Abstract
Context
Women diagnosed with ovarian cancer are at risk for reduced quality of life (QOL). It is imperative to further define these declines to interpret treatment outcomes and design appropriate clinical interventions.
Objectives
The primary objective of this study was to compare data obtained from ovarian cancer patients with normative data to assess the degree to which QOL differs from the norm. Secondary objectives were to examine demographic variables and determine if there was a correlation between physical/functional and social/emotional scores during chemotherapy.
Methods
Patients with Stage III/IV ovarian cancer on Gynecologic Oncology Group Protocols 152 and 172 who underwent surgery followed by intravenous paclitaxel and cisplatin completed the Functional Assessment of Cancer Therapy-Ovarian. The Functional Assessment of Cancer Therapy scale includes the four domains of physical, functional, social, and emotional well-being (PWB, FWB, SWB, and EWB, respectively).
Results
Ovarian cancer patients had a total QOL (Functional Assessment of Cancer Therapy-General) score similar to the U.S. female adult population. However, the reported subscale scores were 2.0 points (95% confidence interval [CI] 1.4–2.5, P < 0.001, effect size = 0.37) lower in PWB, 0.9 points (95% CI 0.3–1.5, P = 0.005, effect size = 0.13) lower in FWB, 5.0 points (95% CI 4.6–5.3, P < 0.001, effect size = 0.74) higher in SWB, and 0.8 points (95% CI 0.3–1.2, P < 0.001, effect size = 0.16) lower in EWB. Correlation between the sum of PWB and FWB and the sum of SWB and EWB was r = 0.53 (P < 0.001). Age was positively correlated with EWB (r = 0.193; 95% CI 0.09–0.29).
Conclusion
Ovarian cancer patients have decreased QOL in physical, functional, and emotional domains; however, they may compensate with increased social support. At the time of diagnosis and treatment, patients’ QOL is affected by inherent characteristics. Assessment of treatment outcomes should take into account the effect of these independent variables.
Keywords: Ovarian cancer, chemotherapy, quality of life
Introduction
Quality of life (QOL) is an important component of assessing the effects of cancer, therapy, and survivorship. The Functional Assessment of Cancer Therapy (FACT), which has been under development since 1987, began with a generic core consisting of four subscales made up of individual items that assess domains relevant to QOL.1 It is appropriate for use in patients with any form of cancer and in the general population.2 Normative data obtained from the general population allow researchers to place results obtained from cancer survivors in an appropriate context.3 QOL scores from cancer patients can be compared with those obtained from a control group of patients not suffering from cancer to have appropriate reference values that take into account important sociodemographic variables such as gender, age, and marital and educational status.4 These comparisons may also be useful in understanding patient phenomena such as adaptation to illness.4 In addition, analyses of FACT scores can also be used to assess total QOL differences among cancer groups and domain effects and subscale item differences.
QOL measurement is multidimensional in nature.1 Although traditional use has focused on comparison across treatment groups, relationships exist among the domains of QOL. Factor analysis subscales were created for the FACT, and relationships between physical, functional, emotional, and social domains can be explored to better understand the effect of cancer and treatment for cancer on these components of QOL.1 Specifically, it would be of interest to determine if the physical/functional well-being (PWB/FWB) domains vary in relation to the emotional/social well-being (EWB/SWB) domains in response to cancer treatment. Individual patient characteristics measured before treatment begins (at baseline) affect a patient’s reaction to a cancer diagnosis and treatment and thus influence disease-specific QOL scores.5 This response may then affect physicians’ assessment of how patients respond to treatment and become a factor in determining which future treatments are selected, especially regarding age and performance status (PS).
In a single institutional study, baseline patient characteristics of women with endometrial or ovarian cancer were analyzed to determine the degree to which QOL is affected by baseline differences in demographic variables and global, physical, and mental health.6 Age and educational level were positively correlated with QOL PWB, whereas increasing body mass index (BMI) was negatively correlated. BMI and educational level were negatively correlated with FWB and SWB. Age was positively correlated with EWB. Therefore, assessment of treatment outcome should take into account the effect of these independent variables, which are present before the beginning of treatment.
Women diagnosed with ovarian cancer are at risk for reduced QOL.7 It is imperative to further define these declines to interpret treatment outcomes and design appropriate clinical interventions. A companion article addressed which QOL line items are associated with low QOL in women receiving chemotherapy for ovarian cancer.8 The primary objective of this study was to compare data obtained from ovarian cancer patients with normative data to assess the degree to which QOL differs from the norm. Secondary objectives were to examine demographic variables in relationship to QOL domains and determine if there was a correlation between physical/functional scores and social/emotional scores during chemotherapy.
Methods
Patients
Data for this analysis were obtained from patients enrolled on the control arms of two Gynecologic Oncology Group (GOG) randomized ovarian cancer protocols. All patients enrolled in GOG 1529 and GOG 17210 who were randomized into the six-cycle intravenous (IV) paclitaxel and cisplatin control arms were included (Fig. 1); all patients underwent identical dosing. GOG 152 measured QOL in a randomized trial of interval secondary cytoreduction in suboptimally debulked women with advanced ovarian carcinoma. GOG 172 measured QOL in a randomized study of IV paclitaxel and cisplatin vs. IV paclitaxel, intraperitoneal (IP) cisplatin, and IP paclitaxel in optimally debulked Stage III epithelial ovarian cancer patients. GOG 152 enrolled 208 patients with no interval cytoreduction, and GOG 172 included 210 patients entered into the IV cisplatin and paclitaxel arm. Patients receiving IP therapy on GOG 172 and those who had secondary debulking surgery on GOG 152 were not included in the analysis. Participating institutions obtained institutional review board approval of the protocols before enrolling any patients; all patients provided written informed consent consistent with all federal, state, and local requirements before they received any protocol therapy. Treatment information and outcome have been previously reported.11,12
Fig. 1.

GOG 152 and 172 trial schema.
Procedures
For the current report, the baseline QOL measure in GOG 152 (before Cycle 4 of chemotherapy) and the second QOL measurement from GOG 172 (before chemotherapy Cycle number 4) were analyzed. Thus, both QOL measures were done mid-regimen. The QOL measure chosen (before chemotherapy Cycle 4, after the third cycle) was used to provide an assessment of QOL during mid-treatment chemotherapy after several cycles have already been administered.
QOL was measured with the Functional Assessment of Cancer Therapy-Ovarian (FACT-O) questionnaire. The FACT-O scale includes the Functional Assessment of Cancer Therapy-General (FACT-G) along with12additionalquestions specific to ovarian cancer patients. The FACT-G, Version 4, is a 27-item core questionnaire evaluating the domains of physical, functional, family-social, and emotional well-being (PWB, seven items; FWB, seven items; SWB, seven items; and EWB, six items, respectively).1 The Trial Outcome Index (TOI) represents the combination of the PWB and FWB subscales along with disease-specific questions and is computed as the sum of the PWB, FWB, and ovarian subscale domains.2 Questions are answered on a five-point Likert scale, and items are summed to give scores for each domain.1 Reliability, validity, and sensitivity to change of the FACT-G have been demonstrated in a variety of settings, and relative scores can be compared with normative data.2 Basen-Engquist et al.13 demonstrated reliability and validity of the FACT-O instrument in ovarian cancer patients. GOG 152 used Version 2 of the FACT-G, and the EWB score only included five items; therefore, this domain score was estimated by assuming that the sixth item was missing.14
Normative data for the adult female population are provided for comparison.3 Brucker et al.3 evaluated normative data from the FACT-G from a sample of the general U.S. adult population (n = 1075) and a sample of adult patients with cancer (n = 2236). Reference data from an instrument used in other samples can help to aid in the interpretation and understanding of a particular group’s results.3 Minimally important differences (MIDs) are considered the “smallest difference” in FACT score that patients perceive as important, either beneficial or harmful.1 Ranges of MIDs based on distribution and anchor-based approaches have been identified to aid interpretation of changes over time and for sample size calculations and are established as 2–3 points for FACT-G domain subscales and 3–7 points for total FACT-G score.15,16 Mean differences between the two protocols ranged from 0.3 to 1.7, and thus, data from the two protocols were combined as there were no MIDs between the two studies. Data from both protocols were compared with normative data from the U.S. adult female population and with those from the female cancer patients12 to identify MIDs (3 points). An effect size for the difference in means between the normative population12 and the combined GOG 152/172 data set was calculated for each FACT-G domain. The standard deviation from the normative population (control group) was used for this calculation. Similarly, effect sizes for the difference in means between the female cancer patient sample and the combined GOG 152/172 data set were computed. QOL is an important issue to cancer patients, and QOL effect size in the 0.2–0.5 range is considered meaningful, especially in the context of normative results.15
To examine the impact of age on QOL, Pearson’s correlation coefficient between TOI, EWB, and age was calculated. The effect of PS was examined by comparing TOI and EWB between patients with a PS of 0 vs. ≥ 1 by use of Student’s t-test for independent samples. PS was available at different time points for the two studies: before Cycle 1 (at baseline) for patients on GOG 172 and before Cycle 4 for those on GOG 152, and comparisons were done separately by protocol. To explore the relationship between SWB/EWB and FWB/PWB, each patient’s scores on the SWB and EWB were summed, and scores for the PWB and FWB were summed. PWB and FWB, along with SWB and EWB domains, were grouped, as they have similar health concepts.17 Pearson’s correlation coefficient was used to examine the linear association between the sum of the SWB/EWB and the sum of PWB/FWB scores. SAS version 9.1 (SAS Institute, Cary, NC) was used for analysis.
Results
There were 208 eligible, suboptimally debulked patients enrolled on the arm of six cycles of cisplatin and paclitaxel without interval debulking in GOG 152 from June 6, 1994 to January 29, 2001.9 There were 210 eligible, optimally debulked patients enrolled for the six cycles of IV cisplatin and paclitaxel arm in GOG 172 from March 23, 1998 to January 29, 2001.10 The mean days from surgery to Cycle 4 in GOG 152 was 77 days. The mean days from surgery to Cycle 4 was 92 days in GOG 172. Before Cycle 4, 189 patients (91%) in GOG 152 and 172 patients (82%) in GOG 172 provided valid QOL assessments for all subscales of the FACT-O.8
Mean domain and total scores for women in GOG 152 and 172 are compared with those for the U.S. adult female population and normative data from female cancer patients, which are presented in Table 1. The ovarian cancer patients had a total FACT-G score similar to the U.S. female adult population. However, the reported subscale scores were 2.0 points (95% confidence interval [CI] 1.4–2.5, P < 0.001, effect size = 0.37) lower in PWB, 0.9 points (95% CI 0.3–1.5, P = 0.005, effect size = 0.13) lower in FWB, 5.0 points (95% CI 4.6–5.3, P < 0.001, effect size = 0.74) higher in SWB, and 0.8 points (95% CI 0.3–1.2, P < 0.001, effect size = 0.16) lower in EWB. When ovarian cancer patients were compared with the female cancer patient sample, subscale differences ranged from 0.1 (EWB) to 2.5 (FWB). Normative data from the female cancer patients were higher for PWB and FWB, whereas SWB was higher in the GOG combined data.
Table 1.
QOL Domain and Total Scores Reported Before Cycle 4 in GOG 152 and GOG 172 Compared with the U.S. Adult Female Population
| Domain | GOG 152 (n = 189) |
GOG 172 (n = 172) |
Combined (n = 361) |
U.S. Adult Female Populationa
|
Effect Sizeb | Female Cancer Samplea
|
Effect Sizeb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| PWB | 20.9 | 5.4 | 19.2 | 5.4 | 20.1 | 5.4 | 22.1 | 5.4 | 0.37 | 21.6 | 5.8 | 0.26 |
| FWB | 17.8 | 5.3 | 16.9 | 6.6 | 17.4 | 5.9 | 18.3 | 6.9 | 0.13 | 19.5 | 6.6 | 0.32 |
| SWB | 24.6 | 3.4 | 24.9 | 3.4 | 24.8 | 3.4 | 19.8 | 6.8 | 0.74 | 22.3 | 5.3 | 0.47 |
| EWB | 18.8 | 4.2 | 18.4 | 3.9 | 18.6 | 4.0 | 19.4 | 5.1 | 0.16 | 18.7 | 4.5 | 0.02 |
| FACT-G | 82.2 | 13.6 | 79.5 | 14.5 | 80.9 | 14.1 | 79.6 | 18.6 | 0.07 | 82.1 | 16.3 | 0.07 |
SD = standard deviation.
From Brucker et al.3
Effect size for difference in means (combined) vs. U.S. adult female population or female cancer sample. SD for the U.S. adult female population or the female cancer patient sample was used as the denominator to calculate the effect size.
The correlation coefficient between the sum of PWB and FWB and the sum of SWB and EWB was r = 0.53 (P < 0.001 for a test of the hypothesis of a zero correlation; Fig. 2). Age was positively correlated with EWB (r = 0.193; 95% CI 0.09–0.29) but not significantly correlated with TOI (r = 0.027; 95% CI −0.08 to 0.13) (Table 2). EWB was not associated with patients’ PS. The TOI score was not associated with baseline PS (GOG 172) but may be associated with PS in GOG 152 when the TOI was reported (before Cycle 4) as seen in Table 3.
Fig. 2.
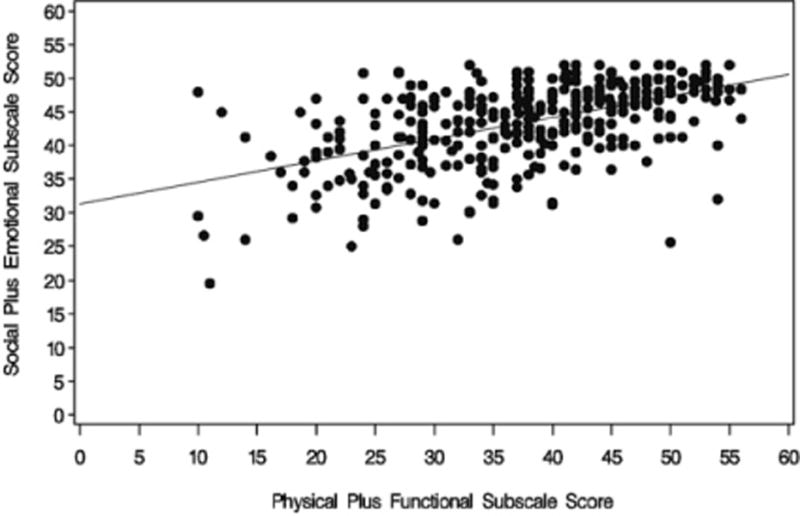
Scatter plot of physical and function subscale scores vs. social and emotional subscale scores, r = 0.53.
Table 2.
Correlation Coefficients of TOI and EWB with Age
| Variable | With Variable | n | Correlation | 95% CI |
|---|---|---|---|---|
| TOI | Age | 361 | 0.027 | −0.077 to 0.130 |
| EWB | Age | 361 | 0.19344 | 0.0918 to 0.291 |
Table 3.
Association of TOI and EWB with PS by Protocol
| GOG 172
| ||||
|---|---|---|---|---|
| GOG PSa
|
||||
| Variable | 0 (n = 75) | ≥ 1 (n = 97) | Difference | 95% CI |
| TOI | 71.5 ± 15.4 | 70.7 ± 15.0 | 0.79 | −3.8 to 5.4 |
| EWB | 18.3 ± 4.1 | 18.5 ± 3.8 | −0.20 | −1.4 to 1.0 |
| GOG 152
| ||||
|---|---|---|---|---|
| GOG PSb
|
||||
| Variable | 0 (n = 107) | ≥ 1 (n = 82) | Difference | 95% CI |
| TOI | 75.3 ± 13.0 | 70.5 ± 14.3 | 4.8 | 0.9 to 8.7 |
| EWB | 19.3 ± 3.8 | 18.3 ± 4.6 | 1.0 | −0.2 to 2.2 |
Only 15 patients have a status of 2. They are combined with PS of 1.
PS was evaluated before Cycle 1 (at baseline) for GOG 172 patients.
PS was evaluated before Cycle 4 for GOG 152 patients.
Discussion
GOG 152 and 172 were designed to assess the effect of adjuvant chemotherapy in ovarian cancer patients. This ancillary study was conducted to obtain an increased understanding of the QOL in ovarian cancer patients undergoing treatment compared with those without cancer and to explore the relationships among domains and patients’ clinical variables.
Reference values of the general population are needed for interpretation of QOL scores so that scores obtained by cancer patients can be compared with those of a control group that does not have cancer.4 For example, if an ovarian cancer patient scores 20 out of 28 in the physical domain of a QOL questionnaire, what does this mean unless the “normal” is known? A comparison with the maximum possible score of 28 might underestimate the patient’s perceived QOL because healthy controls do not score 28. At first glance, it appears that ovarian cancer patients had FACT-G scores similar to the U.S. female adult population. However, the reported subscale scores were lower in PWB and higher in SWB.
Clearly, the cancer journey alters patients’ QOL because some domains decline, whereas others rise.8 The FWB and PWB scores in the GOG patients were lower than normative data and data from female cancer patients as this may be because of the time point chosen (during chemotherapy) or the lingering effects of laparotomy. EWB scores of ovarian cancer patients were similar to those of female cancer patients in general. Decreased EWB in the current combined GOG trials was related to feeling nervous and worrying about dying.8 Regarding their psychosocial needs, others also have found higher SWB scores in women with cancer.3,6 Brucker et al.3 observed a mean SWB score of 22.3 in female cancer patients of all types, lower than the score of 24.8 observed in the current combined GOG trials. Higher SWB scores may be related to patient characteristics or primary disease. However, it may simply be that patients with cancer receive more social support during adjuvant therapy.
This study provides preliminary information suggesting that the PWB/FWB domains are related (significantly correlated) to the EWB/SWB domains. What is unclear is whether decreases in PWB or FWB result in decreases in SWB and EWB or whether women with low SWB or EWB are more susceptible to treatment effects and, thus, have a lower PWB or FWB. Therefore, a clinical intervention in the PWB/FWB subscales may have a positive effect on the EWB/SWB scales and/or improve global QOL.
Demographic variables and clinical factors impact QOL. Previous studies have identified lower QOL scores among those with poor PS; decreased PWB, EWB, and FWB; younger age; and lower socioeconomic status (SES).18,19 Of these variables, age and PS were available for analysis from GOG 152 and 172 and age results were consistent with the literature. The TOI score reported during chemotherapy was found not to be associated with baseline PS (assessed before treatment) but associated with PS at the time the TOI score was reported. Comparisons using TOI (compared with global QOL) with EWB, age, and PS were used to focus on tangible areas that may be amenable to change. Nevertheless, our supposition that individual factors impact QOL further adds to our companion study, which indicated that women in the lowest scoring quartile of the FACT-O have a higher prevalence of symptom distress and selected the worst categories of items related to symptoms and side effects related to treatment.8
Strengths of this study include an innovative approach to QOL analysis in a cooperative group setting, size of the study sample, and well-controlled data collection. Limitations of this study include lack of racial heterogeneity. In addition, the GOG collects limited demographic and clinical factors; therefore, we were unable to perform a more robust comparison of several variables. Additional study is needed to examine other factors that may account for additional variation of QOL in this patient population, such as SES and psychosocial factors. Although it is not conventional to compare cancer patients with the normal population and to use the TOI to assess individual differences, it is reasonable to implement practical applications to QOL data.5,17
If a relationship among QOL domains and variables exists, then providing targeted therapy, such as focusing on emotional and social support, may reduce the effect on physical and functional domains and vice versa. Treating a patient’s anxiety and fear with positive adaptation skills may improve specific symptoms along with EWB.8,20 Or, we can hypothesize by targeting and improving fatigue; it would increase other domains followed by global QOL.8 QOL, at baseline, is predictive of survival in ovarian cancer patients.21 Therefore, interventions that improve QOL in specific identified areas also may improve overall quality and quantity of survival. To fully elucidate the potential benefits of a multidisciplinary QOL intervention trial, a large randomized controlled trial is required.
Acknowledgments
The following GOG member institutions participated in the related treatment study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Colorado Gynecologic Oncology Group, UCCC, University of Mississippi Medical Center, Colorado Foundation for Medical Care, University of California Medical Center at Los Angeles, University of Washington Medical Center, University of Pennsylvania Cancer Center, Milton S. Hershey School of Medicine of the Pennsylvania State University, University of Cincinnati College of Medicine, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Health Science Center at Dallas, Indiana University Cancer Center, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush University Medical Center, State University of New York Downstate Medical Center, University of Kentucky, Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, M.D. Anderson Cancer Center, University of Massachusetts Memorial Medical Center, Fox Chase Cancer Center, Medical University of South Carolina, Women’s Cancer Center, University of Oklahoma Health Science Center, University of Virginia Health Science Center, Tacoma General Hospital, Thomas Jefferson University Hospital, Mayo Clinic, Tampa Bay Cancer Consortium, Gynecologic Oncology Network/Brody School of Medicine, and Ellis Fischel Cancer Center.
This work was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469) and the GOG Statistical and Data Center (CA 37517).
Footnotes
Presented at the Society of Gynecologic Oncologists 2008 Annual Meeting, Tampa, FL, March 9–12, 2008.
References
- 1.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy (FACT) scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 2.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the functional assessment of cancer therapy-general (FACT-G) Eval Health Prof. 2005;28:192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 4.Holzner B, Kemmler G, Cella D, et al. Normative data for function assessment of cancer therapy—general scale and its use for the interpretation of quality of life scores in cancer survivors. Acta Oncol. 2004;43:153–160. doi: 10.1080/02841860310023453. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson GW, Moinpour CM. Individual differences in quality-of-life treatment response. Med Care. 2002;40:39–53. doi: 10.1097/00005650-200206001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Gil KM, Gibbons HE, Jenison EL, Hopkins MP, von Gruenigen VE. Baseline characteristics influencing quality of life in women undergoing gynecologic oncology surgery. Health Qual Life Outcomes. 2007;5:25. doi: 10.1186/1477-7525-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenzel L, Vergote I, Cella D. Quality of life in patients receiving treatment for gynecologic malignancies: special considerations for patient care. Int J Gynaecol Obstet. 2003;83(Suppl 1):211–229. doi: 10.1016/s0020-7292(03)90123-8. [DOI] [PubMed] [Google Scholar]
- 8.von Gruenigen VE, Gil KM, Huang H, et al. Assessment of factors that contribute to decreased quality of life in Gynecologic Oncology Group ovarian cancer trials. Cancer. 2009;115:4857–4864. doi: 10.1002/cncr.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenzel L, Huang HQ, Monk BJ, Rose PG, Cella D. Quality-of-life comparisons in a randomized trial of interval secondary cytoreduction in advanced ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23:5605–5612. doi: 10.1200/JCO.2005.08.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D, Gynecologic Oncology Group Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:437–443. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong DK, Bundy B, Wenzel L, et al. Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;54:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, Nerenstone S, Brandy MF, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351:2489–2497. doi: 10.1056/NEJMoa041125. [DOI] [PubMed] [Google Scholar]
- 13.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the Functional Assessment of Cancer Therapy—Ovarian. J Clin Oncol. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 14.Fairclough DL, Cella DF. Functional Assessment of Cancer Therapy (FACT-G): non-response to individual questions. Qual Life Res. 1996;5:321–329. doi: 10.1007/BF00433916. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Hahn E, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002;11:207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
- 16.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences. Eval Health Prof. 2005;28:172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ. Statistical considerations for use of composite health-related quality-of-life scores in randomized trials. Qual Life Res. 2004;13:717–723. doi: 10.1023/b:qure.0000021686.47079.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan GJ, Counte MA, Cella DF, et al. An analysis of the impact of demographic, clinical, and social factors on health-related quality of life. Value Health. 1999;2:308–318. doi: 10.1046/j.1524-4733.1999.24006.x. [DOI] [PubMed] [Google Scholar]
- 19.Movsas B, Scott C, Watkins-Bruner D. Pretreatment factors significantly influence qualify of life in cancer patients: a Radiation Therapy Oncology Group (RTOG) analysis. Int J Radiat Oncol Biol Phys. 2006;65:830–835. doi: 10.1016/j.ijrobp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin SS. Surviving cancer or other serious illness: a review of individual and community resources. CA Cancer J Clin. 2008;58:60–64. doi: 10.3322/CA.2007.0001. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel LB, Donnelly JP, