have a random distribution at the posterior pole as against a more patterned vasotropic distribution of hypofluorescent spots along the course of choroidal vessels in bird shot chorioretinopathy[65,66,67] but the distribution was similar to the spots seen in MC on ICGA.[82,83]
The lesions of MEWDS appear in the intermediate phase of ICGA as against birdshot where the lesions appear in the early and late phases of ICGA.[65,66,67] There was a peripapillary ring of hypofluorescence which was also seen in patients with MC.[82,83] The peripapillary hypofluorescence seen in MEWDS had a punctate outer margin compared to a more discrete line of abnormal fluorescence seen in MC.[82,83] The wide spread involvement documented with ICGA explains the associated electroretinography findings and the peripapillary hypofluorescence accounts for the enlargement of the blind spot as seen in this patient. In the patient of MEWDS in our series there was no clinical or fluorescein angiographic explanation to account for the visual loss, but the changes seen on ICG angiography supported the hypothesis that this clinical disorder involves the deeper structures of the posterior segment, either the RPE or the underlying inner choroid.
The hypofluorescence seen in APMPPE can be because of cloudy cytoplasm of the RPE blocking choroidal fluorescence in the early phases of the FFA as proposed by Gass.[100] APMPPE also had many associations with both ocular and systemic inflammatory diseases and angiographic choroidal fluorescence may in fact be caused by blockage of choroidal fluorescence by inflamed tissue and inflammatory cells induced by vasculitis.[100] The facts supporting the hypothesis of blockage of choroidal fluorescence because of inflammatory tissue could be attributed to the variability in size and shape of the lesions and the failure of the acute lesion to stain with fluorescein from the periphery. Previous authors have published data stating early hypofluorescent lesions of APMPPE on FFA getting smaller before showing late hyperflourescence which was very much similar to what was observed in three patients of APMPPE seen in our series of 25 patients.[84,85,86,87] Choroidal vascular occlusion is another possible explanation for the choroidal fluorescence seen on angiography which can be due to vasculitis of the choroidal arterioles resulting in obstruction leading to choriocapillaris non perfusion as well as RPE edema.[84,85,86,87] There is also the possibility that in APMPPE both choroidal vascular occlusion and blockage of choroidal fluorescence by inflamed tissue and inflammatory cells may be present.[84,85,86,87]
Hypofluorescent areas were noted in both the FFA and ICGA outside the areas of clinically obvious placoid lesions, suggesting the areas of hypoflourescence are not due to masking of choriocapillaris fluorescence by the abnormal RPE. ICGA of APMPPE in this study were similar to the FFA with areas of hypoflourescence in the ICGA corresponding to those seen on an FFA except for the fact that in the ICGA of APMPPE fluorescence from the large choroidal vessels passing through the hypofluorescent area was seen regularly, in contrast to fluorescein angiograms where it is an infrequent occurrence. The fact that these large choroidal vessels were seen in high contrast in the hypofluorescent areas suggests overlying choriocapillaris non- perfusion.[84,85,86,87]
Vogt Koyanagi Harada disease is an immune mediated inflammatory condition against choroidal and other melanocytes that causes severe granulomatous uveitis in the eye. In the posterior segment, the primary target is in the choroid.[101] Since the availability of ICGA allowing detection of choroidal lesions, several reports have been published on the choroidal involvement in VKH disease.[63,64] One characteristic ICGA sign in all patients with active disease is a pronounced choroidal inflammatory vasculopathic feature involving both the small and larger choroidal vessels. The angiographic sign indicating small vessel involvement was the perfusion delay of the choriocapillaris seen in the very early angiographic phase.[63,64] The characteristic aspect of fuzzy and indistinct choroidal vessels seen in all patients was interpreted as leakage from diffusely inflamed vessels, resulting in the diffusely inflamed vessel walls, resulting in the diffuse late choroidal hyperflourescence also present in both the patients in our series.[63,64] The other characteristic sign seen in these two patients was the presence of hypofluorescent dark dots which can be classified as either partial thickness or full thickness granulomas. Regularly sized, evenly distributed hypofluorescent dark dots that had no significant corresponding signs on FA were interpreted as partial thickness granulomas when they remained isofluorescent in the late angiographic phase and as full thickness granulomas when they remain hypofluorescent.[63,64] Choriocapillaris non perfusion probably plays a minor role in the physio-pathologic process of these hypofluorescent lesions, although choriocapillaris compression by some of the thicker granulomas may occur.[63,64]
Conclusions
The most common finding on FA that corresponded to areas of active choroiditis is of early hypoflourescence with late hyperflourescence: whereas on ICGA it was hypofluorescent spots which was showing areas of involvement albeit in some cases it was revealing more extensive involvement as against FA. In inflammatory disorders less information is obtained from the analysis of early phases as against the altered pattern of filling in intermediate and late phases of ICGA and also very limited information about activity of the disease is revealed merely based on ICGA.
