Abstract
OBJECTIVE:
It is essential to identify a serological marker of injury in order to study the pathophysiology of intestinal ischemia reperfusion. In this work, we studied the evolution of several serological markers after intestinal ischemia reperfusion injury in rats. The markers of non-specific cell damage were aspartate aminotransferase, alanine aminotransaminase, and lactic dehydrogenase, the markers of inflammation were tumor necrosis factor alpha, interleukin-6, and interleukin-1 beta, and the markers of intestinal mucosal damage were intestinal fatty acid binding protein and D-lactate. We used Chiús classification to grade the histopathological damage.
METHODS:
We studied 35 Wistar rats divided into groups according to reperfusion time. The superior mesenteric artery was clamped for 30 minutes, and blood and biopsies were collected at 1, 3, 6, 12, 24, and 48 hours after reperfusion. We plotted the mean ± standard deviation and compared the baseline and maximum values for each marker using Student's t-test.
RESULTS:
The maximum values of interleukin-1 beta and lactic dehydrogenase were present before the maximal histopathological damage. The maximum tumor necrosis factor alpha and D-lactate expressions coincided with histopathological damage. Alanine aminotransaminase and aspartate aminotransferase had a maximum expression level that increased following the histopathological damage. The maximum expressions of interluken-6 and intestinal fatty acid binding protein were not significantly different from the Sham treated group.
CONCLUSION:
For the evaluation of injury secondary to acute intestinal ischemia reperfusion with a 30 minute ischemia period, we recommend performing histopathological grading, quantification of D-lactate, which is synthesized by intestinal bacteria and is considered an indicator of mucosal injury, and quantification of tumor necrosis factor alpha as indicators of acute inflammation three hours after reperfusion.
Keywords: Ischemia/Reperfusion Injury, Intestine, Interleukin-1 Beta, Tumor Necrosis Factor-Alpha, Lactate Dehydrogenase, Chiu Score, D-Lactate
INTRODUCTION
Intestinal ischemia reperfusion (IR), or transient hypoxia followed by re-oxygenation, is a component of the pathogenesis of all diseases that involve a reduction or redistribution of bowel blood flow, including shock, neonatal necrotizing enterocolitis, intestinal transplant, and mesenteric ischemia. As such, IR can complicate surgical procedures in the elderly and for open-heart surgery patients (1–3). In clinical practice, symptoms are often vague, making a definitive diagnosis of acute intestinal ischemia notoriously difficult (4). When accompanied by clinical signs of peritonitis, this usually indicates necrosis of the intestinal wall, and the suspicion of intestinal ischemia is traditionally confirmed by laparotomy (5).
In humans, the systematic search for serological markers of intestinal ischemia to optimize early diagnosis has identified molecules such as D-lactate (6), glutathione S-transferase (GST) (7), and intestinal fatty acid binding protein (iFABP) (8,9). However, a combination of markers, or a “diagnostic intestinal ischemia panel,” reflecting different aspects of bowel viability has not been identified (10). The temporal association between IR and serum markers is also not well understood. The identification of a set of serum markers (SM) that could detect or predict the outcome of an ischemic insult would be essential for studying the pathophysiology of IR.
Numerous publications have reported the effects of drugs and therapeutic modalities on IR injury based on histological damage (11,12) and alterations of serum levels of molecules that are associated with cell damage, endothelial activation, inflammation, and oxidative stress (13–15). However, the evaluation times are often fixed or arbitrary, and the temporal dynamics of histological and serological injury markers remain obscure. Nonetheless, in most studies of experimental IR, histopathological examination is the gold standard. In 1970, Chiu reported a graded scale of damage produced by ischemia in the intestinal mucosa based on hematoxylin and eosin staining (12). Later, Parks and Sonnino reported scores, and Park reported a modification to the original Chiu score (16). The Chiu and Chiu/Parks classifications are currently recommended for comparing results reported in the literature (11,17).
