Abstract
OBJECTIVES:
There are no data in the literature with regard to the acute effects of different styles of music on the geometric indices of heart rate variability. In this study, we evaluated the acute effects of relaxant baroque and excitatory heavy metal music on the geometric indices of heart rate variability in women.
METHODS:
We conducted this study in 21 healthy women ranging in age from 18 to 35 years. We excluded persons with previous experience with musical instruments and persons who had an affinity for the song styles. We evaluated two groups: Group 1 (n = 21), who were exposed to relaxant classical baroque musical and excitatory heavy metal auditory stimulation; and Group 2 (n = 19), who were exposed to both styles of music and white noise auditory stimulation. Using earphones, the volunteers were exposed to baroque or heavy metal music for five minutes. After the first music exposure to baroque or heavy metal music, they remained at rest for five minutes; subsequently, they were re-exposed to the opposite music (70-80 dB). A different group of women were exposed to the same music styles plus white noise auditory stimulation (90 dB). The sequence of the songs was randomized for each individual. We analyzed the following indices: triangular index, triangular interpolation of RR intervals and Poincaré plot (standard deviation of instantaneous beat-by-beat variability, standard deviation of the long-term RR interval, standard deviation of instantaneous beat-by-beat variability and standard deviation of the long-term RR interval ratio), low frequency, high frequency, low frequency/high frequency ratio, standard deviation of all the normal RR intervals, root-mean square of differences between the adjacent normal RR intervals and the percentage of adjacent RR intervals with a difference of duration greater than 50 ms. Heart rate variability was recorded at rest for 10 minutes.
RESULTS:
The triangular index and the standard deviation of the long-term RR interval indices were reduced during exposure to both music styles in the first group and tended to decrease in the second group whereas the white noise exposure decreased the high frequency index. We observed no changes regarding the triangular interpolation of RR intervals, standard deviation of instantaneous beat-by-beat variability and standard deviation of instantaneous beat-by-beat variability/standard deviation in the long-term RR interval ratio.
CONCLUSION:
We suggest that relaxant baroque and excitatory heavy metal music slightly decrease global heart rate variability because of the equivalent sound level.
Keywords: Autonomic Nervous System, Auditory Stimulation, Cardiovascular System, Music
INTRODUCTION
Exposure to classical music presents positive effects on the cardiovascular system (1). Bernardi et al. (1) studied 24 healthy young adults and evaluated the effects of music with vocals (for Puccini “Turandot”), orchestra (Beethoven's “Ninth Symphony”) and progressive crescendos (Bach's Cantata BWV 169 “Gott soll allein mein Herze haben”) on heart rate (HR), respiratory rate, blood pressure and middle cerebral artery flow. The authors indicated that specific musical auditory stimulation may synchronize intrinsic cardiovascular regularity, thereby modulating cardiovascular physiology.
Similarly, the “Mozart effect” refers to the enhanced performance or neurophysiological activity that is associated with listening to Mozart's musical auditory stimulation. The effect can be observed in the spatial IQ tests performed before and after listening to Mozart. (2).
Exposure to heavy metal music presents negative effects related to stress. The responses induced by heavy metal music exposure include sleep disorders, fatigue, exhaustion and immunologic activity impairment (3). We hypothesized that whereas relaxant music auditory stimulation reduces the sympathetic nervous system activity, heavy metal music auditory stimulation increases the sympathetic nervous system activity.
As a noninvasive method for investigating the autonomic nervous system (ANS), heart rate variability (HRV) describes the oscillations of the intervals between consecutive heartbeats (RR intervals), which is influenced by the sinus node (4).
The methods used for analyzing HRV include the geometric methods — triangular index (RRtri), triangular interpolation of NN interval histogram (TINN) and Poincaré plot. These methods convert RR intervals into geometric patterns and allow us to analyze HRV through the geometric or graphical properties of the resulting pattern (4,5). The RRtri and TINN are calculated from the construction of a histogram of the density of normal RR intervals, which contains the length of the RR intervals on the x-axis and the frequency with which they occur on the y-axis. Joining the points of the histogram columns forms a shape like a triangle from which these indices are extracted (4).
The Poincaré plot is a two-dimensional graphical representation of the correlation between consecutive RR intervals, in which each interval is plotted against the following interval. We can qualitatively analyze the data by assessing the shape formed by its attractor, which shows the degree of complexity of the RR intervals. We can quantitatively analyze the Poincaré plot by fitting an ellipse to the shape formed by the plot, which yields the following indices: SD1, SD2 and the SD1/SD2 ratio. The Poincaré plot analysis is based on nonlinear dynamics (4).