To interpret the ICGA images, clinical and FA correlation is essential. To make the most of this observational approach, a standardization of procedures is absolutely necessary. We have tried here to analyze the ICGA findings in variety of chorioretinal inflammatory disorders and to an extent tried to standardize the findings in different posterior uveitic entities.
Because indocyanine green dye has wavelength characteristics that can penetrate even dense hemorrhages, it is unlikely that a dysfunctional retinal pigment epithelium is the sole abnormality in this disorders. It is more likely that these hypofluorescent spots represent inflammatory lesions of the deeper choroid. These inflammatory choroidal foci could alter the choroidal circulation, diverting choroidal blood flow around these lesions and accounting for hypofluorescence seen on indocyanine green angiography.
As can be seen in the different white spot syndromes, ICGA provides the clinician with a powerful additional tool in choroidal inflammatory disorders. It can provide adjunctive information useful in establishing a more definitive diagnosis in intraocular inflammatory chorioretinal diseases associated with multiple spots.
With all non invasive tools like Optical coherence tomography, fundus autofluorescence, its quite intriguing if ICGA will become a follow-up parameter to evaluate disease progression and therapy effectiveness in posterior uveitis in which case its advantages over this non invasive tests have to be firmly established.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Streilein JW. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–89. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 2.Listhaus AD, Freeman WR. Fluorescein angiography in patients with posterior uveitis. Int Ophthalmol Clin. 1990;30:297–308. doi: 10.1097/00004397-199030040-00021. [DOI] [PubMed] [Google Scholar]
- 3.Herbort CP. Fluorescein and indocyanine green angiography for uveitis. Middle East Afr J Ophthalmol. 2009;16:168–87. doi: 10.4103/0974-9233.58419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbort CP, Bodaghi B, Lehoang P. Indocyanine green angiography in ocular inflammatory diseases: Principles, schematic interpretation, semiology and clinical value. J Fr Ophtalmol. 2001;24:423–47. [PubMed] [Google Scholar]
- 5.Flower RW, Hochheimer BF. A clinical technique and apparatus for simultaneous angiography of the separate retinal and choroidal circulations. Invest Ophthalmol. 1973;12:248–61. [PubMed] [Google Scholar]
- 6.Flower RW, Hochheimer BF. Clinical infrared absorption angiography of the choroid. Am J Ophthalmol. 1972;73:458–9. doi: 10.1016/0002-9394(72)90079-7. [DOI] [PubMed] [Google Scholar]
- 7.Benson RC, Kues HA. Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol. 1978;23:159–63. doi: 10.1088/0031-9155/23/1/017. [DOI] [PubMed] [Google Scholar]
- 8.Guyer DR, Puliafito CA, Monés JM, Friedman E, Chang W, Verdooner SR. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology. 1992;99:287–91. doi: 10.1016/s0161-6420(92)31981-5. [DOI] [PubMed] [Google Scholar]
- 9.Herbort CP, LeHoang P, Guex-Crosier Y. Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard angiographic protocol. Ophthalmology. 1998;105:432–40. doi: 10.1016/S0161-6420(98)93024-X. [DOI] [PubMed] [Google Scholar]