In this article, we were interested in identifying combinations of serum and histopathological studies at different time points. Therefore, we assessed the evolution of histopathological injury and serum markers of non-specific cell damage: aspartate aminotransferase (AST), alanine aminotransaminase (ALT), and lactic dehydrogenase (LDH), of acute inflammation: tumor necrosis factor alpha (TNF-alpha), interleukin 6 (IL-6), and interleukin-1 beta (IL-1), and of intestinal mucosa damage: intestinal fatty acid binding protein (iFABP) and D-lactate during reperfusion.
MATERIAL AND METHODS
Animals procedures
The animal procedures were performed in accordance with the proper use and care of laboratory animals. The experiments were performed on 35 female Wistar rats weighing 200–250 g. The animals were maintained under standard conditions, including stable room temperature, a 12:12-h light/dark cycle, and access to commercial rat pellets and water ad libitum. The ethics committee of our institution approved this study.
Animal model
In brief, after 12 hours of fasting and anesthesia with a 40 mg/kg intraperitoneal injection (IP) of pentobarbital sodium (Anestesal; Pfizer, Mexico City, Mexico), a midline laparotomy was performed, and ischemia was induced by occlusion of the superior mesenteric artery with vascular clamps. An absent pulse was visually verified in the mesenteric vessels with a brown discoloration of the intestine. After 30 minutes of ischemia, blood flow was restored, and the abdominal wall was sutured.
The animals were divided into 7 groups (n = 5) and were named according to the reperfusion time: Sham group or negative control, which underwent surgery without arterial occlusion, and groups with 1 hour, 3 hours, 6 hours, 12 hours, 24 hours, and 48 hours of reperfusion.
Depending on the group, at the end of the reperfusion time, the rats were anesthetized, the sutures were removed, blood samples were obtained from the aorta, and full-bowel wall samples of the ileum, 15 cm from the ileocecal junction, were obtained. The animals were then euthanized by exsanguination.
Morphological examination
Intestinal biopsies were fixed in 10% formalin, processed with conventional histological techniques, and stained with hematoxylin and eosin. To assess mucosal injury, the Chiu classification was used (6). The evaluation scale was from 0 to 5, where 0 is a normal intestinal mucosa, 1 is the development of Gruenhagen subepithelial spaces, 2 is the extension of the subepithelial space with moderate lifting of the lamina propria, 3 is the expansion of epithelial lifting with destruction of the tips of villi, 4 is destroyed villi with exposure of the lamina propria and dilated capillaries, and 5 is the disintegration of the lamina propria, hemorrhage, and ulceration.
Serum determinations
After exsanguinating the animal, at least 3 mL of blood was collected and allowed to clot, and the serum was separated by centrifugation and stored at −70°C until further study.
The serum levels of TNF-alpha were determined using a Rat TNF-alpha enzyme-linked immunosorbent assay (ELISA) kit (Peprotech, Rocky Hill, NJ, USA). Serum levels of IL-1 were determined using a Rat IL-1 beta ELISA kit (Peprotech). Serum levels of IL-6 were determined using a Rat IL-6 ELISA kit (Peprotech). The serum levels of iFABP were determined using a Rat iFABP ELISA kit (Biotang, Waltham, MA, USA). The serum levels of D-lactate were determined using a EnzyChrom™ D-Lactate Assay Kit (BioAssay Sytems, Hayward, CA, USA).
The serum levels of AST, ALT and LDH activity were determined by reflectance on a Vitros DT60II System Ortho Clinical Diagnostics by Johnson & Johnson (Rochester, New York, USA).
Statistical analysis
Using SPSS V15 software, we plotted the time variations in the parameters studied, identified the peaks (Pmax) for each parameter and compared them against the baseline (Sham) with t tests for two independent samples. A value of p<0.05 was considered to be statistically significant.