Although the beneficial effects of musical auditory stimulation have been reported (6), no previous studies have investigated the short-term effects of classical baroque and heavy metal music on HRV. Additionally, Bernardi and co-workers (1) suggested that the autonomic activity on the heart depends on the time at which the music is heard. Because the HRV analysis requires a minimum of 256 RR intervals, we believe it is important to investigate its behavior during a single exposure to each style of music. Knowing the physiological responses induced by music exposure is important for developing future therapies that might contribute to the prevention of cardiovascular disorders. Therefore, we evaluated the acute effects of relaxant baroque and excitatory heavy metal musical auditory stimulation on the geometric HRV indices in women.
METHOD
Study population
We analyzed 40 healthy female subjects, who ranged between 18 and 35 years of age and were selected from our institution. We divided the subjects into two groups: Group 1 consisted of 21 healthy women, who were exposed to relaxant classical baroque musical and excitatory heavy metal auditory stimulation; and Group 2 consisted of 19 healthy women, who were exposed to both styles of music and white noise auditory stimulation. We informed all the volunteers about the procedures and objectives of the study. After agreeing to participate in our study, the subjects signed an informed consent. All study procedures were approved by the Ethics Committee in Research of the Faculty of Sciences of the Universidade Estadual Paulista, Campus of Marilia (Case No. CEP-2011-382) and were in accordance with resolution 196/96 National Health 10/10/1996.
Exclusion criteria
We considered the following exclusion criteria: auditory and cardiopulmonary disorders, neurological and other impairments that might prevent the subject from performing procedures and treatment with drugs that might influence cardiac autonomic regulation. We excluded subjects with previous experience with musical instruments or classical ballet music and volunteers who like heavy metal and baroque music styles because this musical preference might affect their cardiovascular responses (7).
Initial evaluation
Before the experimental procedure, we recorded data on the volunteers by collecting the following information: age, gender, weight, height and body mass index (BMI). We measured weight with a digital scale (W 200/5, Welmy, São Paulo/SP, Brazil) with a precision of 0.1 kg. We measured height with a stadiometer (ES 2020, Sanny, São Paulo/SP, Brazil) with a precision of 0.1 cm and 2.20 m of extension. We calculated the BMI using the following formula: weight (kg)/height (m2).
HRV analysis
The R-R intervals, which were recorded with a portable HR monitor (with a sampling rate of 1000 Hz), were downloaded to the Polar Precision Performance program (v. 3.0, Polar Electro, Finland). The software enabled us to visualize the HR and extract a cardiac period (R-R interval) file in “txt” format. After digital filtering complemented with manual filtering to eliminate premature ectopic beats and artifacts, we used at least 256 R–R intervals for the data analysis. We included only data series with more than 95% sinus rhythm (4,8). To calculate the indices, we used HRV analysis software (Kubios HRV v.1.1 for Windows, Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Kuopio, Finland).
Time and frequency domain indices of HRV
To analyze the HRV in the frequency domain, the low frequency (LF = 0.04 to 0.15 Hz) and high frequency (HF = 0.15 to 0.40 Hz) spectral components were used in ms2 and normalized units. In addition, the ratio between these components (LF/HF) represents a value that is relative to each spectral component in relation to the total power minus the very low frequency (VLF) components. We calculated the spectral analysis using the Fast Fourier Transform algorithm (9).
The time domain was analyzed by means of the standard deviation of normal-to-normal (SDNN) R-R intervals, the percentage of the adjacent RR intervals with a difference of duration greater than 50 ms (pNN50) and root-mean square of differences (RMSSD) between the adjacent normal RR intervals in a given time interval (9).
Geometric HRV indices
The HRV analysis was performed using the following geometrical methods: RRtri, TINN and Poincaré plot (SD1, SD2 and SD1/SD2 ratio). The RRtri was calculated from the construction of a density histogram of RR intervals, which contains the horizontal axis of all possible RR intervals measured on a discrete scale with 7.8125 ms boxes (1/128 seconds) and on the vertical axis, the frequency with which each occurred. The union of points of the histogram columns forms a triangle-like shape. The RRtri was obtained by dividing the number of RR intervals used to construct the histogram by their modal frequency (i.e., the RR interval that most frequently appeared on RR) (4).
The TINN consists of the measure of the base of a triangle. The method of least squares is used to determine the triangle. The RRtri and the TINN express the overall variability of the RR intervals (4).
The Poincaré plot is a map of points in Cartesian coordinates that is constructed from the values of the RR intervals. Each point is represented on the x-axis by the previous normal RR interval and on the y-axis by the following RR interval.
For the quantitative analysis of the plot, an ellipse was fitted to the points of the chart, with the center determined by the average RR interval. The SD1 indices were calculated to measure the standard deviation of the distances of the points from the diagonal y = x, and SD2 measures the standard deviation of the distances of points from the line y = -x+RRm, where RRm is the average RR interval. The SD1 is an index of the instantaneous recording of the variability of beat-to-beat and represents the parasympathetic activity, whereas the SD2 index represents the long-term HRV and reflects the overall variability. The SD1/SD2 shows the ratio between the short- and long-term variation among the RR intervals (10).
