Abstract
Objective
To examine the effects of individual and local level socioeconomic status (SES) and health care access characteristics on the number of self-report physician visits for systemic lupus erythematosus (SLE).
Methods
Data derived from 755 adult participants from the 2004 to 2007 Lupus Outcomes Study (LOS) resulted in a sample of 2,926 repeated-measures observations. The outcome measure was the number of physician visits in the prior 12 months. Information on disease activity and manifestations, demographics, health insurance, and specialty of the participants’ main SLE physician was collected through yearly LOS interviews. Local area measures including neighborhood poverty, the number of subspecialists per capita, and hospital market areas were added from secondary data sources. We used a mixed model with repeated measures to estimate the number of physician visits for SLE by SES and health care access characteristics, as well as the extent of concentrated poverty and number of subspecialists per capita in the local community, and whether these relationships varied by specific hospital market area. Multivariate models were adjusted for demographic and health status covariates.
Results
LOS respondents reported a mean ± SD of 11.8 ± 10.7 (range 0–52) physician visits for SLE. After adjustment, having less than a high school education, receiving care in a health maintenance organization, being treated by a generalist, and living in a community of concentrated poverty were associated with a significantly lower number of physician visits for SLE. These relationships varied by hospital market areas.
Conclusion
Beyond health status, the number of physician visits for SLE varies by SES, neighborhood poverty, and characteristics of the health care system.
INTRODUCTION
In recent years, the literature establishing disparities in outcomes by race/ethnicity and socioeconomic status (SES) in systemic lupus erythematosus (SLE) has expanded with strong evidence that both characteristics are important determinants of outcomes. In addition, there is a growing consensus that at least some of the effect of race/ethnicity may be due to differences in SES (1– 6). Recently, it has been suggested that the geographic area in which one resides can affect outcomes in SLE (7,8), and in one study the geographic effect was independent of the personal SES and race/ethnicity of the individual (8).
Although most analysts would argue that health care cannot account for all of the disparity in outcomes by these characteristics, a growing body of literature is exploring the extent to which disparities in health care access and utilization exist in SLE (6,9). For example, it has been observed that hospitals and physicians treating a higher volume of persons with SLE achieve better outcomes (10,11) and that uninsured persons with SLE receiving care at low-volume centers experienced poorer outcomes (10). Elderly and poor persons with SLE also had more avoidable SLE hospitalizations (7). With respect to ambulatory care, those persons with lower incomes and the elderly were less likely to receive rheumatology care (12,13), while those receiving Medicaid had to travel much longer distances for their principal lupus care (14). Similarly, managed care had more of an impact in reducing health care utilization of persons with SLE among those in public insurance programs than among those in private plans (15), which is consistent with a heightened effect of managed care among the elderly, disabled, and poor.
As the nation implements health care reform, the role that differences among health care markets play in health care utilization has garnered more attention (16–18). For example, variations in hospital capacity, the supply of health personnel, and the extent of managed care penetration have been associated with health care utilization (19,20). Similarly, the capacity and willingness of health care providers to integrate and coordinate care have also been associated with variations in utilization (17).
However, to our knowledge, there are no studies in SLE that show the effect of local differences in health care characteristics on health care utilization among persons with this condition. The present study is designed to estimate such effects, after taking into account sociodemographic and medical characteristics of the individual with SLE. The study also estimates the incremental effect of living in an area of concentrated poverty beyond the effect of low SES at the individual level.
MATERIALS AND METHODS
Data sources
The Lupus Outcomes Study (LOS) is a longitudinal study of 957 individuals with a physician diagnosis of SLE that was further confirmed by an independent medical record review based on American College of Rheumatology criteria (21,22). Details about enrollment and data collection have been reported previously (23) and are summarized briefly here. Participants were recruited through health care settings (34%) and nonclinical sources, including patient support groups and conferences, newsletters, and Web sites; 73% were from California and the remaining participants were from 38 other states. LOS interviews have been conducted annually by telephone since September 2002. They included measures capturing demographic characteristics, SES, nature of health insurance, disease activity, functional and cognitive status, and health care utilization. On average, 95% of eligible participants from each wave were re-interviewed in the subsequent wave.
To describe the SES and health care delivery system characteristics in the participants’ geographic areas, data were appended from the 2000 US Census, the Health Resources and Services Administration’s Area Resource File (24), and the Dartmouth Institute’s Health Care Atlas (25). The census data were linked at the block group level to LOS records through geocoding procedures conducted by Sonoma Technology using the Environmental Systems Research Institute ArcGIS software, as described in a prior publication (8).
The research protocol was approved by the Committee on Human Research, University of California, San Francisco. All participants gave their informed consent to be part of the study.
