Abstract
OBJECTIVE:
Frailty syndrome can be defined as a state of vulnerability to stressors resulting from a decrease in functional reserve across multiple systems and compromising an individual's capacity to maintain homeostasis. The purpose of this study was to determine the prevalence of frailty and its association with social and demographic factors, functional capacity, cognitive status and self-reported comorbidities in a sample of community-dwelling older individuals who are clients of a healthcare plan.
METHODS:
We evaluated 847 individuals aged 65 years or older who lived in the northern area of the city of Rio de Janeiro, Brazil. The subjects were selected by inverse random sampling and stratified by gender and age. To diagnose frailty, we used the scale proposed by the Cardiovascular Health Study, which consisted of the following items: low gait speed, grip strength reduction, feeling of exhaustion, low physical activity and weight loss. The data were collected between 2009 and 2010, and the frailty prevalence was calculated as the proportion of individuals who scored positive for three or more of the five items listed above. To verify the association between frailty and risk factors, we applied a logistic regression analysis.
RESULTS:
The prevalence of frailty syndrome was 9.1% (95% confidence interval [CI], 7.3-11.3); 43.6% (95% CI, 40.3-47) of the individuals were considered robust, and 47.3% (95% CI 43.8-50.8) were considered pre-frail (p<0.001). The frail individuals tended to be older (odds ratio [OR] 13.2, 95% CI, 8.7-20) and have lower education levels (OR 2.1, 95% CI, 1-4.6), lower cognitive performance (OR 0.76, 95% CI, 0.73-0.79) and reduced health perception (OR 65.8, 95% CI, 39.1-110.8). Frail individuals also had a greater number of comorbidities (OR 6.6, 95% CI, 4.4-9.9) and worse functional capacity (OR 3.8, 95% CI, 2.9-5).
CONCLUSION:
The prevalence of frailty was similar to that seen in other international studies and was significantly associated with educational level, cognition, comorbidities, functional capacity, perception of health and old age.
Keywords: Aged, Frail Elderly, Prevalence
INTRODUCTION
An aging population is a challenge that affects both rich and poor countries. It is estimated that approximately one million people worldwide turn 60 years old each month (1). Furthermore, the projected growth of this group will increase in coming years, and the need for resources to care for older individuals will, therefore, increase proportionately. This growth will also lead to an increased incidence of degenerative and disabling diseases, thereby generating the need for a greater collective understanding of how to care for this population. Only a minority of this elderly population, however, is responsible for the majority of multi-professional attention and health expenses (2).
Frailty syndrome is a condition that has been widely studied in recent decades (3). This syndrome can be viewed as a reduction in the physiological reserves of multiple organic systems and is an independent predictor of mortality, morbidity and institutionalization (4-9). Moreover, it is estimated that 10% to 27% of the population over 65 years is frail (10). Such figures also increase with age, such that in the population older than 85 years of age, the prevalence of frailty can reach 45% (11).
Although it is generally believed that an experienced health professional can reliably identify a fragile elderly patient, there is still no consensus regarding the clinical definition of frailty (12-16). Some studies have observed that this condition may be associated with the presence of common markers, such as falls, functional loss and disability (17-19). Although several methods have been proposed, two models with different conceptual and operational frameworks currently compete to explain previously collected empirical data (20,21). The first model suggests that an accumulation of physiological limitations and deficits in different domains can identify frail patients (20,22,23), whereas the second model proposes using a scale comprised of five items: weight loss, muscular weakness, low gait speed, low physical activity level and feeling exhausted. According to the second model, the frailty “phenotype” is characterized by the presence of three or more of these five items (21),.
The current study sought to use the criteria proposed by Fried et al., which was based on the data from the Cardiovascular Health Study (CHS) (21), to determine the prevalence of frailty syndrome in an elderly community dwelling in the city of Rio de Janeiro, Brazil and to evaluate the social, demographic and health factors associated with frailty.
MATERIALS AND METHODS
Study and Sample Population
This cross-sectional descriptive study used the baseline data from the Frailty in Brazilian Older People Study (Fragilidade em Idosos Brasileiros [FIBRA-RJ]).