- 10.Lim JI, Flower RW. Indocyanine green angiography. Int Ophthalmol Clin. 1995;35:59–70. [PubMed] [Google Scholar]
- 11.Bischoff PM, Flower RW. Ten years experience with choroidal angiography using indocyanine green dye: A new routine examination or an epilogue? Doc Ophthalmol. 1985;60:235–91. doi: 10.1007/BF00157827. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff PM, Niederberger HJ, Török B, Speiser P. Simultaneous indocyanine green and fluorescein angiography. Retina. 1995;15:91–9. doi: 10.1097/00006982-199515020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Brown N, Strong R. Infrared fundus angiography. Br J Ophthalmol. 1973;57:797–802. doi: 10.1136/bjo.57.10.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kogure K, David NJ, Yamanouchi U, Choromokos E. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol. 1970;83:209–14. doi: 10.1001/archopht.1970.00990030211015. [DOI] [PubMed] [Google Scholar]
- 15.Hochheimer BF. Angiography of the retina with indocyanine green. Arch Ophthalmol. 1971;86:564–65. doi: 10.1001/archopht.1971.01000010566014. [DOI] [PubMed] [Google Scholar]
- 16.Craandijk A, Van Beek CA. Indocyanine green fluorescence angiography of the choroid. Br J Ophthalmol. 1976;60:377–85. doi: 10.1136/bjo.60.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flower RW. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Invest Ophthalmol. 1973;12:881–95. [PubMed] [Google Scholar]
- 18.Kogure K, David NJ, Yamanouchi U, Choromokos E. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol. 1970;83:209–14. doi: 10.1001/archopht.1970.00990030211015. [DOI] [PubMed] [Google Scholar]
- 19.Hochheimer BF. Angiography of the retina with indocyanine green. Arch Ophthalmol. 1971;86:564–65. doi: 10.1001/archopht.1971.01000010566014. [DOI] [PubMed] [Google Scholar]
- 20.Flower RW, Hochheimer BF. Clinical infrared absorption angiography of the choroid. Am J Ophthalmol. 1972;73:458–9. doi: 10.1016/0002-9394(72)90079-7. [DOI] [PubMed] [Google Scholar]
- 21.Miki T, Shiraki K, Kohno T, Moriwaki M, Obana A. Computer assisted image analysis using the subtraction method in indocyanine green angiography. Eur J Ophthalmol. 1996;6:30–8. doi: 10.1177/112067219600600108. [DOI] [PubMed] [Google Scholar]
- 22.Herbort CP. Posterior uveitis: New insights provided by indocyanine green angiography. Eye. 1998;12:757–9. doi: 10.1038/eye.1998.198. [DOI] [PubMed] [Google Scholar]
- 23.Hochheimer BF, D’Anna SA. Angiography with new dyes. Exp Eye Res. 1978;27:1–16. doi: 10.1016/0014-4835(78)90048-9. [DOI] [PubMed] [Google Scholar]
- 24.Freeman WR, Bartsch DU, Mueller AJ, Banker AS, Weinreb RN. Simultaneous indocyanine green and fluorescein angiography using a confocal scanning laser ophthalmoscope. Arch Ophthalmol. 1998;116:455–63. doi: 10.1001/archopht.116.4.455. [DOI] [PubMed] [Google Scholar]
- 25.Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: Indications and interpretation: An evidence-based update. Ophthalmology. 2003;110:15–21. doi: 10.1016/s0161-6420(02)01563-4. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi K, de Laey JJ. Indocyanine green angiography of choroidal neovascular membranes. Ophthalmologica. 1985;190:30–9. doi: 10.1159/000309489. [DOI] [PubMed] [Google Scholar]