RESULTS
Histopathological changes
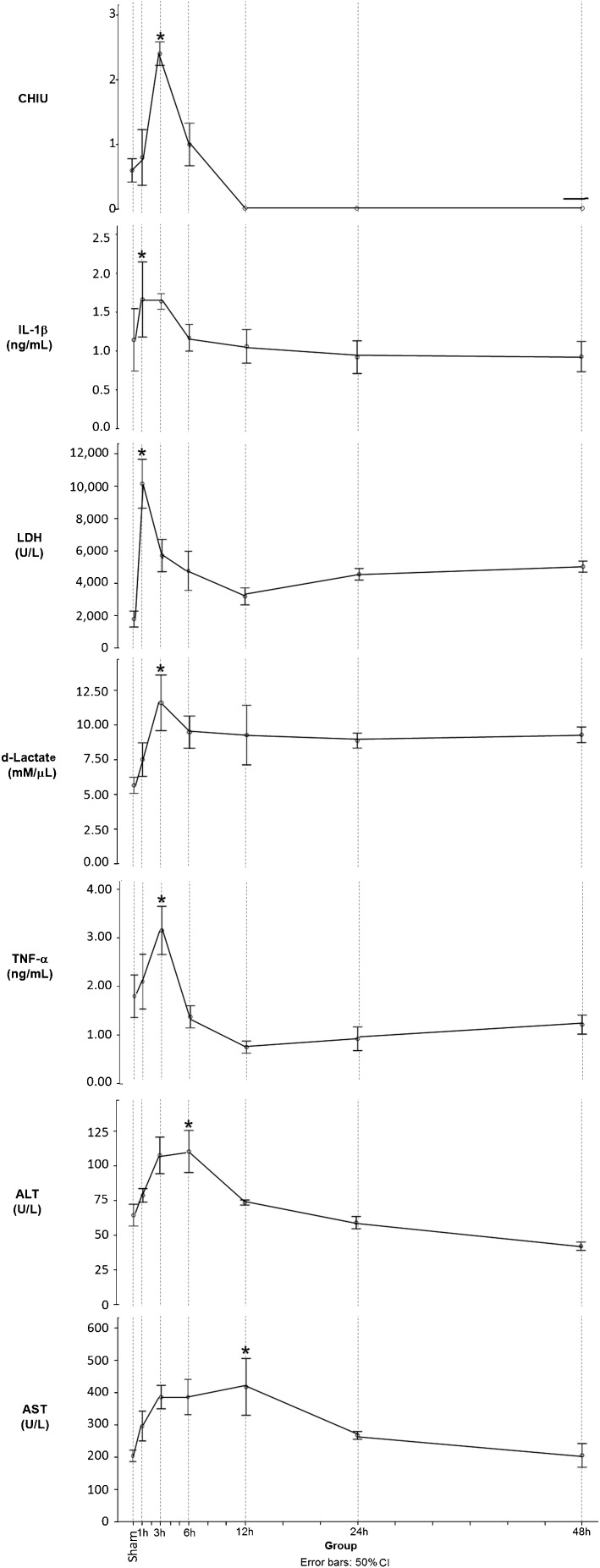
The histomorphological damage to the intestinal epithelium according to the Chiu scale was evident in our model at one hour, was maximal at three hours, and returned to baseline levels after 12 hours of reperfusion. The damage was undetectable at 24 and 48 hours (Figure 1).
Figure 1.
Temporal evolution of serum marker levels and histological damage at different times of reperfusion. Chiu: histopathological damage scored according to Chiu, LDH: lactic dehydrogenase, IL-1: interleukin-1 beta, AST: aspartate aminotransferase, ALT: alanine aminotransaminase, TNF-a: tumor necrosis factor alpha. *p<0.05 versus Sham.
Non-specific markers of cell damage
Of the non-specific markers of cell damage, LDH peaked at one hour of reperfusion, AST peaked at 6 hours and ALT peaked at 12 hours. The latter two showed a significant elevation at 3 hours (Figure 1).
TNF-alpha, IL-1beta, IL-6, iFABP and D-lactate
The markers whose levels coincided with the peak of injury (Pmax) were TNF-alpha and IL-1beta, which peaked at one hour and remained elevated until 3 hours. No differences were observed at 6 hours compared to the control group. Serum D-lactate did not decrease after reaching its maximum at 3 hours (Figure 1). The levels of IL-6 peaked at 12 hours of reperfusion but did not differ from the controls. The iFABP concentration did not significantly differ from the controls at any time.