Adults of both genders who lived in the northern area of the city of Rio de Janeiro and were older than 65 years and had been enrolled in a health maintenance organization for at least 12 months were selected to participate in the study. In total, 9,769 individuals fulfilled these criteria. The exclusion criteria consisted of severe functional and/or cognitive disabilities that profoundly compromised mobility, intellectual functions and understanding of the test instructions, thereby making it impossible to assess the performance of such participants using the five items of the frailty scale.
The sample was stratified by age and gender based on the corresponding ratios of the source population strata. The group comprised 847 people. Inverse random sampling was used to select individuals in each stratum, except for those older than 95 years, who were all included. This sampling strategy was performed because a large number of replacement elements in each stratum were required to achieve the calculated sample size.
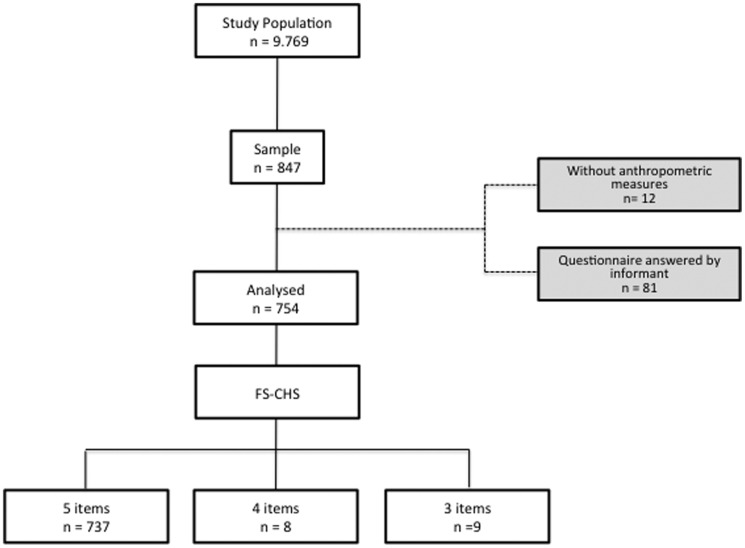
Because of the limitations associated with specific functional deficits or neuropsychiatric problems, 81 individuals required an informant to fill out the evaluation questionnaire. As per the exclusion criteria, these 81 patients did not participate in the analysis (Figure 1).
Figure 1.
Sampling selection for individuals aged 65 or older who were inhabitants of the northern region of Rio de Janeiro in 2010.
Individuals who fulfilled at least three of the five items of the Frailty Scale of the Cardiovascular Health Study (FS-CHS) were included. According to this criterion, 12 individuals were excluded from the analysis, but 17 were included despite having fulfilled only three or four items. Seven of these included individuals did not finish the gait speed test, two did not complete the hand grip strength test, and eight others were unable to do both tests. A total of 754 individuals were included in the analysis.
Data Collection
This study was approved by the Ethics Committee in Research of Pedro Ernesto University Hospital, Rio de Janeiro, Brazil.
The interviews were performed at the subjects' homes between January 2009 and January 2010. After signing an informed consent form, each subject underwent a health survey that assessed the following information: social and demographic characteristics, health habits, presence of weight loss, self-reported co-morbidities (e.g., heart disease, lung disease, arterial hypertension, stroke, diabetes, cancer, depression, osteoporosis and osteoarthritis), and functional capacity, which was evaluated by assessing the performance of basic and instrumental activities of daily living (27,28) and a cognitive evaluation, which was done by applying the Mini Mental State Examination (MMSE) (29).
For the diagnosis of frailty, the five items proposed by Fried at al. were considered: non intentional weight loss; feeling of exhaustion; low palmar grip strength; low level of physical activity and low gait speed. Non-intentional weight loss was evaluated by means of self-report. The individuals who lost more than 4.5 kg during the last year were considered as positive.
The feeling of exhaustion was assessed by the answers given to two items of the Center of Epidemiological Study Center Scale (CES-D): “Have you felt that you had to make an effort to do your customary tasks?”�and “Were you unable to proceed when doing your things?”�(30). Those individuals who stated “always” and “in most cases” to at least one of these questions were considered as positive.