- 27.Destro M, Puliafito CA. Indocyanine green videoangiography of choroidal neovascularization. Ophthalmology. 1989;96:846–53. doi: 10.1016/s0161-6420(89)32826-0. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K, Hasegawa Y, Tazawa Y, de Laey JJ. Clinical application of indocyanine green angiography to choroidal neovascularization. Jpn J Ophthalmol. 1989;33:57–65. [PubMed] [Google Scholar]
- 29.Hayashi K, de Laey JJ. Indocyanine green angiography of submacular choroidal vessels in the human eye. Ophthalmologica. 1985;190:20–9. doi: 10.1159/000309488. [DOI] [PubMed] [Google Scholar]
- 30.Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: Observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen SM, Shen JH, Smiddy WE. Laser energy and dye fluorescence transmission through blood in vitro. Am J Ophthalmol. 1995;119:452–7. doi: 10.1016/s0002-9394(14)71231-0. [DOI] [PubMed] [Google Scholar]
- 32.Baker KJ. Binding of sulfobromophthalein (BSP) sodium and indocyanine green (ICG) by plasma alpha-1 lipoproteins. Proc Soc Exp Biol Med. 1966;122:957–63. doi: 10.3181/00379727-122-31299. [DOI] [PubMed] [Google Scholar]
- 33.Paumgartner G. The handling of indocyanine green by the liver. Schweiz Med Wochenschr. 1975;105:1–30. [PubMed] [Google Scholar]
- 34.Wheeler HO, Cranston WI, Meltzer JI. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med. 1958;99:11–4. doi: 10.3181/00379727-99-24229. [DOI] [PubMed] [Google Scholar]
- 35.Rapaport E, Ketterer SG, Wiegand BD. Hepatic clearance of indocyanine green. Ophthalmology. 1994;101:529–33. [Google Scholar]
- 36.American Academy of Ophthalmology. Indocyanine choroidal green angiogram (Ophthalmic procedures assessment) Ophthalmology. 1998;105:1564–9. [Google Scholar]
- 37.Slakter JS, Yannuzzi LA, Guyer DR, Sorenson JA, Orlock DA. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995;6:25–32. doi: 10.1097/00055735-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Ito YN, Mori K, Young-Duvall J, Yoneya S. Aging changes of the choroidal dye filling pattern in indocyanine green angiography of normal subjects. Retina. 2001;21:237–42. doi: 10.1097/00006982-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Yoneya S, Komatsu Y, Mori K, Deguchi T, Saitoh T, Young-Duvall J. The improved image of indocyanine green angiography in young healthy volunteers. Retina. 1998;18:30–6. doi: 10.1097/00006982-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Flower RW. Extraction of choriocapillaris hemodynamic data from ICG fluorescence angiograms. Invest Ophthalmol Vis Sci. 1993;34:2720–9. [PubMed] [Google Scholar]
- 41.Flower RW. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Invest Ophthalmol. 1973;12:881–95. [PubMed] [Google Scholar]
- 42.Klein GJ, Baumgartner RH, Flower RW. An image processing approach to characterizing choroidal blood flow. Invest Ophthalmol Vis Sci. 1990;31:629–37. [PubMed] [Google Scholar]
- 43.Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. 1992. Retina. 2012;32:191. doi: 10.1097/iae.0b013e31823f98c7. [DOI] [PubMed] [Google Scholar]
- 44.Gharbiya M, Pantaleoni FB, Grandinetti F, Gabrieli CB. Indocyanine green angiographic findings in idiopathic choroidal neovascularisation. Eye. 1999;13:621–8. doi: 10.1038/eye.1999.170. [DOI] [PubMed] [Google Scholar]
- 45.Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Hope-Ross M, Orlock DR. Digital indocyanine-green videoangiography of occult choroidal neovascularization. Ophthalmology. 1994;101:1727–35. doi: 10.1016/s0161-6420(13)31433-x. [DOI] [PubMed] [Google Scholar]
- 46.Slakter JS, Yannuzzi LA, Sorenson JA, Guyer DR, Ho AC, Orlock DA. A pilot study of indocyanine green videoangiography-guided laser photocoagulation of occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1994;112:465–72. doi: 10.1001/archopht.1994.01090160041020. [DOI] [PubMed] [Google Scholar]
- 47.Ho AC, Fisher YL, Slakter JS, Guyer DR, Sorenson JA, Yannuzzi LA. Intraoperative indocyanine green videoangiography in subretinal surgery. Arch Ophthalmol. 1994;112:872–4. doi: 10.1001/archopht.1994.01090190014006. [DOI] [PubMed] [Google Scholar]
- 48.Sorenson JA, Yannuzzi LA, Slakter JS, Guyer DR, Ho AC, Orlock DA. A pilot study of digital indocyanine green videoangiography for recurrent occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1994;112:473–9. doi: 10.1001/archopht.1994.01090160049021. [DOI] [PubMed] [Google Scholar]
- 49.Yannuzzi LA, Sorenson JA, Guyer DR, Slakter JS, Chang B, Orlock D. Indocyanine green videoangiography: Current status. Eur J Ophthalmol. 1994;4:69–81. doi: 10.1177/112067219400400201. [DOI] [PubMed] [Google Scholar]
- 50.Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Hanutsaha P, Spaide RF, et al. Classification of choroidal neovascularization by digital indocyanine green videoangiography. Ophthalmology. 1996;103:2054–60. doi: 10.1016/s0161-6420(96)30388-6. [DOI] [PubMed] [Google Scholar]
- 51.Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–10. doi: 10.1097/00006982-199515020-00003. [DOI] [PubMed] [Google Scholar]
- 52.Akman A, Kadayifçilar S, Oto S, Aydin P. Indocyanine green angiographic features of traumatic choroidal ruptures. Eye. 1998;12:646–50. doi: 10.1038/eye.1998.162. [DOI] [PubMed] [Google Scholar]
- 53.Parodi MB, Ravalico G. Detection of retinal arterial macroaneurysms with indocyanine green videoangiography. Graefes Arch Clin Exp Ophthalmol. 1995;233:119–21. doi: 10.1007/BF00241482. [DOI] [PubMed] [Google Scholar]
- 54.Quaranta M, Cohen SY, Krott R, Sterkers M, Soubrane G, Coscas GJ. Indocyanine green videoangiography of angioid streaks. Am J Ophthalmol. 1995;119:136–42. doi: 10.1016/s0002-9394(14)73865-6. [DOI] [PubMed] [Google Scholar]
- 55.Scheider A, Neuhauser L. Fluorescence characteristics of drusen during indocyanine-green angiography and their possible correlation with choroidal perfusion. Ger J Ophthalmol. 1992;1:328–34. [PubMed] [Google Scholar]
- 56.Schwartz D. Fluorescence characteristics of drusen during indocyanine-green angiography and their possible correlation with choroidal perfusion. Surv Ophthalmol. 1995;4:67–74. doi: 10.1016/s0039-6257(05)80099-2. [DOI] [PubMed] [Google Scholar]
- 57.Shields CL, Shields JA, de Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79:237–45. doi: 10.1136/bjo.79.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wroblewski JJ, Gitter KA, Cohen G, Schomaker K. Indocyanine green angiography in Stargardt's flavimaculatus. Am J Ophthalmol. 1995;120:208–18. doi: 10.1016/s0002-9394(14)72609-1. [DOI] [PubMed] [Google Scholar]