Significant differences were documented between the sham group and Pmax in injury scores, non-specific injury markers (LDH, AST and ALT), the inflammatory markers TNF-alpha and IL-1beta, and the specific injury marker D-lactate. There were no significant differences in the concentrations of iFABP or IL-6 (Table 1).
Table 1.
Serum Markers and Cell Damage Sham group vs pMax.
| Sham X±DE | (pMax) X±DE | p-value** | |
| Chiu | 0.6±0.54 | 2.40±0.54 | 0.001 |
| LDH | 1129.00±190.08 | 10174.00±4558.73 | 0.01 |
| AST | 204.60±54.66 | 301.75±72.08 | 0.05 |
| ALT | 54.50±10.21 | 110.40±45.9 | 0.05 |
| IL-1 beta | 0.64±0.52 | 1.63±0.30 | 0.009 |
| IL-6 | 3.65±1.19 | 5.30±2.97 | 0.28 |
| TNF-alpha | 1.23±0.23 | 3.17±1.49 | 0.04 |
| iFABP | 21.50±5.95 | 24.10±4.53 | 0.46 |
| D-lactate | 5.25±1.70 | 8.92±0.86 | 0.009 |
Chiu: histopathological damage scored according to Chiu. LDH: lactic dehydrogenase in U/L. AST: aspartate aminotransferase in U/L. ALT: alanine aminotransaminase in U/L. IL-1 beta: interleukin-1 beta in ng/mL, IL-6: interleukin-6 in ng/mL, TNF-alpha: tumor necrosis factor alpha in ng/mL, D-lactate in mM/mcgL, iFABP: intestinal fatty acid binding protein in pg/mL. P-value in independent t tests.
DISCUSSION
The intestinal mucosa is particularly sensitive to IR injury. We observed severe mucosal injury beginning at 1 hour of perfusion, reaching maximal severity at 3 hours and subsiding at 24 hours. This is consistent with other reports that morphological intestinal repair begins shortly after 3 hours of reperfusion and is complete around 24 hours (18–20). During reperfusion, the cascade of events that seeks to limit damage and maintain homeostasis involves all the components of the digestive tract: mucous cells, fibroblasts, neurons, endothelial cells and the immune system (21). Previous studies have correlated the duration of ischemia with histological damage to the intestinal mucosa (22). At 30 minutes of ischemia, cellular reserves of oxygen are consumed (12), and the mucosa loses its ability to accumulate sugars and amino acids against a concentration gradient (23). Histological lesions secondary to ischemia are exacerbated by reperfusion. The period of time of ischemia, the type of ischemia (arterial, venous, arterial and venous) (24), and the time to reperfusion all determine the severity of injury.
The concentrations of LDH and IL-1 increased before the lesion was identified histopathologically. LDH is a non-specific marker of cell injury and has been associated with ischemic events in the intestine (25), while interleukin-1 beta is considered a marker of acute inflammation, resulting in the activation of macrophages during early stages of tissue injury. Serum levels of LDH, but not IL-1beta, have been shown to remain elevated 72 hours after intestinal IR in rats (26). Peak serum concentrations of D-lactate and TNF-alpha coincided with histopathological injury. TNF-alpha, in concordance with previous reports (24), increased upon injury. Both TNF and IL-1 are essential in the physiopathology of IR and are widely used in studies evaluating intestinal IR injury (27). Additionally, the intestine has been identified as a source of TNF during IR-mediated damage (28). However, these are non-specific markers of inflammation and systemic injury, which makes their diagnostic utility questionable.
Intestinal gut barrier dysfunction is an important aspect of the physiopathology of IR injury. Intestinal bacteria produce D-lactate, and its presence in serum reflects a major failure of the mucosal barrier (25). In a systematic literature review of clinical studies, D-lactate was indeed found to be one of the most well studied and reliable biomarkers of intestinal ischemia (10). However, in a rat model of gut IR induced by aortic clamping, D-lactate was not elevated in the serum after 1 hour of reperfusion following 40 minutes of ischemia, consistent with our results (29). We did find that D-lactate was elevated at 3 hours, which coincided with severe mucosal injury and, presumably, gut barrier dysfunction. This suggests that D-lactate sensitivity is time-dependent and correlates to structural mucosal injury.
Interestingly, D-lactate remained elevated at 48 hours. This could indicate prolonged functional impairment and bacterial translocation independent of mucosal morphological integrity. Support for this idea comes from previous studies that showed elevated D-lactate at 48 hours of reperfusion and correlated to bacteremia following 1 hour of intestinal ischemia in rats (30). Human studies have confirmed persistent elevations of D-lactate 48 hours after reperfusion during colonic ischemia (31). The temporal dynamics of tight-junction alterations in the intestinal mucosa, thought to be a basis for barrier dysfunction after IR (32), could partly explain this result. Additionally, experimental studies in primates have shown that serum D-lactate levels can predict mortality in models of hemorrhagic shock (33). This adds to the possible clinical value of D-lactate as a biomarker in settings of intestinal IR.
Another specific mucosal injury marker, iFABP (25), was unrelated to histopathology during the acute phase in our study. Rat models of acute intestinal IR have shown elevations of iFABP early in the course of intestinal mucosal injury (34). Clinical studies have documented an elevation of iFABP as a specific marker for mucosal injury. Although the source of iFABP is assumed to be the cells of the intestinal mucosa, its utility is based on the identification of a chronic process of local lesions (8,9,25). However, recent clinical studies have found elevations of iFABP during acute intestinal necrosis (35). Not all studies have been consistent: iFABP was not correlated to intestinal IR at any time point (1, 3 and 6 hours reperfusion) in a recent study in rabbits (36). Together with our results, this suggests that the role of iFABP as a biomarker for intestinal ischemia remains unclear.
We identified ALT and AST as molecules that remained elevated even after histopathological injury had subsided, peaking at 6 and 24 hours post-ischemia. A previous study showed that AST and LDH elevations occurred early after IR, but no ALT elevations were evident at 120 minutes of reperfusion (37). This suggested that the origin of AST and LDH was indeed the intestine and not the liver. In our study, we also found delayed ALT elevations. During reperfusion, peripheral organs, such as the lung and liver, displayed injury as a result of polymorphonuclear leukocyte infiltration and the release of inflammatory mediators into the bloodstream (38). Liver and lung injury indeed occur after intestinal IR, and the elevation of these enzymes appears to reflect remote organ damage (39).
The use of fixed time models of intestinal IR injury using serum markers as surrogates for histopathological analysis should be discouraged. Both serum markers and histology are important in grading the severity of IR injury. Additionally, the chosen markers should ideally coincide with mucosal injury. Studying the changes in concentration of these markers during experimentation will allow for a better understanding of the process. When planning a study on IR injury, it is essential to identify the times at which measurements will be carried out, as this could reflect different aspects of the physiopathology.
Our study has several limitations. We only used one ischemia time (30 minutes), making it impossible to directly establish correlations between the serum markers and local or systemic injury. We used a sublethal model of intestinal IR and could not make associations with mortality. We did not directly measure for mucosal barrier permeability, and we did not carry out immunohistochemical or ultrastructural investigations of the injured mucosa. These are areas of future research.
In conclusion, AST, LDH, IL-1 and TNF-alpha are elevated early after reperfusion, and in the case of TNF-alpha, correlate well with maximal mucosal injury. However, they are non-specific markers of tissue injury (AST, LDH) or part of the systemic inflammatory response (IL-1, TNF-alpha). Their utility as diagnostic tools is thus limited, but they could reflect injury severity. We were surprised not to find changes in IL-6 levels considering the evidence of its involvement in IR injury (26–40). D-lactate is a more promising clinical biomarker of intestinal injury and could be used to evaluate functional (barrier) alterations. It coincided with maximal injury in our study and remained elevated after 48 hours. However, D-lactate was not elevated in the hyperacute phase of intestinal ischemia (1 hour of reperfusion). The specific roles of other markers, such as iFABP, coagulation parameters (41), oxidative stress (42), and adhesion molecules (40), requires further investigation.
ACKNOWLEDGMENTS
We would like to thank Gilberto Arevalo Martinez, MVZ, for providing the animals and ensuring their care. We would also like to thank the staff at the Liver Unit, UANL for management of the serum samples. The project was financed by the Department of Physiology of the School of Medicine, the Liver Unit and The Pathology and Cytopathology Service of the “Dr. José Eleuterio González” University Hospital, Universidad Autónoma de Nuevo León. This work was supported by the Programa de Apoyo a la Investigación Científica y Tecnológica of UANL (PAICYT-UANL: SA339-10).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Venkateswaran RV, Charman SC, Goddard M, Large SR. Lethal mesenteric ischaemia after cardiopulmonary bypass: A common complication. Eur J Cardiothorac Surg. 2002;22(4):534–8. doi: 10.1016/s1010-7940(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 2.Schutz A, Eichinger W, Breuer M, Gansera B, Kemkes BM. Acute mesenteric ischemia after open heart surgery. Angiology. 1998;49(4):267–73. doi: 10.1177/000331979804900404. [DOI] [PubMed] [Google Scholar]
- 3.Acosta-Merida MA, Marchena-Gomez J, Cruz-Benavides F, Hernandez-Navarro J, Roque-Castellano C, Rodriguez-Mendez A, et al. Factores predictivos de necrosis masiva intestinal en la isquemia mesentérica aguda. Cir Esp. 2007;81(3):144–9. doi: 10.1016/s0009-739x(07)71286-1. [DOI] [PubMed] [Google Scholar]
- 4.Van der Voort PH. The incomplete puzzle of vasoactive medication in (abdominal) sepsis. Crit Care Med. 2006;34(5):1565–6. doi: 10.1097/01.CCM.0000216187.00379.12. [DOI] [PubMed] [Google Scholar]
- 5.Woo K, Mayor K, Kohanzadeh S, Allins AD. Laparotomy for visceral ischemia and gangrene. Am Surg. 2007;73(10):1006–8. [PubMed] [Google Scholar]
- 6.Garcia J, Smith FR, Cucinell SA. Urinary D-lactate excretion in infants with necrotizing enterocolitis. J Pediatr. 1984;104(2):268–70. doi: 10.1016/s0022-3476(84)81010-0. [DOI] [PubMed] [Google Scholar]
- 7.Khurana S, Corbally MT, Manning F, Armenise T, Kierce B, Kilty C. Glutathione S-transferase: a potential new marker of intestinal ischemia. J Pediatr Surg. 2002;37(11):1543–8. doi: 10.1053/jpsu.2002.36181. [DOI] [PubMed] [Google Scholar]
- 8.Mannoia K, Boskovic DS, Slater L, Plank MS, Angeles DM, Gollin G. Necrotizing enterocolitis is associated with neonatal intestinal injury. J Pediatr Surg. 2011;46(1):81–5. doi: 10.1016/j.jpedsurg.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 9.Edelson MB, Sonnino RE, Bagwell CE, Lieberman JM, Marks WH, Rozycki HJ. Plasma intestinal fatty acid binding protein in neonates with necrotizing enterocolitis: a pilot study. J Pediatr Surg. 1999;34(10):1453–7. doi: 10.1016/s0022-3468(99)90102-1. [DOI] [PubMed] [Google Scholar]
- 10.Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg. 2009;33(7):1374–83. doi: 10.1007/s00268-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 11.Quaedackers JS, Beuk RJ, Bennet L, Charlton A, oude Egbrink MG, Gunn AJ, et al. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the emall bowel. Transplantation Proceedings. 2000;32(6):1307–10. doi: 10.1016/s0041-1345(00)01238-0. [DOI] [PubMed] [Google Scholar]
- 12.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. Arch Surg. 1970;101(4):478–83. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 13.Block T, Nilsson TK, Björck M, Acosta S. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Invest. 2008;68(3):242–8. doi: 10.1080/00365510701646264. [DOI] [PubMed] [Google Scholar]
- 14.El-Awady SI, El-Nagar M, El-Dakar M, Ragab M, Elnady G. Bacterial translocation in an experimental intestinal obstruction model. C-reactive protein reliability. Acta Cir Bras. 2009;24(2):98–106. doi: 10.1590/s0102-86502009000200005. [DOI] [PubMed] [Google Scholar]
- 15.Kurimoto Y, Kawaharada N, Ito T, Morikawa M, Higami T, Asai Y. An experimental evaluation of the lactate concentration following mesenteric ischemia. Surgery Today. 2010;38(10):926–30. doi: 10.1007/s00595-007-3737-8. [DOI] [PubMed] [Google Scholar]
- 16.Park PO, Haglung U, Bulkley GB, Fâlt K. The secuence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107(5):574–80. [PubMed] [Google Scholar]
- 17.Oltean M, Olausson M. The Chiu/Park scale for grading intestinal ischemia-reperfusion: if it ain`t broke don`t fix it! Intensive Care Med. 2010;36(6):1095. doi: 10.1007/s00134-010-1811-y. [DOI] [PubMed] [Google Scholar]
- 18.Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, et al. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11(35):5485–91. doi: 10.3748/wjg.v11.i35.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone WC, Bjorling DE, Southard JH, Galbreath EJ, Lindsay WA. Evaluation of intestinal villus height in rats after ischemia and reperfusion by administration of superoxide dismutase, polyethylene glycol-conjugated superoxide dismutase, and two 21-aminosteroids. Am J Vet Res. 1992;53(11):2153–6. [PubMed] [Google Scholar]
- 20.Illyés G, Hamar J. Sequence of morphological alterations in a small intestinal ischaemia/reperfusion model of the anesthetized rat. A light microscopy study. Int J Exp Pathol. 1992;73(2):161–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Gastrointest Liver Physiol. Am J Physiol. 1997;273(4 Pt 1):G769–75. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- 22.Haglund U. Experimental Shock and Gastronintestinal Involmement. In: Jensen S L, editor. Essentials of Esperimental Surgery. Amsterdam: Harwood academic publishers; 1996. pp. 33/1–33/10. 1a ed. [Google Scholar]
- 23.Archer SY, Hodin RA. Intestinal Regeneration and Adaptation Models. In: Wilmore D W, Souba W W, editors. Surgical Research. San Diego: Academic Press; 2001. pp. 557–71. [Google Scholar]
- 24.Guzman-de la Garza FJ, Camara-Lemaroy CR, Alargon-Galvan G, Cordero-Perez P, Muñoz-Espinosa LE, Fernandez-Garza NE. Different patterns of intestinal response to injury after arterial, venous or arteriovenous occlusion in rats. World J Gastroenterol. 2009;15(31):3901–7. doi: 10.3748/wjg.15.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Noord D, Mensink PB, de Knegt RJ, Ouwendijk M, Francke, van Vuuren AJ, et al. Serum markers and instestinal mucosal injury in chronic gastrointestinal ischemia. Dig Dis Sci. 2011;56(2):506–12. doi: 10.1007/s10620-010-1303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hei ZQ, Gan XL, Huang PJ, Wei J, Shen N, Gao WL. Influence of Ketotifen, Cromolyn Sodium, and Compound 48/80 on the survival rates after intestinal ischemia reperfusion injury in rats. BMC Gastroenterol. 2008;8:42. doi: 10.1186/1471-230X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto S, Tanabe M, Wakabayashi G, Shimazu M, Matsumoto K, Kitajima M. The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res. 2001;99(1):134–41. doi: 10.1006/jsre.2001.6106. [DOI] [PubMed] [Google Scholar]
- 28.Grotz MR, Deitch EA, Ding J, Xu D, Huang Q, Regel G. Intestinal cytokine response after gut ischemia. Role of gut barrier failure. Ann Surg. 1999;229(4):478–86. doi: 10.1097/00000658-199904000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collange O, Tamion F, Chanel S, Hue G, Richard V, Thuilliez C, et al. D-lactate is not a reliable marker of gut ischemia-reperfusion in a rat model of supraceliac aortic clamping. Crit Care Med. 2006;34(5):1415–9. doi: 10.1097/01.CCM.0000214517.24064.35. [DOI] [PubMed] [Google Scholar]
- 30.Wu GH, Wang H, Zhang YW, Wu ZH, Wu ZG. Glutamine supplemented parenteral nutrition prevents intestinal ischemia- reperfusion injury in rats. World J Gastroenterol. 2004;10(17):2592–4. doi: 10.3748/wjg.v10.i17.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assadian A, Assadian O, Senekowitsch C, Rotter R, Bahrami S, Fürst W, et al. Plasma D-lactate as a potential early marker for colon ischaemia after open aortic reconstruction. Eur J Vasc Endovasc Surg. 2006;31(5):470–4. doi: 10.1016/j.ejvs.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Inoue K, Oyamada M, Mitsufuji S, Okanoue T, Takamatsu T. Different changes in the expression of multiple kinds of tight-junction proteins during ischemia-reperfusion injury of the rat ileum. Acta Histochem Cytochem. 2006;39(2):35–45. doi: 10.1267/ahc.05048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobhian B, Kröpfl A, Hölzenbein T, Khadem A, Redl H, Bahrami S. Increased circulating D-lactate levels predict risk of mortality after hemorrhage and surgical trauma in baboons. Shock. 2012;37(5):473–7. doi: 10.1097/SHK.0b013e318249cb96. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman JM, Sacchettini J, Marks C, Marks WH. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery. 1997;121(3):335–42. doi: 10.1016/s0039-6060(97)90363-9. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen Windsant IC, Hellenthal FA, Derikx JP, Prins MH, Buurman WA, Jacobs MJ, et al. Circulating Intestinal Fatty Acid-Binding Protein as an Early Marker of Intestinal Necrosis After Aortic Surgery: A Prospective Observational Cohort Study. Ann Surg. 2012;255(4):796–803. doi: 10.1097/SLA.0b013e31824b1e16. [DOI] [PubMed] [Google Scholar]
- 36.Dundar ZD, Cander B, Gul M, Karabulut KU, Kocak S, Girisgin S, et al. Serum intestinal fatty acid binding protein and phosphate levels in the diagnosis of acute intestinal ischemia: an experimental study in rabbits. J Emerg Med. 2012;42(6):741–7. doi: 10.1016/j.jemermed.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Caglayan F, Caglayan O, Gunel E, Elcuman Y, Cakmak M. Intestinal ischemia-reperfusion and plasma enzyme levels. Pediatr Surg Int. 2002;18(4):255–7. doi: 10.1007/s003830100666. [DOI] [PubMed] [Google Scholar]
- 38.Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Neutrophil and nonneutrophil mediated injury in intestinal ischemia reperfusion. Ann Surg. 1993;218(4):444–54. doi: 10.1097/00000658-199310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cámara-Lemarroy CR, Guzmán-de la Garza FJ, Alarcón-Galván G, Cordero-Pérez P, Muñoz-Espinosa LE, Fernández-Garza NE. Effects of thalidomide and pentoxyphylline over local and remote organ injury after intestinal ischemia/reperfusion. Transplant Proc. 2010;42(5):1624–6. doi: 10.1016/j.transproceed.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 40.Braun F, Hosseini M, Wieland E, Sattler B, Müller AR, Fändrich F, et al. Kinetics and localization of interleukin-2, interleukin-6, heat shock protein 70, and interferon gamma during intestinal-rerfusion injury. Transplant Proc. 2004;36(2):267–9. doi: 10.1016/j.transproceed.2004.01.082. [DOI] [PubMed] [Google Scholar]
- 41.Guzman-de la Garza FJ, Camara-Lemaroy CR, Ballesteros-Elizondo RG, Alarcon-Galvan G, Cordero Perez P, Fernandez- Garza NE. Ketamine and the Myenteric Plexus in Intestinal Ischemia/Reparfusion Injury. Dig Dis Sci. 2010;55(7):1878–85. doi: 10.1007/s10620-009-0976-0. [DOI] [PubMed] [Google Scholar]
- 42.Li Ch, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2001;282(2):C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]