Grip strength was measured using a manual dynamometer (JAMAR Model J00105) in the dominant upper limb by requesting each participant to apply the highest force possible in three attempts. The individuals in the first quintile (after adjusting for gender and body mass index [BMI]) were considered positive for this frailty item.
The level of physical activity was evaluated by applying the Minnesota Leisure-Time Physical Activities (MLTPA) instrument, which assesses the amount of physical activity performed by the individual and the estimated caloric expenditure. Individuals with a caloric expenditure in the first quintile were considered positive for this item.
Gait speed was measured using a chronometer to clock the time spent to walk a 4.6-meter-long circuit. The individuals in the first quintile, after adjusting for their respective heights and genders, were considered positive for this item.
Statistical Analysis
The descriptive analyses of all variables were calculated with a 95% CI and a statistical significance of p<0.05. Given the strategy of sample selection, different weights were allotted to each age stratum by considering a variation coefficient for frailty of 15% for an estimated prevalence of 0.07, with a 95% CI. Contingency tables were used for the comparisons between the scores for the frailty criteria, and Chi-squared, analysis of variance (ANOVA), and Kruskal-Wallis tests were performed. Univariate binary logistic regression analyses were performed to test the association between each co-variable and the binary response variable. The discriminatory ability of the multiple logistic regression model was evaluated with the Hosmer-Lemeshow test calculation. The data analysis was performed using the SPSS version 19.9, IBM software, 2009, Chicago.
RESULTS
We analyzed a total sample of 754 individuals, of which 66.9% were women, 62.6% were Caucasian and 44.1% were either married or living with a partner (Table 1). The mean patient age was 76.6 years (±6.9 years), and the mean educational level was 10.02 years (±5 years). The mean per capita income expressed in referential minimum wages (MW) was 6.3 MW. Of the nine self-reported comorbidities, those with the highest prevalence were systemic arterial hypertension (64.7%), osteoarthritis (35.9%), osteoporosis (27.1%) and diabetes mellitus (22.1%). A mean number of 1.87 falls (±1.43 falls) was recorded, and the Mini-Mental State Examination (MMSE) mean score was 25.47 (±3.37). The caloric expenditure cut-off point was 254.66 Kcal/week for the men and 184.94 Kcal/week for the women. The results for hand grip strength and gait speed are shown in Tables 3 and 4, respectively.
Table 1.
Sociodemographic and health characteristics of individuals aged 65 years or older who were inhabitants of the northern region of Rio de Janeiro, Brazil in 2010.
| Variables | Frequency n (%) | 95% CI (%)§) |
| Gender | ||
| Female | 529 (66.9) | 65.8 - 68 |
| Male | 225 (33.1) | 32 - 34.2 |
| Age group | ||
| 65 to 74 | 320 (37) | 35.6 – 38.5 |
| 75 to 84 | 334 (45.1) | 43.3 – 47 |
| ≥85 | 100 (17.8) | 16.7 – 19 |
| Race/color | ||
| Caucasian | 468 (62.6) | 59 – 66 |
| Non-Caucasian | 286 (37.4) | 34 – 41 |
| Marital status | ||
| Married/Living with partner | 324 (44.1) | 41 - 47.3 |
| Divorced/Separated | 58 (7.5) | 5.8 - 9.6 |
| Single | 83 (10.7) | 8.7 - 13.1 |
| Widower | 289 (37.7) | 34.5 - 40.9 |
| Education level (years) | ||
| Illiterate | 17 (3) | 2.1 – 4.4 |
| 1 to 5 | 183 (25.2) | 22.4 – 28.2 |
| 6 to 11 | 286 (37.5) | 34.3 – 40.9 |
| ≥12 | 268 (34.3) | 31.2 – 37.5 |
| Income (MW)+ | ||
| 0 to 2 | 121 (16.4) | 13.9 - 19.3 |
| 2.1 to 5 | 250 (34.4) | 31 – 38 |
| >5.1 | 349 (49.2) | 45.6 - 52.8 |
| Comorbidities | ||
| 0 and 1 | 304 (41) | 37.6 - 44.6 |
| 2 and 3 | 365 (48.1) | 44.5 - 51.6 |
| >4 | 85 (10.9) | 8.9 -13.3 |
| Functional capacity¶ | ||
| Independent | 609 (80.9) | 77.9 - 83.5 |
| Dependent | 145 (19.1) | 16.5 - 22.1 |
| Health self-perception | ||
| Very Bad - Bad | 37 (4.8) | 3.5 - 6.6 |
| Regular | 293 (38.5) | 35.1 - 42.1 |
| Good - Very Good | 424 (56.7) | 53.1 - 60.2 |
p<0.001.
+ Income in minimum wages (MW) = US$ 232.5 from 01/2009 to 01/2010.
¶ Basic activities of daily living.
Table 3.
Gait speed cut-off points of individuals aged 65 years or older who were inhabitants of the northern region of Rio de Janeiro, Brazil in 2010.
| Gender | Height (m) | Course Time (s) |
| Male | ≤1.68 | ≥7 |
| >1.68 | ≥6.3 | |
| Female | ≤1.54 | ≥7.6 |
| >1.54 | ≥6.6 | |
Table 4.
Univariate and multivariate logistic regression for the FS-CHS and the associated variables among individuals aged 65 years or older who were inhabitants of the northern region of Rio de Janeiro, Brazil in 2010 (n = 398).
| Univariate | Multivariate | |||||||
| OR | (%) 95% CI | p-value | OR | (%) 95% CI | p-value | |||
| Gender | ||||||||
| Male | ||||||||
| Female | 1.97 | 1.65 | 2.37 | <0.001 | 0.93 | 0.68 | 1.28 | 0.68 |
| Age group | ||||||||
| 65 to 74 | <0.001 | |||||||
| 75 to 84 | 4 | 3.2 | 5 | 3.2 | 2.4 | 4.4 | 0.000 | |
| ≥85 | 24.5 | 18.7 | 32 | 13.2 | 8.7 | 20 | 0.000 | |
| Race/color | ||||||||
| Non-Caucasian | ||||||||
| Caucasian | 1.1 | 0.9 | 1.3 | 0.2 | 2.7 | 2.1 | 3.5 | 0.000 |
| Marital status | ||||||||
| Married/Living with partner | ||||||||
| Divorced/Separated | 0.69 | 0.44 | 1.07 | 0.098 | 1.12 | 0.62 | 2.01 | 0.69 |
| Single | 3.1 | 2.4 | 4.0 | 0.000 | 7.6 | 5.1 | 11.2 | 0.000 |
| Widower | 2.7 | 2.2 | 3.2 | 0.000 | 2.6 | 1.9 | 3.7 | 0.000 |
| Education level (years) | ||||||||
| ≥12 | ||||||||
| 6 to 11 | 1.42 | 1.12 | 1.71 | <0.001 | 1.31 | 0.98 | 1.84 | 0.06 |
| 1 to 5 | 3.13 | 2.56 | 3.74 | .56 | 0.39 | 0.80 | 0.002 | |
| Illiterate | 8.43 | 5.14 | 14.22 | 2.12 | 1 | 4.62 | 0.048 | |
| Income + | ||||||||
| 0 to 2 | <0.001 | |||||||
| 2.1 to 5 | 0.21 | 0.16 | 0.26 | 0.34 | 0.23 | 0.48 | 0.000 | |
| >5 | 0.28 | 0.23 | 0.35 | 0.43 | 0.30 | 0.62 | 0.000 | |
| Comorbidities | ||||||||
| 0 and 1 | <0.001 | |||||||
| 2 and 3 | 3.6 | 2.9 | 4.4 | 3.3 | 2.5 | 4.5 | 0.000 | |
| >4 | 11.9 | 9.2 | 15.4 | 6.6 | 4.4 | 9.9 | 0.000 | |
| Functional capacity | ||||||||
| Independent | ||||||||
| Dependent ¶ | 3.7 | 3.1 | 4.4 | <0.001 | 3.9 | 2.9 | 5.1 | 0.000 |
| Health self-perception | ||||||||
| Good - Very Good | ||||||||
| Regular | 2.9 | 2.4 | 3.5 | <0.001 | 2.5 | 1.9 | 3.3 | 0.000 |
| Very bad - Bad | 24.6 | 17.4 | 34.8 | 65.8 | 39.1 | 110.8 | 0.000 | |
| Falls | ||||||||
| Without falls | <0.001 | |||||||
| 1 to 2 | 2.4 | 2 | 2.9 | 1 | 0.82 | 1.4 | 0.554 | |
| ≥3 | 4.8 | 3.5 | 6.8 | 2.2 | 1.3 | 3.4 | 0.002 | |
| MMSE | 0.7 | 0.71 | 0.75 | <0.001 | 0.76 | 0.73 | 0.79 | 0.000 |
OR: Odds Ratio.
CI: Confidence Interval.
FS-CHS: Frailty Scale-Cardiovascular Health Study.
MMSE: Mini-Mental State Examination.
+ Income in minimum wages (MW) = US$ 232.5from 01/2009 to 01/2010.
¶ Functional Dependence in >1 Basic activities of daily living.
The prevalence of frailty syndrome was 9.1% (95% CI, 7.3-11.3); 43.6% were characterized as robust (95% CI, 40.3-47), and 47.3% (95% CI, 43.8-50.8) were characterized as pre-frail (p<0.001). The mean age was higher among frail patients, with a seven-year difference between the frail and non-frail subjects. Frail individuals also demonstrated poorer cognitive performance, and women and widowers were more commonly frail. There was also an increased proportion of frail individuals among those with lower levels of education (≤8 years) and lower incomes (up to 2 minimum wages [MW]).
For the entire sample, the item most affected by frailty was the time spent to walk the 4.6-m course (30.2%), followed by handgrip strength (24.1%); furthermore, the prevalence of these items among frail individuals revealed similar values of 87.7% and 68.8%, respectively.
The logistic regression results are shown in Table 4. In the multivariate model, all variables analyzed, with the exception of gender, educational level between 6 and 11 years, and divorced or separated marital status, were statistically significant.
DISCUSSION
In this study, the prevalence of frailty among individuals aged 65 years or older who were clients of a health care organization was 9.1%, and frailty was associated with advanced age, Caucasian race, female gender, single or widower status, low income, three or more falls in the year prior to the interview, low educational level, poor cognitive performance, an increased number of comorbidities, low health self-perception and functional dependence.
Numerous operational criteria to identify frail elderly persons have been reported in the literature. However, we observed great diversity in the composition of the items that compose the instruments used to classify individuals as frail (31). The present study addressed this discrepancy using an instrument that is widely accepted in the scientific community. For the last 10 years, the concept of the frailty phenotype, as proposed by Linda Fried and co-workers (21), has dominated this area of scientific research, and many efforts have been made to confirm the reproducibility and validity of this method (25,32). Although this method consists essentially of biological items, some authors believe that the instrument can be affected by other domains, such as cognition (33). Moreover, there remains much discussion as to what constitutes the real nature of FS-CHS, what it represents and whether its content is valid (34). For this reason, evaluating different elderly populations using these items, testing alternative hypotheses and reflecting on the meaning of the produced empirical data remains the task of many scientists in the field of human aging.
The prevalence of frailty varies worldwide, depending on the population studied. In Europe, Asia and North America, data indicate that this value ranges from 4.9% to 27.3% (10,24,25),.
In Brazil, until recently, few studies have investigated the prevalence and factors associated with frailty syndrome. Yassuda et al. (39) reported a 7% frailty prevalence in a community sample in a neighborhood of São Paulo City, Brazil. In that study, the socio-demographic characteristics and distribution were similar to those reported in our study, and this previous study was the first to examine the Brazilian population using the FS-CHS and adopt specific cut points for the sample in relation to each of the items. Duarte et al. (40) measured frailty in the Saúde, Bem-estar e Envelhecimento (SABE) longitudinal study and reported a prevalence of 12.9%, which was associated with low educational levels, worse health perception, symptoms of depression, and hospitalizations. However, to measure caloric expenditures, the authors used a different instrument than that proposed by Fried et al. (21). In addition, Santos (41) evaluated frailty syndrome characteristics among older individuals of a community by applying the five proposed criteria and using the same cut-off points as the CHS for the manual grip strength and gait speed; the authors reported a 13.3% frailty prevalence (41).
Studies that have attempted to apply the FS-CHS methodology are difficult to compare because there is much variation in the composition and format of the instrument used to measure each item of the scale (10,37,42,43). For example, the “loss of weight” item in the study by Xue and co-workers and the “exhaustion feeling” in the study from Boyd et al. represent such variable items (24,26). According to Xue et al., self-reporting may be subject to errors, and BMI may be a better indicator of weight loss (24). To verify the feeling of exhaustion, Boyd et al. used one analogical visual scale rather than two questions from the CES-D (26). Moreover, previous evaluations of energy expenditure have also differed greatly between studies (35,37).
In the Study of Osteoporotic Fractures (SOF) (32), Ensrud et al. found a significant association between the performance of an instrument composed of three items (including get up from a chair five times, self-reported exhaustion and self-reported weight loss) and negative outcomes on health. The authors also compared this index to a modified version of the FS-CHS and concluded that the two scales were equal in terms of accuracy, although the first method demonstrated the advantage of simplicity. For Ribeiro-Filho et al., there is no evidence supporting a fundamental postulate of this study, which is that the modified version of the CHS retains the same clinometric performance characteristics as the original (44). The “free choice” of items to make up each new version of the FS-CHS has been a constant in the series of papers that appeared since the original publication in 2001. This freedom of choice has generally been determined by the availability of variables in the databases of various studies, many of which had not been initially designed for the main purpose of studying frailty syndrome.
The present study stratified the caloric expenditure cut-off points, palmar grip strength and course time according to the percentiles given by gender, BMI and height (Table 2). Differences in the values of these items were observed when compared to those reported by Fried et al. (21); both handgrip strength and caloric expenditure demonstrated lower values in our sample. Using equal cut-off points for samples with distinct phenotypic characteristics may lead to rating biases that could jeopardize the results.
Table 2.
Cut-off points for handgrip strength of individuals aged 65 years or older who were inhabitants of the northern region of Rio de Janeiro, Brazil in 2010.
| Gender | IMC (Kg/m2) | Handgrip Strength (Kgf) |
| Male | ≤22.40 | 16.8 |
| 22.40<BMI≤25.51 | 23.3 | |
| 25.51<BMI≤28.33 | 23.3 | |
| >28.33 | 23.4 | |
| Female | ≤24.12 | 13.3 |
| 24.12<BMI≤26.92 | 14 | |
| 26.92<BMI≤30.26 | 14 | |
| >30.26 | 14.7 |
Multivariate logistic regression showed a strong association between frailty, functional limitation, health self-perception, number of comorbidities and age, which corroborates the existing data in the literature (34). Furthermore, regarding cognition, there was a meaningful association with frailty (OR 0.79; 95% CI, 0.76-0.83). Although it is not present in the instrument proposed by the CHS, cognition is undoubtedly an important item to be considered in future modification proposals (45).
Another interesting finding was the significant relationship between marital status and frailty, as bachelors and widowers had a greater frailty risk (Table 3). In this regard, Guerra et al. (46,47) evaluated risk factors associated with the probability of hospital admission and concluded that the lack of a social support network (as a consequence of the individual's marital status) and the housing distance from urban centers were factors associated with frailty. From the viewpoint of the peculiarities of Brazilian culture, this factor is important to be considered in studies of repeated hospital admission and frailty (48).
Some limitations must be discussed for the present study. First, the cross-sectional study design prevented establishing a causal nexus between the studied variables and frailty syndrome. A second limitation refers directly to the items proposed in the CHS and used in this study. Although all proposed measures were adopted, the use of the MLPTA was shown to be problematic for the field inquiry because it is an instrument that has been validated in another country and references activities that are atypical in Brazilian culture; therefore, it may lead to errors in estimating weekly caloric expenditure.
ACKNOWLEDGMENTS
This study was supported by the National Research Council, Brazil (Conselho Nacional de Pesquisa [CNPq]), under grant No. 555087/2006-9, and the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro, Brazil (Fundação Carlos Chagas de Apoio à Pesquisa [FAPERJ]), under grant No. E-26/171.469/2006.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Camarano AA. Rio de Janeiro: IPEA; 1999. Muito além dos 60: os novos idosos brasileiros. [Google Scholar]
- 2.Castro M, Travassos C. Factors associated with readmission to a general hospital in Brazil. Cad Saude Publica. 2005;21(4):1186–200. doi: 10.1590/s0102-311x2005000400021. [DOI] [PubMed] [Google Scholar]
- 3.Swinne C, Cornete P, Schoevaerdts D, Latteur V, Melon C. Frailty in the medical literature. Age and Ageing. 1998;27(3):411–3. [Google Scholar]
- 4.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age and Ageing. 1997;26(4):315–8. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- 5.Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123(11):1457–60. doi: 10.1016/s0047-6374(02)00082-9. [DOI] [PubMed] [Google Scholar]
- 6.Nourhashemi F, Andrieu S, Gillette-Guyonnet S, Vellas B, Albarede JL, Grandjean H. Instrumental activities of daily living as a potential marker of frailty: a study of 7364 community-dwelling elderly women (the EPIDOS study) J Gerontol A Biol Sci Med Sci. 2001;56(7):M448–53. doi: 10.1093/gerona/56.7.m448. [DOI] [PubMed] [Google Scholar]
- 7.Ottenbacher KJ, Ostir GV, Peek MK, Snih SA, Raji MA, Markides KS. Frailty in Older Mexican Americans. Journal of the American Geriatrics Society. 2005;53(9):1524–31. doi: 10.1111/j.1532-5415.2005.53511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortz WM., 2nd A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57(5):M283–8. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 9.Xue QL, Fried LP, Glass TA, Laffan A, Chaves PH. Life-space constriction, development of frailty, and the competing risk of mortality: the Women's Health And Aging Study I. Am J Epidemiol. 2008;167(2):240–8. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 10.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675–81. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 12.Rockwood K, Hogan DB, MacKnight C. Conceptualisation and measurement of frailty in elderly people. Drugs Aging. 2000;17(4):295–302. doi: 10.2165/00002512-200017040-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Mahallati A, Simonsick EM. Frailty and the foolishness of Eos. J Gerontol A Biol Sci Med Sci. 2006;61(3):260–1. doi: 10.1093/gerona/61.3.260. [DOI] [PubMed] [Google Scholar]
- 14.Whitson HE, Purser JL, Cohen HJ. Frailty thy name is .. Phrailty? J Gerontol A Biol Sci Med Sci. 2007;62(7):728–30. doi: 10.1093/gerona/62.7.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bortz W. Understanding frailty. J Gerontol A Biol Sci Med Sci. 2010;65(3):255–6. doi: 10.1093/gerona/glp162. [DOI] [PubMed] [Google Scholar]
- 16.Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–2. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8(1):1–17. [PubMed] [Google Scholar]
- 18.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57(3):B115–25. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitz LA. Dynamic models for the study of frailty. Mech Ageing Dev. 2008;129(11):675–6. doi: 10.1016/j.mad.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 23.Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, et al. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007;128(3):250–8. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue Q-L, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63(9):984–90. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 25.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 26.Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118(11):1225–31. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 27.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 28.Lino VT, Pereira SR, Camacho LA, Ribeiro Filho ST, Buksman S. Cross-cultural adaptation of the Independence in Activities of Daily Living Index (Katz Index) Cad Saude Publica. 2008;24(1):103–12. doi: 10.1590/s0102-311x2008000100010. [DOI] [PubMed] [Google Scholar]
- 29.Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. Suggestions for utilization of the mini-mentalstate examination in Brazil. Arq Neuropsiquiatr. 2003;61(3B):777–81. doi: 10.1590/s0004-282x2003000500014. [DOI] [PubMed] [Google Scholar]
- 30.Batistoni SST, Neri AL, Cupertino APFB. [Validity of the Center for Epidemiological Studies Depression Scale among Brazilian elderly] Rev Saude Publica. 2007;41(4):598–605. doi: 10.1590/s0034-89102007000400014. [DOI] [PubMed] [Google Scholar]
- 31.Hogan DB, MacKnight C, Bergman H Steering Committee CIoFaA. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(3 Suppl):1–29. [PubMed] [Google Scholar]
- 32.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 33.Sarkisian CA, Gruenewald TL, John Boscardin W, Seeman TE. Preliminary evidence for subdimensions of geriatric frailty: the MacArthur study of successful aging. J Am Geriatr Soc. 2008;56(12):2292–7. doi: 10.1111/j.1532-5415.2008.02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Garcia FJ, Gutierrez Avila G, Alfaro-Acha A, Amor Andres MS, De Los Angeles De La Torre Lanza M, Escribano Aparicio MV, et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J Nutr Health Aging. 2011;15(10):852–6. doi: 10.1007/s12603-011-0075-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen C-Y, Wu S-C, Chen L-J, Lue B-H. The prevalence of subjective frailty and factors associated with frailty in Taiwan. Arch Gerontol Geriatr. 2010;50 Suppl 1:S43–7. doi: 10.1016/S0167-4943(10)70012-1. [DOI] [PubMed] [Google Scholar]
- 37.Castell Alcalá MV, Otero Puime A, Sánchez Santos MT, Garrido Barral A, González Montalvo JI, Zunzunegui MV. Prevalence of frailty in an elderly Spanish urban population. Relationship with comorbidity and disability. Aten Primaria. 2010;42(10):520–7. doi: 10.1016/j.aprim.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hébert R, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–7. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 39.Yassuda MS, Lopes A, Cachioni M, Falcao DV, Batistoni SS, Guimaraes VV, et al. Frailty criteria and cognitive performance are related: data from the FIBRA study in Ermelino Matarazzo, Sao Paulo, Brazil. J Nutr Health Aging. 2013;16(1):55–61. doi: 10.1007/s12603-012-0003-6. [DOI] [PubMed] [Google Scholar]
- 40.Duarte Y, Lebrao ML, Corona LP, Nunes DP, Santos JL, Alexandre TS. Frailty syndrome in Brazilian older adults. Journal of Epidemiology and Community Health. 2011;65(Suppl 1):A105. [Google Scholar]
- 41.Santos EG. Belo Horizonte: UFMG; 2008. Perfil de Fragilidade em Idosos Comunitários de Belo Horizonte - um estudo transversal. [Google Scholar]
- 42.Avila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Le Goff M, Ritchie K, et al. Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008;63(10):1089–96. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rockwood K, Mitnitski A. Frailty, fitness, and the mathematics of deficit accumulation. Reviews in Clinical Gerontology. 2007;17:1–12. [Google Scholar]
- 44.Ribeiro-Filho ST, Lourenço RA, Moreira VG. Comparing indexes of frailty: the cardiovascular health study and the study of osteoporotic fractures. J Am Geriatr Soc. 2010;58(2):383–5. doi: 10.1111/j.1532-5415.2009.02690.x. author reply 5-6. [DOI] [PubMed] [Google Scholar]
- 45.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–61. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 46.Guerra HL, Firmo JO, Uchoa E, Lima-Costa MF. The Bambui Health and Aging Study (BHAS): factors associated with hospitalization of the elderly. Cad Saude Publica. 2001;17(6):1345–56. doi: 10.1590/s0102-311x2001000600005. [DOI] [PubMed] [Google Scholar]
- 47.Guerra HL, Vidigall PG, Lima-Costa MF. Biomedical factors associated with hospitalization of older adults: The Bambuí Health and Aging Study (BHAS) Cad Saude Publica. 2003;19(3):829–38. doi: 10.1590/s0102-311x2003000300015. [DOI] [PubMed] [Google Scholar]
- 48.Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–7. doi: 10.1111/j.1532-5415.1993.tb06175.x. [DOI] [PubMed] [Google Scholar]
- 49.Lustosa LP. Tradução e adaptação transcultural do Minessota Leisure Time Activities Questionnaire em idosos. Geriatria & Gerontologia. 2011;5(2):57–65. [Google Scholar]