- 59.Piccolino FC, Borgia L, Zinicola E. Indocyanine green angiography of circumscribed choroidal hemangiomas. Retina. 1996;16:19–28. doi: 10.1097/00006982-199616010-00005. [DOI] [PubMed] [Google Scholar]
- 60.Yannuzzi LA, Hope-Ross M, Slakter JS, Guyer DR, Sorenson JA, Ho AC, et al. Analysis of vascularized pigment epithelial detachments using indocyanine green videoangiography. Retina. 1994;14:99–113. doi: 10.1097/00006982-199414020-00003. [DOI] [PubMed] [Google Scholar]
- 61.Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16:203–13. doi: 10.1097/00006982-199616030-00004. [DOI] [PubMed] [Google Scholar]
- 62.Yuzawa M, Kawamura A, Haruyama M, Matsui M. Indocyanine green video-angiographic findings in choroidal osteoma. Eur J Ophthalmol. 1994;4:191–8. doi: 10.1177/112067219400400401. [DOI] [PubMed] [Google Scholar]
- 63.Herbort CP, Mantovani A, Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: Angiographic signs and utility in patient follow-up. Int Ophthalmol. 2007;27:173–82. doi: 10.1007/s10792-007-9060-y. [DOI] [PubMed] [Google Scholar]
- 64.Bouchenaki N, Herbort CP. The contribution of indocyanine green angiography to the appraisal and management of Vogt-Koyanagi- Harada disease. Ophthalmology. 2001;108:54–64. doi: 10.1016/s0161-6420(00)00428-0. [DOI] [PubMed] [Google Scholar]
- 65.Guex CY, Herbort CP. Prolonged retinal arterio-venous circulation time by fluorescein but not by indocyanine green angiography in birdshot chorioretinopathy. Ocul Immunol Inflamm. 1997;5:203–6. doi: 10.3109/09273949709116895. [DOI] [PubMed] [Google Scholar]
- 66.Howe LJ, Stanford MR, Graham EM, Marshall J. Choroidal abnormalities in birdshot chorioretinopathy: An indocyanine green angiography study. Eye (Lond) 1997;11:554–9. doi: 10.1038/eye.1997.142. [DOI] [PubMed] [Google Scholar]
- 67.Fardeau C, Herbort CP, Kullmann N, Quentel G, LeHoang P. Indocyanine green angiography in birdshot chorioretinopathy. Ophthalmology. 1999;106:1928–34. doi: 10.1016/S0161-6420(99)90403-7. [DOI] [PubMed] [Google Scholar]
- 68.Miyanaga M, Kawaguchi T, Miyata K, Horie S, Mochizuki M, Herbort CP. Indocyanine green angiography findings in initial acute pretreatment Vogt-Koyanagi-Harada disease in Japanese patients. Jpn J Ophthalmol. 2010;54:377–82. doi: 10.1007/s10384-010-0853-6. [DOI] [PubMed] [Google Scholar]
- 69.Bozzoni-Pantaleoni F, Gharbiya M, Pirraglia MP, Accorinti M, Pivetti-Pezzi P. Indocyanine green angiographic findings in Behçet disease. Retina. 2001;21:230–6. doi: 10.1097/00006982-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Matsuo T, Sato Y, Shiraga F, Shiragami C, Tsuchida Y. Choroidal abnormalities in Behçet disease observed by simultaneous indocyanine green and fluorescein angiography with scanning laser ophthalmoscopy. Ophthalmology. 1999;106:295–300. doi: 10.1016/S0161-6420(99)90069-6. [DOI] [PubMed] [Google Scholar]
- 71.Klaeger A, Tran VT, Hiroz CA, Morisod L, Herbort CP. Indocyanine green angiography in Behçet's uveitis. Retina. 2000;20:309–14. doi: 10.1097/00006982-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 72.Auer C, Bernasconi O, Herbort CP. Indocyanine green angiography features in toxoplasmic retinochoroiditis. Retina. 1999;19:22–9. doi: 10.1097/00006982-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Bernasconi O, Auer C, Herbort CP. Recurrent toxoplasmic retinochoroiditis. Significance of perilesional satellite dark dots seen by indocyanine green angiography. Ocul Immunol Inflamm. 1997;5:207–11. doi: 10.3109/09273949709116896. [DOI] [PubMed] [Google Scholar]
- 74.Wolfensberger TJ, Piguet B, Herbort CP. Indocyanine green angiographic features in tuberculous chorioretinitis. Am J Ophthalmol. 1999;127:350–3. doi: 10.1016/s0002-9394(98)00325-0. [DOI] [PubMed] [Google Scholar]
- 75.Herbort CP. Precise monitoring and differentiation of inflammatory events by indocyanine green (ICG) angiography in a case of recurrent posterior sarcoid uveitis. Ocul Immunol Inflamm. 2000;8:303–6. doi: 10.1076/ocii.8.4.303.6458. [DOI] [PubMed] [Google Scholar]
- 76.Wolfensberger TJ, Herbort CP. Indocyanine green angiographic features in ocular sarcoidosis. Ophthalmology. 1999;106:285–9. doi: 10.1016/S0161-6420(99)90067-2. [DOI] [PubMed] [Google Scholar]
- 77.Matsuo T, Itami M, Shiraga F. Choroidopathy in patients with sarcoidosis observed by simultaneous indocyanine green and fluorescein angiography. Retina. 2000;20:16–21. doi: 10.1097/00006982-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Cimino L, Auer C, Herbort CP. Sensitivity of indocyanine green angiography for the follow-up of active inflammatory choriocapillaropathies. Ocul Immunol Inflamm. 2000;8:275–83. doi: 10.1076/ocii.8.4.275.6462. [DOI] [PubMed] [Google Scholar]
- 79.Howe L, Stanford M, Graham E, Marshall J. Indocyanine green angiography in inflammatory eye disease. Eye. 1998;12:761–7. doi: 10.1038/eye.1998.199. [DOI] [PubMed] [Google Scholar]
- 80.Giovannini A, Ripa E, Scassellati-Sforzolini B, Ciardella A, Tom D, Yannuzzi L. Indocyanine green angiography in serpiginous choroidopathy. Eur J Ophthalmol. 1996;6:299–306. doi: 10.1177/112067219600600314. [DOI] [PubMed] [Google Scholar]
- 81.Giovannini A, Mariotti C, Ripa E, Scassellati-Sforzolini B. Indocyanine green angiographic findings in serpiginous choroidopathy. Br J Ophthalmol. 1996;80:536–40. doi: 10.1136/bjo.80.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slakter JS, Giovannini A, Yannuzzi LA, Scassellati-Sforzolini B, Guyer DR, Sorenson JA, et al. Indocyanine green angiography of multifocal choroiditis. Ophthalmology. 1997;104:1813–9. doi: 10.1016/s0161-6420(97)30022-0. [DOI] [PubMed] [Google Scholar]
- 83.Vadalà M, Lodato G, Cillino S. Multifocal choroiditis: Indocyanine green angiographic features. Ophthalmologica. 2001;215:16–21. doi: 10.1159/000050820. [DOI] [PubMed] [Google Scholar]
- 84.Howe LJ, Woon H, Graham EM, Fitzke F, Bhandari A, Marshall J. Choroidal hypoperfusion in acute posterior multifocal placoid pigment epitheliopathy. An indocyanine green angiography study. Ophthalmology. 1995;102:790–8. doi: 10.1016/s0161-6420(95)30955-4. [DOI] [PubMed] [Google Scholar]
- 85.Park D, Schatz H, McDonald HR, Johnson RN. Indocyanine green angiography of acute multifocal posterior placoid pigment epitheliopathy. Ophthalmology. 1995;102:1877–83. doi: 10.1016/s0161-6420(95)30780-4. [DOI] [PubMed] [Google Scholar]
- 86.Dhaliwal RS, Maguire AM, Flower RW, Arribas NP. Acute posterior multifocal placoid pigment epitheliopathy. An indocyanine green angiographic study. Retina. 1993;13:317–25. doi: 10.1097/00006982-199313040-00009. [DOI] [PubMed] [Google Scholar]
- 87.Uyama M, Matsunaga H, Matsubara T, Fukushima I, Takahashi K, Nishimura T. Indocyanine green angiography and pathophysiology of multifocal posterior pigment epitheliopathy. Retina. 1999;19:12–21. doi: 10.1097/00006982-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Ie D, Glaser BM, Murphy RP, Gordon LW, Sjaarda RN, Thompson JT. Indocyanine green angiography in multiple evanescent white-dot syndrome. Am J Ophthalmol. 1994;117:7–12. doi: 10.1016/s0002-9394(14)73008-9. [DOI] [PubMed] [Google Scholar]
- 89.Obana A, Kusumi M, Miki T. Indocyanine green angiographic aspects of multiple evanescent white dot syndrome. Retina. 1996;16:97–104. doi: 10.1097/00006982-199616020-00002. [DOI] [PubMed] [Google Scholar]
- 90.Tsukamoto E, Yamada T, Kadoi C, Hayasaka S, Nagaki Y, Hayasaka Y. Hypofluorescent spots on indocyanine green angiography at the recovery stage in multiple evanescent white dot syndrome. Ophthalmologica. 1999;213:336–8. doi: 10.1159/000027449. [DOI] [PubMed] [Google Scholar]
- 91.Prünte C, Niesel P. Quantification of choroidal blood-flow parameters using indocyanine green video-fluorescence angiography and statistical picture analysis. Graefes Arch Clin Exp Ophthalmol. 1988;226:55–8. doi: 10.1007/BF02172719. [DOI] [PubMed] [Google Scholar]
- 92.Auer C, Herbort CP. Indocyanine green angiographic features in posterior scleritis. Am J Ophthalmol. 1998;126:471–6. doi: 10.1016/s0002-9394(98)00119-6. [DOI] [PubMed] [Google Scholar]
- 93.Caccavale A, Mignemi L. Fluorescein and indocyanine green angiography findings in a case of poststreptococcal syndrome with erythema nodosum and posterior uveitis. Retina. 2001;21:669–72. doi: 10.1097/00006982-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 94.Arevalo JF, Fuenmayor-Rivera D, Giral AE, Murcia E. Indocyanine green videoangiography of multifocal Cryptococcus neoformans choroiditis in a patient with acquired immunodeficiency syndrome. Retina. 2001;21:537–41. doi: 10.1097/00006982-200110000-00023. [DOI] [PubMed] [Google Scholar]
- 95.Jones BE, Jampol LM, Yannuzzi LA, Tittl M, Johnson MW, Han DP, et al. Relentless placoid chorioretinitis: A new entity or an unusual variant of serpiginous chorioretinitis? Arch Ophthalmol. 2000;118:931–8. [PubMed] [Google Scholar]
- 96.de Laey JJ. Placoid epitheliopathy and serpiginous choroidopathy. Bull Soc Belge Ophtalmol. 1989;230:105–22. [PubMed] [Google Scholar]
- 97.Gupta V, Agarwal A, Gupta A, Bambery P, Narang S. Clinical characteristics of serpiginous choroidopathy in North India. Am J Ophthalmol. 2002;134:47–56. doi: 10.1016/s0002-9394(02)01501-5. [DOI] [PubMed] [Google Scholar]
- 98.Blumenkranz MS, Gass JD, Clarkson JG. Atypical serpiginous choroiditis. Arch Ophthalmol. 1982;100:1773–5. doi: 10.1001/archopht.1982.01030040753008. [DOI] [PubMed] [Google Scholar]
- 99.Slakter JS, Giovannini A, Yannuzzi LA, Scassellati-Sforzolini B, Guyer DR, Sorenson JA, et al. Indocyanine green angiography of multifocal choroiditis. Ophthalmology. 1997;104:1813–9. doi: 10.1016/s0161-6420(97)30022-0. [DOI] [PubMed] [Google Scholar]
- 100.Gass JD. St. Louis: Mosby; 1987. Acute posterior multifocal placoid pigment Epitheliopathy. Stereoscopic Atlas of Macular diseases; pp. 504–9. [Google Scholar]
- 101.Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39:265–92. doi: 10.1016/s0039-6257(05)80105-5. [DOI] [PubMed] [Google Scholar]