The plot was qualitatively analyzed using HRV analysis software based on the figures formed by its attractor. The expected shapes were described by Tulppo et al. (10) as:
1) Figures in which an increase in the dispersion of RR intervals is observed with increased intervals, characteristic of a normal plot.
2) Small figures with beat-to-beat global dispersion without increased long-term dispersion of RR intervals.
Measurement of the auditory stimulation
The equivalent sound levels were measured in a soundproof room, using an SV 102 audiodosimeter (Svantek, Finland). The audiodosimeter was programmed to collect measurements in the "A" weighting circuit, indicating a slow response.
The measurement was made during a session, which lasted 4 minutes 50 seconds for the relaxant classical baroque music and 5 minutes 15 seconds for the excitatory heavy metal music. We used the insert-type microphone (microphone in real ear), which was placed inside the auditory canal of the subject, just below the microphone, and connected to the personal stereo.
Before each measurement, we calibrated the microphones using the calibrator acoustic CR: 514 model (Cirrus Research Plc.).
Experimental protocol
Data were collected in a room with the temperature set between 21°C and 25°C and relative humidity regulated between 50% and 60%. The volunteers were instructed not to drink alcoholic or caffeinated beverages for 24 hours before the evaluation. The data were collected on an individual basis between 8 AM and 12 PM to minimize the confounding effects of the circadian rhythm. The procedures necessary for the data collection were explained on an individual basis; the subjects were instructed to remain at rest and avoid talking during the data collection.
After the initial evaluation, we placed the heart monitor belt over the subject's thorax, aligned with the distal third of the sternum, and the Polar RS800CX heart rate receiver (Polar Electro, Finland) was placed on the wrist. The subjects were seated and remained at rest with spontaneous breathing for 10 minutes with the earphones turned off.
After 10 minutes of rest, the subjects were exposed to excitatory heavy metal (Gamma Ray's “Heavy Metal Universe”) or relaxant baroque (Pachelbel's "Canon in D Major”) musical auditory stimulation for 5 minutes each. Subsequently, the individuals remained at rest for 5 minutes and thereafter were exposed to musical auditory stimulation for 5 minutes. The sequence of songs was randomized for each individual. In an additional protocol with a different group of women, the subjects were exposed to both styles of music and white noise auditory stimulation (90 dB) to investigate whether the variation in the equivalent sound level of the songs influences HRV.
Statistical analysis
Standard statistical methods were used to calculate the means and standard deviations. The normal Gaussian distribution of the data was verified by the Shapiro-Wilk goodness-of-fit test (z value of >1.0). For parametric distributions, we applied the one-way ANOVA for repeated-measures followed by the Bonferroni post-test. For nonparametric distributions, we used the Friedman test followed by Dunn's post-test. We compared the geometric indices of HRV between the three moments (Group 1, control condition vs. classical baroque vs. excitatory heavy metal; Group 2, control condition vs. classical baroque vs. excitatory heavy metal vs. white noise). The differences were considered significant when the probability of a Type I error was less than 5% (p<0.05). We used the Software GraphPad StatMate version 2.00 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
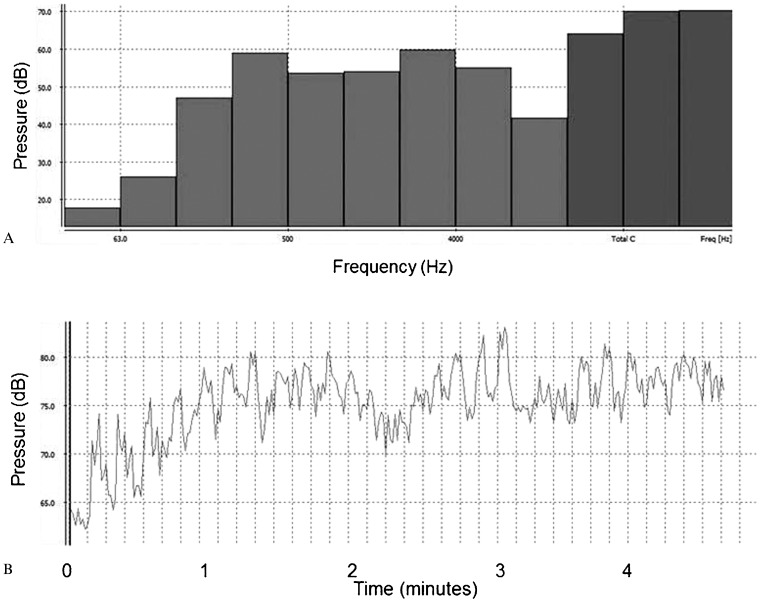
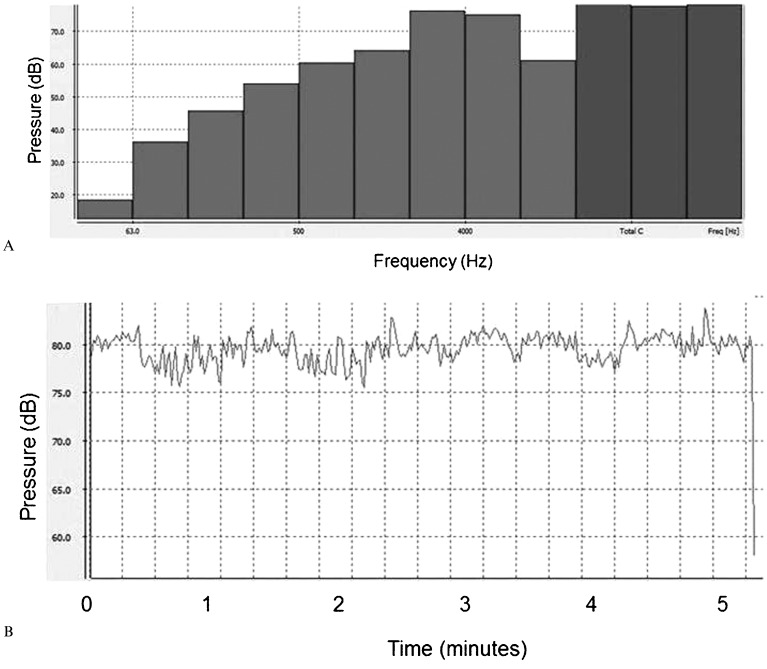
The volunteers were exposed to an equivalent sound level between approximately 70 and 80 dB. Figure 1 shows the measurement for the baroque music; Figure 2 presents the equivalent sound level during heavy metal music stimulation.
Figure 1.
Equivalent sound level of auditory musical stimulation in the baroque style.
Figure 2.
Equivalent sound level of auditory musical stimulation in the heavy metal style.
Table 1 presents the basal diastolic (DAP) and systolic (SAP) arterial pressures, HR, mean RR, weight, height and BMI of the volunteers.
Table 1.
Baseline diastolic (DAP) and systolic arterial pressure (SAP), heart rate (HR), mean RR interval, weight, height and body mass index (BMI) of the volunteers.
| Variable | Value |
| Age (years) | 25.9±4 |
| Height (m) | 1.62±0.09 |
| Weight (kg) | 67±10 |
| BMI (kg/m2) | 25±4 |
| HR (bpm) | 77.1±14 |
| Mean RR (ms) | 780±118 |
| SAP (mmHg) | 112±10 |
| DAP (mmHg) | 68±7 |
Table 2 demonstrates that the SD1 index when listening to the two musical styles tended to be reduced compared with the control group but showed no significant changes (Friedman test followed by Dunn's post-test, p = 0.09). The same result occurred with the index TINN, which tended to be decreased in response to exposure to relaxant baroque and excitatory heavy metal music (Friedman test followed by Dunn's post-test, p = 0.2). In contrast, the RRtri showed a significant reduction during exposure to relaxant baroque music and excitatory heavy metal music (ANOVA followed by Bonferroni's post-test, p = 0.03). Moreover, the SD2 index showed a significant reduction during both relaxant baroque and excitatory heavy metal music compared with the control condition (Friedman test followed by Dunn's post-test, p = 0.04). With regard to the SD1/SD2 ratio, we observed no significant changes (ANOVA followed by Bonferroni's post-test, p = 0.56).
Table 2.
Average values followed by their standard deviations for analysis of geometric indices of HRV.
| Index | Control | Baroque Music | Heavy Metal Music |
| RRTri | 13.2±4 | 11.6±4*) | 10.3±2*) |
| TINN | 162.8±106 | 150±109 | 106.9±83 |
| SD1 | 28.9±16 | 27±13 | 27.7±17 |
| SD2 | 62.7±19 | 52±20*) | 47±9*) |
| SD1/SD2 | 0.452±9.18 | 0.527±0.22 | 0.563±0.22 |
RRtri, triangular index; TINN, triangular interpolation of RR intervals; SDI, standard deviation of the instantaneous variability of the beat-to-beat heart rate; SD2, standard deviation of long-term continuous RR interval variability; SD1/SD2 ratio, ratio between the short- and long-term variation of the RR intervals.
*p<0.05 vs. control.
To analyze whether the changes in the equivalent sound level influences HRV, we applied an additional inclusion of white noise auditory stimulation. As shown in Table 3, the SD1 index was not different between the four moments (Friedman test followed by Dunn's post-test, p = 0.5). The TINN index (Friedman test followed by Dunn's post-test, p = 0.1) and RRTri (ANOVA followed by Bonferroni's post-test, p = 0.1) tended to be decreased at the time of exposure to excitatory heavy metal music compared with control condition. The SD2 index tended to decrease during both relaxant baroque and white noise compared with the control condition (Friedman test followed by Dunn's post-test, p = 0.09). Regarding the SD1/SD2 ratio, we observed no significant changes (ANOVA followed by Bonferroni's post-test, p = 0.39).
Table 3.
Average values and their standard deviations for the analysis of the time domain, frequency domain and geometric HRV indices.
| Index | Control | Baroque Music | Heavy Metal Music | White Noise |
| RRTri | 13.2±4 | 12.1±3 | 11.8±3 | 12.4±4 |
| TINN | 214.6±63 | 205±22 | 177.1±56 | 211±95 |
| SD1 | 27.2±13 | 25.5±13 | 24.4±10 | 23.4±11 |
| SD2*1) | 61.3±21 | 54.1±22 | 63.4±35 | 55.6±21 |
| SD1/SD2*) | 0.44±0.12 | 0.46v0.13 | 0.42±0.15 | 0.42±0.14 |
| SDNN (ms) | 49±17 | 45.6±17 | 43.4±16 | 44.4±17 |
| RMSSD (ms) | 38.3±19 | 35±17 | 32.4±13 | 31.9±15 |
| pNN50 (%) | 19.2±16 | 15±15 | 13.1v12 | 12.1±13 |
| LF (ms2)) | 693.9±727 | 691.2±1109 | 692.4±923 | 717.8±655 |
| LF (nu) | 49.9±19 | 51.4±18 | 54.5±15 | 59.5±17 |
| HF (ms2)) | 785.1±739 | 723.9±811 | 513.9±459 | 534.4±550 |
| HF (nu) | 49.9±20 | 48.4±18 | 45.3±15 | 40.4±17*) |
| LF/HF | 1.6±1.8 | 1. 53±1.62 | 1.58±1.33 | 2.2±2 |
RRTri, triangular index; TINN, triangular interpolation of RR intervals; SD1, standard deviation of the instantaneous variability of the beat-to-beat heart rate; SD2, standard deviation of long-term continuous RR interval variability; SD1/SD2 ratio, ratio between the short- and long-term variations of RR intervals; SDNN, standard deviation of normal-to-normal R-R intervals; RMSSD, root-mean square of differences between adjacent normal RR intervals in a time interval; pNN50, percentage of adjacent RR intervals with a difference of duration greater than 50 ms; LF, low frequency; HF, high frequency; LF/HF, low-frequency/high-frequency ratio.
*p<0.05 vs. control.
In the time domain, there was no significant difference between the four moments regarding the SDNN (ANOVA followed by Bonferroni's post-test, p = 0.37), RMSSD (ANOVA followed by Bonferroni's post-test, p = 0.3) and pNN50 (ANOVA followed by Bonferroni's post-test, p = 0.17) indices (Table 3). In the frequency domain analysis, we observed no changes regarding LF in either normalized (Friedman test followed by Dunn's post-test, p = 0.12) or absolute (Friedman test followed by Dunn's post-test, p = 0.2) units and HF in absolute units (Friedman test followed by Dunn's post-test, p = 0.19). The HF index was reduced in absolute units during exposure to white noise compared with the control condition (Friedman test followed by Dunn's post-test, p = 0.04). In contrast, the LF/HF ratio tended to be increased in the same situation compared with the control condition, but this result did not reach significance (Friedman test followed by Dunn's post-test, p = 0.08).
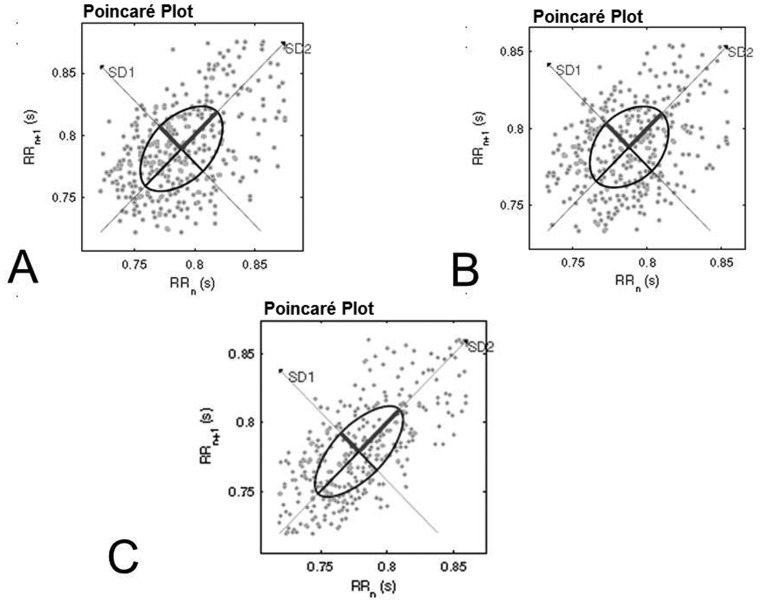
Figure 3 shows an example of the Poincaré plot patterns from one subject during no music (A), relaxant baroque musical auditory stimulation (B) and excitatory heavy metal musical auditory stimulation (C).
Figure 3.
Visual pattern of the Poincaré plot observed in one subject during the control condition (A), exposure to relaxant baroque music (B) and exposure to excitatory heavy metal music (C).
DISCUSSION
Considering the relevance of musical therapy and auditory stimulation during rehabilitation (13-15), we evaluated the acute effects of excitatory heavy metal and relaxant baroque music on the geometric, time and frequency domain indices of HRV in two groups of healthy women. The results obtained by the HRV geometric indices in the Group 1 showed that exposure to both styles of music decreased the HRV because the RRTri and SD2 indices decreased. In the Group 2, the RRTri and SD2 indices did not show significant differences, although they tended to be reduced during similar conditions. The frequency domain analysis indicated that the parasympathetic activity on the heart decreased during white noise auditory stimulation. We suggest that acute exposure to relaxant classical baroque and excitatory heavy metal musical auditory stimulation slightly reduces the HRV due to their equivalent sound level.
In this study, we reported that the SD1 index was unchanged during exposure to both styles of music. This index represents the transverse axis of the Poincaré plot; it indicates the standard deviation of the instantaneous variability of the beat-to-beat HR. This index represents the influence of the parasympathetic activity on the sinoatrial node (4). A reduction in vagal modulation has been observed in another study related to chronic obstructive pulmonary disease (COPD), in which the SD1 index was reduced in volunteers with COPD compared with subjects without the disease (10), which suggests an increased sympathetic tone in the patients with COPD. Moreover, in a study of obese and eutrophic children (12), the authors evaluated the SD1 index and observed a significant reduction in the obese subjects compared with the control group. This reduction is associated with an increased risk of morbidity and mortality from all causes and the development of various risk factors. Nonetheless, we found no effects of acute relaxant baroque music and excitatory heavy metal music on the SD1 index.
Based on our data, the SD2 index in the first group was decreased during exposure to both relaxant baroque and excitatory heavy metal musical auditory stimulation compared with no music stimulation. However, in the second group, this index tended to decrease but did not reach significance. The SD2 index expresses the overall variability of RR intervals. This long-term component of HRV usually accounts for all other HR changes, including changes associated with the baroceptor reflex loop and thermoregulation, when analyzed for 24 hours (4). Our results suggest that this stimulation presents slight effects on the global variability of HR.
Chuang and co-workers investigated the effects of long-term, 8-month music therapy intervention on autonomic function in anthracycline-treated breast cancer patients. The authors observed that the musical therapy improved the time and frequency domain indices of HRV. The authors' protocol was divided into two activities. During the first part of the study, the subjects were exposed to popular Taiwanese songs with moderate, pleasant rhythms and tempos. The second part of the study was spent learning how to play diverse musical instruments, such as hand bells, ukuleles, egg shakers, Cadeson bongos, metallophones and recorders. Our findings suggest that the relaxant baroque musical auditory stimulation acutely reduces the overall HRV.
The reduction of the RRTri indices in response to acute relaxant baroque and excitatory heavy metal music in the first group and the absence of significance in the second group support the hypothesis that acute musical auditory stimulation has slight effects on global HRV. The RRTri presented a close association with the standard deviation of all RR intervals and did not suffer from the influence of ectopic beats and artifacts because the artifacts and ectopic beats are located outside the triangle (16). Our group previously reported reduced values of RRTri in adult patients with COPD (10) and in obese children (12), which suggests that decreased RRTri is related to increased cardiovascular impairment risks. Taken together, the subjects in our study tended to present decreased global HRV during exposure to auditory stimulation with music.
The qualitative visual analysis of Poincaré plot revealed slight changes during excitatory heavy metal exposure, showing a greater beat-to-beat dispersion of RR intervals and a greater dispersion of RR intervals over the long term. The qualitative analysis supports the increased responses of the SD2 and RRTri indices during this condition compared with the control; this finding indicates that the global HRV is reduced during heavy metal musical auditory stimulation.
As we anticipated, the excitatory heavy metal music exposure reduce the global HRV. Both the SD2 and RRTri indices were decreased compared with control period, which included seated rest. Nevertheless, another index that corresponds to the global HRV, the TINN, was unchanged during relaxant baroque or excitatory heavy metal musical auditory stimulation. We believe that the acute effects of the music selected induced slight but significant responses, as observed with the geometric HRV indices. Although previous studies have evaluated the effect of different musical styles on stress, the influence of different styles of musical auditory stimulation on physiological responses has not been widely investigated. The existing studies observed the relaxing effect of classical music whereas genres such as techno music, hip hop and heavy metal are commonly associated with physiological arousal (17,18). We believe that acute excitatory heavy metal music can acutely induce stress responses and reduce HRV, as observed by analyzing the geometric indices.
As a main finding, both styles of music reduced the geometric HRV indices, which represent the overall variability of the RR intervals. We wonder whether acoustic stimulation reduces the global HRV. Conversely, Roy and co-workers (19) investigated the effects of a novel auditory binaural stimulus, called rotating acoustic stimulus, on the cardiac autonomic responses. The authors observed a decrease in the HR and an increase in the time domain RMSSD, SD1, SD2 and the SD1/SD2 ratio after the stimulation. They suggested that rotating acoustic stimulation may be a beneficial stimulus for cardiac autonomic regulation. Music presents different effects on HRV compared with different styles of auditory stimulation.
Classical music tends to relax the body and possibly stimulates the parasympathetic nervous system (6). Nevertheless, there is no direct evidence of a relationship between acute classical music and specific components of HRV. The elegant study performed by Bernardi and et al. (1) observed that during relaxant classical musical auditory stimulation, there is a moment during which sympathetic activation is accompanied by increases in cerebral blood flow velocity and arterial blood pressure, tachycardia and skin vasoconstriction. The authors (1) showed that sympathetic and parasympathetic activation depends on the music period. In our study, the volunteers were exposed to the same music that contains the same rhythm and decibel level. However, the music stretches that may influence the ANS with more intensity were not separated because the analysis of HRV requests a minimum of 256 RR intervals; if we separate the music stretch, the RR interval number would not reach this number at rest. Our group is presently studying a protocol to verify this important issue.
An important variable that we investigated was the music intensity. The absence of white noise is a limitation of many studies that have investigated the effects of music on the cardiovascular system. We reported that exposure to white noise significantly decreased the parasympathetic activity on the heart, which is indicated by the reduction of HF index, and tended to increase the sympathetic activity (p = 0.08). Nakamura et al. (20) observed that the number of c-Fos–reactive cells increased in the auditory cortexes of rats exposed to white noise compared with nonstimulated rats. In another study (21), the same group observed no effect of white noise auditory stimulation on gastric vagal nerve activity. In both cases, the animals were anesthetized using urethane, which may have influenced the responses compared with the conscious state. Our findings suggest that the equivalent sound level is involved in the global HRV decrease caused by exposure to excitatory heavy metal musical auditory stimulation.
The responses observed in our study may be explained by a physiological mechanism associated with the brain (15). A previous study performed in rats indicated that musical auditory stimulation decreases the renal sympathetic activity and arterial blood pressure through histaminergic neurons that are located at the suprachiasmatic nucleus of the hypothalamus (20). The dopamine release in the mesolimbic reward system, specifically the nucleus accumbens, was proposed to be involved in emotional stimulation when listening to music (22). Another investigation in rats indicated that musical auditory stimulation enhances calcium/calmodulin-dependent dopamine synthesis in the brain, thus decreasing blood pressure (23). An important issue is the style of music used by the authors because each study used different music. Thus, we must be careful when we interpret data.
In our study, we investigated only women because the literature indicates that there are differences between men and women regarding their physiological responses to musical auditory stimulation (16). During excitatory heavy metal music exposure, women presented a higher increase in the sympathetic nervous system responses compared with men. The sympathetic responses were evaluated by analyzing skin conductance and finger temperature. Men presented more intense autonomic responses after heavy metal musical auditory stimulation, as observed by an increased secretion of salivary amylase. This response is induced by sympathetic and parasympathetic nerve activities stimulation (17). Therefore, our data should not be extrapolated to men.
In conclusion, relaxant baroque and excitatory heavy metal musical auditory stimulation present slight effects on the global HRV, as observed through an analysis of the geometric HRV indices. We suggest that the acute effects of relaxant music are different from its chronic effects on HRV and that the equivalent sound level is also involved in this mechanism. Our results report the transient nature of music-related patterns and suggest that additional investigations regarding the relationship between musical auditory stimulation and cardiac autonomic regulation are necessary to expand the potential practice of music stimulation in therapeutic applications.
ACKNOWLEDGMENTS
Our study received financial support from FAPESP.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Bernardi L, Porta C, Casucci G, Balsamo R, Bernardi NF, Fogari R, et al. Dynamic interactions between musical, cardiovascular, and cerebral rhythms in humans. Circulation. 2009;30(25):3171–80. doi: 10.1161/circulationaha.108.806174. [DOI] [PubMed] [Google Scholar]
- 2.Rauscher FH, Shaw GL, Ky KN. Listening to Mozart enhances spatial temporal reasoning: towards a neurophysiological basis. Neurosci Lett. 1995;185(1):44–7. doi: 10.1016/0304-3940(94)11221-4. [DOI] [PubMed] [Google Scholar]
- 3.Sutoo D, Akiyama K. Music improves dopaminergic neurotransmission: demonstration based on the effect of music on blood pressure regulation. Brain Res. 2004;1016(2):255–62. doi: 10.1016/j.brainres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1993;93(5):1043–65. [PubMed] [Google Scholar]
- 5.Vanderlei LC, Pastre CM, Freitas Júnior IF, Godoy MF. Analysis of cardiac autonomic modulation in obese and eutrophic children. Clinics. 2010;65(8):789–92. doi: 10.1590/S1807-5932201000080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang CY, Han WR, Li PC, Song MY, Young ST. Effect of Long-Term Music Therapy Intervention on Autonomic Function in Anthracycline-Treated Breast Cancer Patients. Integrat Cancer Ther. 2011;10(4):312–6. doi: 10.1177/1534735411400311. [DOI] [PubMed] [Google Scholar]
- 7.Harrer G, Harrer H. In: Music, Emotion and Autonomic Function. MacDonald Critchley, editor. 1977. [Google Scholar]
- 8.Carvalho TD, Pastre CM, de Godoy MF, Fereira C, Pitta FO, de Abreu LC, et al. Fractal correlation property of heart rate variability in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6(1):23–8. doi: 10.2147/COPD.S15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pivatelli FC, Dos Santos MA, Fernandes GB, Gatti M, de Abreu LC, et al. Sensitivity, specificity and predictive values of linear and nonlinear indices of heart rate variability instable angina patients. Int Arch Med. 2012;5(1):31. doi: 10.1186/1755-7682-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias de Carvalho T, Marcelo Pastre C, Claudino Rossi R, de Abreu LC, Valenti VE, Marques Vanderlei LC. Geometric index of heart rate variability in chronic obstructive pulmonary disease. Rev Port Pneumol. 2011;17(6):260–5. doi: 10.1016/j.rppneu.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Tulppo MP, Mäkikallio TH, Seppänen T, Laukkanen RT, Huikuri HV. Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am J Physiol. 1998;274(2 Pt 2):H424–9. doi: 10.1152/ajpheart.1998.274.2.H424. [DOI] [PubMed] [Google Scholar]
- 12.Vanderlei LC, Pastre CM, Freitas IF, Jr, Godoy MF. Geometric indexes of heart rate variability in obese and eutrophic children. Arq Bras Cardiol. 2010;95(1):35–40. doi: 10.1590/s0066-782x2010005000082. [DOI] [PubMed] [Google Scholar]
- 13.Valenti VE, Guida HL, Vanderlei LCM, Roque AL, Ferreira LL, Ferreira C, et al. Relationship between cardiac autonomic regulation and auditory mechanisms: importance for growth and development. J Hum Growth Develop. 2013;23(1):94–8. [Google Scholar]
- 14.Brown LA, de Bruin N, Doan JB, Suchowersky O, Hu B. Novel challenges to gait in Parkinson's disease: the effect of concurrent music in single- and dual-task contexts. Arch Phys Med Rehabil. 2009;90(9):1578–83. doi: 10.1016/j.apmr.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Valenti VE, Guida HL, Frizzo AC, Cardoso AC, Vanderlei LC, Abreu LC. Auditory stimulation and cardiac autonomic regulation. Clinics. 2012;67(8):955–8. doi: 10.6061/clinics/2012(08)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Bio Eng Comput. 2006;44(11):1031–51. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 17.Nater UM, Abbruzzese E, Krebs M, Ehlert U. Sex differences in emotional and psychophysiological responses to musical stimuli. Int J Psychophysiol. 2006;62(2):300–8. doi: 10.1016/j.ijpsycho.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki A, Booker A, Kapur V, Tilt A, Niess H, Lillemoe KD, Warshaw AL, Conrad C. The impact of music on metabolism. Nutrition. 2012;28(11-12):1075–80. doi: 10.1016/j.nut.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Roy B, Choudhuri R, Pandey A, Bandopadhyay S, Sarangi S, Kumar Ghatak S. Effect of rotating acoustic stimulus on heart rate variability in healthy adults. Open Neurol J. 2012;6(1):71–7. doi: 10.2174/1874205X01206010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura T, Tanida M, Niijima A, Hibino H, Shen J, Nagai K. Auditory stimulation affects renal sympathetic nerve activity and blood pressure in rats. Neurosci Lett. 2007;12416(1):107–12. doi: 10.1016/j.neulet.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Tanida M, Niijima A, Nagai K. Effect of auditory stimulation on parasympathetic nerve activity in urethane-anesthetized rats. In Vivo. 2009;23(3):415–9. [PubMed] [Google Scholar]
- 22.Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14(2):257–62. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- 23.Sutoo D, Akiyama K. Music improves dopaminergic neurotransmission: demonstration based on the effect of music on blood pressure regulation. Brain Res. 2004;61016(2):255–62. doi: 10.1016/j.brainres.2004.05.018. [DOI] [PubMed] [Google Scholar]