Study population
The present study incorporates data from the second through the fifth interviews collected from 2004 to 2007 representing 815, 823, 797, and 728 annual participants, respectively. The unit of analysis for this study is a person-year with a total potential of 3,163 observations. We excluded observations in the years when the participant resided outside of the US (n = 13) and when the participant reported that they did not have health insurance (n = 56). We dropped 168 observations (5%) with missing data. Our final sample included 2,926 observations representing 755 LOS participants, which is a mean of 3.9 observations per participant.
Dependent variable
The dependent variable is a self-report measure of the total number of ambulatory physician visits for SLE care during the 12-month period preceding the interview. This included visits to generalists, specialist physicians, psychiatrists, and other medical doctors, and excluded nurses, physical therapists, and other allied health professionals.
Fixed-effects variables
The primary independent variables were SES and health care delivery characteristics measured at the individual and local area levels. At the individual level, SES was measured by educational attainment grouped into 3 categories: high school or less; some college, trade school, or associate degree; and baccalaureate degree or beyond. For the local area measure of SES, we first calculated the proportion of households in a census block group living at or below 125% of the Federal Poverty Level (FPL). The top decile of this proportion represents LOS participants residing in a census block group in which at least 30% of the households live at or below 125% of the FPL. We used this top decile as a cut point to define an area of concentrated poverty. The US Census Bureau defines a poverty area as a census tract in which at least 20% of households are below 100% of the FPL (26). In the poverty areas defined in our study, at least 22% of residents fell below 100% of the poverty threshold, making our definition comparable to the measure used by the US Census Bureau.
Health care delivery characteristics were measured at the individual level by self-report of 1) source of insurance as categorized by Medicare, Medicaid, employer-based (including the participant’s or family member’s current or former employer), or privately purchased (by participant or family member), 2) health maintenance organization (HMO) versus other types of health plans (HMO was further defined for the LOS participants as a plan where you must generally receive care from the HMO physician or else the expense is not covered), and 3) whether the participant’s main SLE physician is a specialist or a generalist. The source of insurance and report of HMO status were validated measures derived from the Medical Expenditures Panel Survey (27,28). The measure of specialist or generalist was based on the participant’s response to the following LOS questions: “Who is the main doctor for your lupus? What is the doctor’s specialty? Is he or she a rheumatologist, nephrologist (kidney specialist), internist, general or family practitioner, or some other specialty?” When the participants reported having an internist, general practitioner, or family practitioner, they were classified as having a “generalist,” whereas a report of a rheumatologist or nephrologist resulted in the classification of having a “specialist.” Those participants who reported “some other specialty” (n = 46 [1.6%]) were asked to specify the specialty in a followup question. The qualitative responses were then classified into 2 subgroups: other medical doctor specialty (e.g., neurologist) or other general/allied provider (e.g., nurse practitioner). The other medical doctor specialty subgroup was classified as “specialist,” whereas the other general/allied provider subgroup was classified as “generalist.”
At the local area level, health care delivery was characterized by the supply of internal medicine subspecialists per county using Area Resource File data (24), which include US county records of the number of internal medical subspecialists (including but not limited to rheumatology and nephrology, but excluding general internal medicine medical doctors) who provide direct patient care to civilians. If participants resided in a county that had no subspecialists, then they were assigned the value “zero subspecialists in county.” Of the remaining observations, we calculated the county rates of the number of persons per subspecialist and categorized the distribution into 2 groups: counties with 1 subspecialist per 1,000 – 8,000 population (the bottom quartile of persons per subspecialist) and counties with 1 subspecialist per 8,001–123,000 population (remaining quartiles).
In the analysis, we controlled for potential confounding by including demographic characteristics and health status. Demographic characteristics included age, sex, and race/ethnicity (non-Hispanic white versus all other). We measured health status with the Medical Outcomes Study Short Form 36 health survey physical functioning scale, the Center for Epidemiologic Studies Depression Scale (29), and a self-reported global rating of SLE disease activity in the 3 months preceding the interview (similar to a visual analog scale, but used over the telephone where participants were asked to rate their SLE disease activity on a 0–10 scale). The global rating of SLE was intended as a disease-specific measure of health (30).
We used the prevalence of SLE-related events as a proxy measure for disease severity. Participants were asked if they had had any of the following manifestations or procedures in the preceding 12 months: kidney problems due to SLE, kidney biopsy, kidney transplant, dialysis, bronchoscopy, lung biopsy, pleurisy, pericarditis, hemoptysis, deep vein thrombosis, pulmonary embolism, transient ischemic attack, stroke, heart attack, eye clot, vision loss, other blood clots, or seizures. Since 1 of the 4 yearly interviews assessed only 11 of the 18 above events, we calculated the proportion of events experienced and categorized the distribution into 3 levels: no events, ≤20% of events (corresponding to 2 SDs above the mean), and >20% of events, rather than using a count data. We also controlled for the number of years since SLE diagnosis.
Random-effect variable
We obtained data on hospital service areas (HSAs) from the Dartmouth Atlas of Health Care (25). An HSA is a geographic area defined by a collection of zip codes within which residents receive the majority of their hospital care. After matching LOS records to an HSA by zip code, we found that the LOS sample represented 337 of the 3,436 nationwide HSAs. In order to make inferences to the HSA population, we modeled HSA as a random effect. By analyzing HSA as a random effect we were able to examine whether the relationships between the aforementioned predictors and number of physician visits for SLE vary from HSA to HSA. Of note, the geographic areas of HSAs are not required to be contiguous and may cross political boundaries (25). For example, 2 participants who reside in the same county may reside in 2 different HSAs. As a result, the variable in this study that was measured at the county geographic level (county supply of specialist) was not completely nested within the HSA, which precluded performing a multilevel or hierarchical methods analysis.
Statistical analysis
Exploratory analyses of the dependent variable demonstrated that the total number of physician visits for SLE had a skewed distribution, with a minority of participants with very high physician use. Therefore, we transformed the number of physician visits for SLE to its natural logarithm prior to estimating the models. After estimation we used a smearing factor to transform this measure back to natural terms (31).
To examine the relationships between the number of physician visits for SLE and the independent fixed-and random-effects variables, we ran a series of linear mixed models that accounted for repeated measures. Mixed models were an extension of general linear models that allowed for the simultaneous examination of individual and group (HSA in our study) level variations that were correlated. We presented the results of the fixed-effect variables using least squares (adjusted) group means and 95% confidence intervals (95% CIs). The result for the HSA random effects was presented in the form of a coefficient that represents the proportion of the variance of number of physician visits that can be explained by the HSA-to-HSA variation, after controlling for the fixed variables in the model.
The first set of models examined the bivariate relationship between each predictor variable and the number of physician visits for SLE while controlling for HSA and repeated measures. The second model examined the multivariate relationship between individual level SES and the health care delivery system on the number of physician visits for SLE and the degree to which these relationships varied by HSA. This model controlled for health status (physical function, depressive symptomology, global rating of SLE activity, and disease severity) and repeated measures. The third model built on the previous model by adding local area level predictors.
RESULTS
Sample characteristics
Table 1 shows characteristics of the LOS sample (n = 775) in the baseline year of 2004. The study sample was 93% female, 71% non-Hispanic white, and 85% attained some college education or were college graduates. Five percent of the sample received health insurance through Medicaid, 38% through Medicare, and the remainder through an employer or private insurance. Thirty-three percent of the sample was enrolled in an HMO, and 91% reported that their main SLE physician was a specialist. The mean ± SD age at the time of the interview was 48.5 ± 12.5 years and, on average, the participants were diagnosed at a mean ± SD of 13.8 ± 8.6 years prior to the interview.
Table 1.
Baseline characteristics of 755 Lupus Outcomes Study participants*
| Characteristics | No. (%) | Mean ± SD (range) |
|---|---|---|
| Individual level variables | ||
| Age, years | 48.5 ± 12.5 (19–83) | |
| Women | 700 (93) | |
| Race/ethnicity | ||
| White | 538 (71) | |
| Hispanic | 55 (7) | |
| African American | 55 (7) | |
| Asian/Pacific Islander | 65 (9) | |
| Other | 42 (6) | |
| Socioeconomic status | ||
| Education | ||
| High school graduate or less | 110 (15) | |
| Some college | 351 (46) | |
| College graduate | 294 (39) | |
| Health care | ||
| Primary insurance | ||
| Medicaid | 36 (5) | |
| Medicare | 286 (38) | |
| Privately purchased | 48 (6) | |
| Employer (self or family) | 385 (51) | |
| HMO | 252 (33) | |
| Main lupus physician | ||
| Generalist | 64 (9) | |
| Specialist | 691 (91) | |
| Health status | ||
| SLE-related manifestations/procedures in past year | ||
| None | 353 (47) | |
| ≤20% in past year | 364 (48) | |
| >20% in past year | 38 (5) | |
| SLE activity in past 3 months | 4.1 ± 2.7 (0–10) | |
| SF-36 physical functioning scale | 58.3 ± 29.8 (0–100) | |
| CES-D depressive symptoms score | 15.4 ± 12.8 (0–54) | |
| Years since SLE diagnosis | 13.8 ± 8.6 (1–47) | |
| Contextual level variables | ||
| Socioeconomic status | ||
| Lives in neighborhood poverty area | 59 (8) | |
| Health care | ||
| No. of persons/subspecialist in county | ||
| None | 34 (5) | |
| 8,001–123,000 | 183 (24) | |
| 1,000–8,000 | 538 (71) | |
| No. of SLE medical visits in past year | 11.8 ± 10.7 (0–52) | |
HMO = health maintenance organization; SLE = systemic lupus erythematosus; SF-36 = Medical Outcomes Study Short Form 36 health survey; CES-D = Center for Epidemiologic Studies Depression Scale.
Participants reported moderate levels of disease activity, physical functioning, and depressive symptomatology. Approximately one-half of the participants reported having ≥1 SLE-related manifestations or procedures in the prior year. On average, participants reported a mean ± SD of 11.8 ± 10.7 physician visits for SLE during the prior 12 months.
A large proportion of the LOS sample resided in counties with very high levels of physician specialist work force. While LOS participants were recruited through health care and community-based settings, ~50% of the counties in which LOS participants resided were metropolitan areas with populations of ≥1 million compared with 13% of counties nationwide (data not shown). National studies have documented high per capita specialist physician supply in urbanized areas (20).
Bivariate results
Table 2 presents unadjusted and adjusted average annual number of physician visits for SLE from 2004 to 2007. The unit of analyses is a person-year (n = 2,926 observations), which represents the followup of 755 LOS respondents over a mean of 3.9 years.
Table 2.
Average annual number of physician visits for SLE, with and without adjustment for individual and local factors*
| Bivariate models† (n = 2,926) | Multivariate models‡
|
||
|---|---|---|---|
| Individual factors (n = 2,926) | Individual and local factors (n = 2,926) | ||
| Fixed effects, individual level variables | |||
| Demographics | |||
| Age 18–34 years | 11.8 (10.4–13.3) | 12.5 (11.3–13.8)§ | 12.5 (11.3–3.8)§ |
| Age 35–54 years | 13.3 (12.4–14.3) | 11.9 (11.3–12.6)§ | 11.9 (11.3–2.6)§ |
| Age 55–64 years | 12.9 (11.7–14.1) | 11.1 (10.3–12.0)§ | 11.1 (10.3–1.9)§ |
| Age ≥65 years (reference) | 12.0 (10.6–13.7) | 9.2 (8.2–10.2) | 9.2 (8.2–10.3) |
| Sex | |||
| Male | 10.4 (8.6–12.4) | 10.8 (9.4–12.4) | 10.8 (9.5–12.4) |
| Female | 13.1 (12.3–13.9)§ | 11.5 (11.0–12.0) | 11.5 (11.0–2.0) |
| Race/ethnicity | |||
| White | 12.5 (11.7–13.4) | 11.2 (10.7–11.8) | 11.2 (10.7–1.8) |
| Nonwhite | 13.8 (12.5–15.1) | 11.9 (11.0–12.8) | 12.0 (11.1–2.9) |
| Sociodemographics | |||
| Education | |||
| High school graduation or less | 13.0 (11.7–14.4) | 10.1 (9.3–11.0)§ | 10.1 (9.3–11.1)§ |
| Some college | 13.6 (12.6–14.5)§ | 11.5 (10.9–12.2) | 11.5 (10.9–2.2) |
| College graduate (reference) | 12.0 (11.1–13.0) | 11.9 (11.1–12.6) | 11.8 (11.1–2.6) |
| Health care characteristics | |||
| Insurance primary | |||
| Medicaid | 18.0 (15.3–21.2)§ | 13.1 (11.3–15.1)§ | 13.4 (11.5–5.6)§ |
| Medicare | 15.1 (14.1–16.3)§ | 12.9 (12.1–13.8)§ | 12.9 (12.1–3.7)§ |
| Privately purchased | 10.7 (9.6–12.1) | 10.4 (9.4–11.6) | 10.4 (9.3–11.5) |
| Employer (self or family; reference) | 10.7 (10.0–11.5) | 10.5 (9.9–11.1) | 10.4 (9.9–11.1) |
| HMO | |||
| No HMO | 13.5 (12.7–14.3)§ | 11.8 (11.2–12.4)§ | 11.8 (11.2–2.4)§ |
| HMO | 11.4 (10.6–12.3) | 10.7 (10.0–11.4) | 10.7 (10.0–1.4) |
| Type of main lupus doctor | |||
| Generalist | 9.3 (8.3–10.4) | 7.9 (7.1–8.7) | 7.9 (7.1–8.7) |
| Specialist | 13.2 (12.5–14.0)§ | 11.9 (11.3–12.4)§ | 11.9 (11.3–2.4)§ |
| Fixed effects, local level variables | |||
| Sociodemographics | |||
| Lives in poverty area | |||
| No | 12.8 (12.1–13.6) | 11.6 (11.1–2.1)§ | |
| Yes | 13.7 (11.6–16.2) | 10.0 (8.7–11.3) | |
| Health care characteristics | |||
| Persons/subspecialist in county | |||
| None | 13.5 (10.6–17.2) | 10.8 (9.1–13.0) | |
| 8,001–123,000 | 13.0 (12.1–13.9) | 11.5 (10.9–2.1) | |
| 1,000–8,000 (reference) | 12.4 (11.1–14.0) | 11.4 (10.4–2.5) | |
| Random effects, coefficent | |||
| Variance at HSA level | 0.03§ | 0.03§ | |
| Variance repeated measures | 0.52§ | 0.52§ | |
| Residual | 0.49§ | 0.49§ | |
Values are the mean (95% confidence interval) unless otherwise specified. SLE = systemic lupus erythematosus; HMO = health maintenance organization; HSA = hospital service area.
Models control for repeated measures and HSA random effects.
Covariates include Medical Outcomes Study Short Form 36 health survey physical function scale, Center for Epidemiologic Studies Depression Scale (depressive symptomatology), global rating of SLE activity, and disease severity. Models account for repeated measures and HSA random effects.
Significant at P < 0.05.
The first column shows the unadjusted number of physician visits for SLE. Significant group differences exist among factors previously linked to access to care. LOS participants who had some college education, were not in an HMO, and had a specialist as their main lupus physician reported higher physician use. On average, individuals with Medicaid and Medicare reported significantly more physician visits (18.0 and 15.1, respectively) than those with private or employer-based insurance (10.7 and 10.7, respectively). Neither of the local area level variables was significantly associated with the outcome, although those living in areas of concentrated poverty or in areas with fewer subspecialists appeared to have somewhat higher rates of physician visits. These results accounted for repeated measures and for HSA random effect. They did not take into account health status differences across the various SES and health care system characteristics.
Individual level multivariate results
The second column in Table 2 shows the multivariate relationship of individual level factors and the number of physician visits for SLE, controlling for health status. In this model, all 3 of the health care delivery variables are independently predictive of the number of physician visits for SLE in the 12 months prior to interview. As in the bivariate relationships, receiving care from a specialist and not having membership in an HMO remain associated with more physician visits for SLE, and persons with public insurance have ~20% more such visits than those with private or employer-based insurance. The results indicate that education and age are associated with physician visits for SLE. Individuals with a high school education or less and older adults report fewer physician visits for this condition.
Our second level of inquiry focused on whether the relationship between the individual level predictors and number of physician visits for SLE varies among regional health care markets. We found a small but significant impact of HSAs on such visits, indicating that there is additional variation in physician visits for SLE across health care markets after accounting for the above individual level differences. The HSA-to-HSA variation independently accounts for 3% of the total variation in such visits.
Local area and individual level multivariate results
The third column in Table 2 shows the results when the local area level variables (area of concentrated poverty and number of specialist per county) are added to the multivariate model. Individuals residing in areas of concentrated poverty experienced fewer physician visits for SLE. The variable measuring the number of subspecialists per capita had no independent effect. The HSA random effect remained significant and unaltered after adding the number of subspecialist per capita. This result indicates that the effect of HSAs is independent of the supply of physicians as measured by the number of internal medicine subspecialists per capita.
This final model shows that after accounting for an individual’s health status and demographics, physician use for SLE is related to a complex mix of sociodemographic and health care system factors at the individual (including age, education, source of health insurance, HMO coverage, and specialty of main SLE physician), local community (whether an area meets criterion for concentrated poverty), and regional (the HSA) levels. Of note, the addition of local area level variables and the random effects for the HSA did not diminish the impact of the individual level characteristics, suggesting that the 2 sets independently contribute to rates of physician visits for persons with SLE.
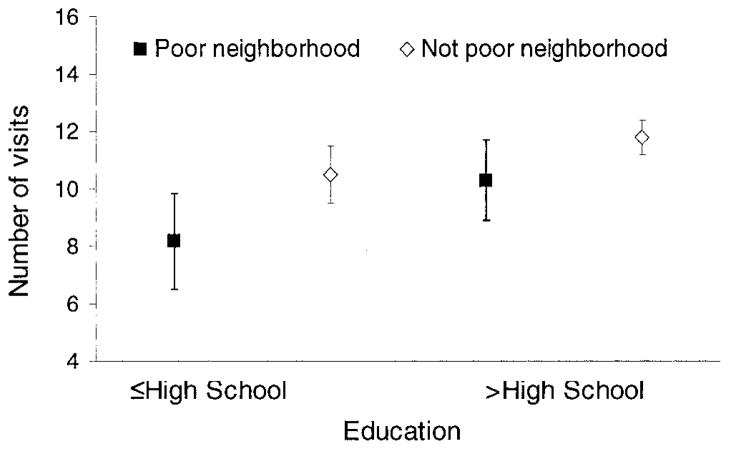
Figure 1 highlights the way that individual SES and community poverty combine to affect the number of physician visits for SLE after adjusting for covariates. Rates are significantly lower for persons with low levels of education living in areas of concentrated poverty compared with those with higher levels of education not living in areas of concentrated poverty. The number of visits to physicians for SLE is in between these 2 extremes for persons with low levels of education not living in areas of concentrated poverty and for those with higher levels of education living in such areas.
Figure 1.
Combined impact of individual and neighborhood socioeconomic status on number of physician visits for systemic lupus erythematosus, adjusting for covariates.
DISCUSSION
This study corroborates with other studies in showing that individual sociodemographic characteristics contribute to the number of physician visits for SLE in the year prior to interview. After adjustment, there are fewer visits with each increment in age, and almost 20% fewer for those participants with a high school education or less than for those with at least a college degree (10.1 versus 11.8 visits per year).
The first contribution of this study was to establish the substantial impact of the health care system itself on the number of physician visits for SLE. Persons receiving their care through an HMO have ~10% fewer physician visits than those seen in non-HMO settings (10.7 versus 11.8 visits). An even stronger effect occurred for the specialty of the main SLE physician, with persons with SLE seen by generalists reporting mean visits of 7.9 (95% CI 7.1– 8.7) for the condition in the 12 months prior to interview, compared with mean visits of 11.9 (95% CI 11.3–12.4) for those seen by rheumatologists, which is a difference of ~50% in relative terms even after taking individual demographics and health status into account.
Second, we found substantial effects of where one lives on the amount of care one receives for SLE. Therefore, even after adjusting for individual-level medical and sociodemographic characteristics, persons living in areas of concentrated poverty reported ~16% fewer physician visits for their SLE. There was also an effect of the HSAs on the number of physician visits for SLE beyond the effect of all other individual and community variables.
There are several ways to interpret the results indicating that community variables have effects beyond an individual’s own low SES on the quantity of physician visits. Communities with high concentrations of poverty may not provide the kinds of social networks that would allow access to specialists with experience in treating fairly rare conditions such as SLE. That such communities also often have high rates of crime may make it difficult to venture out to take advantage of the social networking opportunities that do exist. Alternatively, such communities may not be able to attract sufficient medical expertise to practice locally, and the primary care physicians that are there may be overburdened to the extent that they primarily provide basic care for common conditions.
The impact of community on access, whether due to residence in areas of concentrated poverty, particular HSAs, or both, may be unfortunate because it likely dovetails with problems of access to specialty care for SLE. In our study we found profound differences in the number of physician visits for SLE between those seen by generalist and specialist physicians. Moreover, there is accumulating evidence for rheumatic diseases in general (32–37) and for SLE that care and outcomes differ among levels of specialization (11,13), although part of the specialty effect may be due to experience rather than to training per se.
It is important to note that while population-based studies show differences in utilization across local areas, such differences may not reflect patient needs but rather local capacity for care (20). The evidence also suggests that higher utilization does not necessarily result in better outcomes or quality of care (38,39). The population-based findings are central to current health policy debate. However, the degree to which utilization beyond a certain level (not yet established empirically) benefits populations requiring ongoing specialty care for severe chronic conditions is still uncertain.
Our findings are limited by the use of self-report measurements for both the dependent and independent variables. The measure of SLE activity and our created proxy measure for SLE disease damage have not been psychometrically tested and may not fully adjust for disease activity or severity. A further limitation of this study is that the LOS data cohort is not a representative sample of people with SLE in the US. In particular, it has fewer people with very low SES and a higher proportion of people residing in highly urbanized areas, which may explain the lack of a significant effect of the area supply of subspecialists. However, the size and diversity of the LOS provides a broader picture of the SLE population compared with the typical SLE cohort based in an academic medical setting. While the LOS cohort is racially and ethnically diverse, it does not allow for stratified analyses of the various racial and ethnic groups.
Nonetheless, this study has established that there is a difference in the number of physician visits for SLE by SES, even after adjustment for other characteristics of the condition and the individual’s background. Readdressing this difference may be especially daunting when the individual lives in an area of concentrated poverty and/or an HSA with fewer resources. As several analysts have noted with respect to the efforts in Massachusetts to expand coverage for that state’s population, a policy that has been largely successful, it is difficult to secure equal access until the supply of health care providers grows to match the increased number with coverage (40 – 43). The problem exposed by this study of heightened access problems for those living in select areas is likely to be even more daunting since expertise to treat an uncommon, complex, and severe condition such as SLE is not as widely disseminated as primary care (6). As the era of increased medications approved specifically for SLE approaches, access to specialized expertise to treat the condition becomes even more important. The present study confirmed that the differences in the most basic measure of access, which is the number of physician visits for SLE over a year, are substantial and in part due to the individual’s background, as well as due to the specific nature of the community in which he or she resides.
In a prior study, we established that the community in which one resides affects outcomes in SLE independently of one’s own SES (8). In the present study we showed that the community also affects health care utilization, as measured by the number of physician visits for SLE. The immediate next step in this research agenda is to establish whether there are differences among communities in the quality of care for SLE (44) and then establish whether such differences in the quantity and quality of SLE care account for some of the outcome difference across communities already observed.
Acknowledgments
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants 5R01AR56476 and P60-AR0-53308), the Agency for Healthcare Research and Quality/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant 1-R01-HS0-13893), the Arthritis Foundation, and the State of California Lupus Fund.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Ms Tonner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Tonner, Trupin, Yazdany, Criswell, Katz, Yelin.
Acquisition of data. Tonner, Trupin, Yazdany, Criswell, Katz, Yelin.
Analysis and interpretation of data. Tonner, Trupin, Yazdany, Criswell, Katz, Yelin.
Because Drs. Katz and Yelin are Editors of Arthritis Care & Research, review of this article was handled by the Editor of Arthritis & Rheumatism.
References
- 1.Alarcon GS, McGwin G, Jr, Sanchez ML, Bastian HM, Fessler BJ, Friedman AW, et al. for the LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. XIV. Poverty, wealth, and their influence on disease activity. Arthritis Rheum. 2004;51:73–7. doi: 10.1002/art.20085. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon GS, McGwin G, Jr, Bastian HM, Roseman J, Lisse J, Fessler BJ, et al. for the LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. Arthritis Rheum. 2001;45:191–202. doi: 10.1002/1529-0131(200104)45:2<191::AID-ANR173>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon GS, McGwin G, Jr, Bartolucci AA, Roseman J, Lisse J, Fessler BJ, et al. for the LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum. 2001;44:2797– 806. doi: 10.1002/1529-0131(200112)44:12<2797::aid-art467>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Bastian HM, Roseman JM, McGwin G, Jr, Alarcon GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152– 60. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 5.Odutola J, Ward M. Ethnic and socioeconomic disparities in health among patients with rheumatic disease. Curr Opin Rheumatol. 2005;17:147–52. doi: 10.1097/01.bor.0000151403.18651.de. [DOI] [PubMed] [Google Scholar]
- 6.Demas K, Costenbader K. Disparities in lupus care and outcomes. Curr Opin Rheumatol. 2009;21:102–9. doi: 10.1097/BOR.0b013e328323daad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward MM. Avoidable hospitalizations in patients with systemic lupus erythematosus. Arthritis Rheum. 2008;59:162–8. doi: 10.1002/art.23346. [DOI] [PubMed] [Google Scholar]
- 8.Trupin L, Tonner MC, Yazdany J, Julian LJ, Criswell LA, Katz PP, et al. The role of neighborhood and individual socioeconomic status in outcomes of systemic lupus. J Rheumatol. 2008;35:1782– 8. [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke AE, Petri M, Manzi S, Isenberg DA, Gordon C, Senecal JL, et al. The systemic lupus erythematosus Trination Study: absence of a link between health resource use and health outcome. Rheumatology (Oxford) 2004;43:1016–24. doi: 10.1093/rheumatology/keh229. [DOI] [PubMed] [Google Scholar]
- 10.Ward M. Hospital experience and mortality in patients with systemic lupus erythematosus: which patients benefit most from treatment at highly experienced hospitals? J Rheumatol. 2002;29:1198–206. [PubMed] [Google Scholar]
- 11.Ward M. Association between physician volume and in-hospital mortality in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:1646–54. doi: 10.1002/art.21053. [DOI] [PubMed] [Google Scholar]
- 12.Katz J, Barrett J, Liang M, Kaplan H, Roberts W, Baron J. Utilization of rheumatology physician services by the elderly. Am J Med. 1998;105:312– 8. doi: 10.1016/s0002-9343(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 13.Yazdany J, Gillis JZ, Trupin L, Katz P, Panopalis P, Criswell LA, et al. Association of socioeconomic and demographic factors with utilization of rheumatology subspecialty care in systemic lupus erythematosus. Arthritis Rheum. 2007;57:593–600. doi: 10.1002/art.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillis JZ, Yazdany J, Trupin L, Julian L, Panopalis P, Criswell LA, et al. Medicaid and access to care among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57:601–7. doi: 10.1002/art.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yelin E, Trupin L, Katz P, Criswell LA, Yazdany J, Gillis J, et al. Impact of health maintenance organizations and fee-for-service on health care utilization among people with systemic lupus erythematosus. Arthritis Rheum. 2007;57:508–15. doi: 10.1002/art.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wennberg J. Practice variations and health care reform: connecting the dots. Health Aff (Millwood) 2004;(Suppl Web Exclusives):VAR140–4. doi: 10.1377/hlthaff.var.140. [DOI] [PubMed] [Google Scholar]
- 17.Fisher E, Bynum J, Skinner J. Slowing the growth of health care costs: lessons from regional variation. N Engl J Med. 2009;360:849–52. doi: 10.1056/NEJMp0809794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gawande A. The cost conundrum: what a Texas town can teach us about health care. The New Yorker. 2009 URL: http://www.newyorker.com/reporting/2009/06/01/090601fa_fact_gawande.
- 19.Sirovich B, Gottlieb D, Welch H, Fisher E. Regional variations in health care intensity and physician perceptions of quality of care. Ann Intern Med. 2006;144:641–9. doi: 10.7326/0003-4819-144-9-200605020-00007. [DOI] [PubMed] [Google Scholar]
- 20.Goodman D, Fisher E, Bronner K. Health care costs continue to rise as quality lags: where is the money spent? Lebanon (NH): Dartmouth Institute for Health Policy and Clinical Practice; 2009. [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg MC for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 23.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57:56– 63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services, Office of Research and Planning, and Bureau of Health Professionals. Area resource file. Rockville (MD): Bureau of Health Professionals; 2001. [Google Scholar]
- 25.Dartmouth Medical School. The Dartmouth atlas of health care in the United States. Chicago: American Hospital Publishing; 1999. [Google Scholar]
- 26.Bishaw A. Areas with concentrated poverty: 1999. Washington (DC): US Census Bureau; 2005. [Google Scholar]
- 27.Cohen JW, Monheit AC, Beauregard KM, Cohen SB, Lefkowitz DC, Potter DE, et al. The Medical Expenditures Panel Survey: a national information resource. Inquiry. 1996;33:373– 89. [PubMed] [Google Scholar]
- 28.Kerwin J, Cantor D, Sheridan S. Results of rounds 3 and 4 of managed care cognitive interviews for the household portion of MEPS: report to AHCPR from Westat. Rockville (MD): Westat; 1995. [Google Scholar]
- 29.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385– 401. [Google Scholar]
- 30.Ward M. The rating scale preference measure as an evaluative measure in systemic lupus erythematosus. Lupus. 2000;9:696–701. doi: 10.1191/096120300672399292. [DOI] [PubMed] [Google Scholar]
- 31.Duan N, Manning W, Morris C, Newhouse JP. A comparison of alternative models for the demand for medical care. J Bus Econ Stat. 1983;1:115–26. [Google Scholar]
- 32.Yelin E, Such C, Criswell L, Epstein W. Outcomes for persons with rheumatoid arthritis with a rheumatologist and non-rheumatologist as the main physician for this condition. Med Care. 1998;36:513–22. doi: 10.1097/00005650-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Katz JN, Solomon DH, Schaffer JL, Horsky J, Burdick E, Bates DW. Outcomes of care and resource utilization among patients with knee and shoulder disorders treated by general internists, rheumatologists, or orthopedic surgeons. Am J Med. 2000;108:28–35. doi: 10.1016/s0002-9343(99)00313-7. [DOI] [PubMed] [Google Scholar]
- 34.Solomon DH, Bates DW, Panush RS, Katz JN. Costs, outcomes, and patient satisfaction by provider type for patients with rheumatic and musculoskeletal conditions: a critical review of the literature and proposed methodological standards. Ann Intern Med. 1997;127:52– 60. doi: 10.7326/0003-4819-127-1-199707010-00009. [DOI] [PubMed] [Google Scholar]
- 35.Ward M, Leigh J, Fries J. Progression of functional disability in patients with rheumatoid arthritis: associations with rheumatology subspecialty care. Arch Intern Med. 1993;153:2229–37. [PubMed] [Google Scholar]
- 36.MacLean CH, Louie R, Leake B, McCaffrey DF, Paulus HE, Brook RH, et al. Quality of care for patients with rheumatoid arthritis? JAMA. 2000;284:984–92. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 37.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57:928–34. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 38.Fisher E, Wennberg J. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69–79. doi: 10.1353/pbm.2003.0004. [DOI] [PubMed] [Google Scholar]
- 39.Wennberg J, Bronner K, Skinner J, Fisher E, Goodman D. Inpatient care intensity and patients’ ratings of their hospital experience. Health Aff (Millwood) 2009;28:103–12. doi: 10.1377/hlthaff.28.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holahan J, Blumberg L. Massachusetts health care reform: a look at the issues. Health Aff (Millwood) 2006;25:w432– 43. doi: 10.1377/hlthaff.25.w432. [DOI] [PubMed] [Google Scholar]
- 41.Long S, Masi P. Access and affordability: an update on health reform in Massachusetts, fall 2008. Health Aff (Millwood) 2008;28:w578– 87. doi: 10.1377/hlthaff.28.4.w578. [DOI] [PubMed] [Google Scholar]
- 42.Kingsdale J. Implementing health care reform in Massachusetts: strategic lessons learned. Health Aff (Millwood) 2009;28:w588–94. doi: 10.1377/hlthaff.28.4.w588. [DOI] [PubMed] [Google Scholar]
- 43.Brennan T, Mello M. Incremental health care reform. JAMA. 2009;301:1814– 6. doi: 10.1001/jama.2009.610. [DOI] [PubMed] [Google Scholar]
- 44.Yazdany J, Panopalis P, Gillis JZ, Schmajuk G, MacLean CH, Wofsy D, et al. the Systemic Lupus Erythematosus Quality Indicators Project Expert Panels. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum. 2009;61:370–7. doi: 10.1002/art.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]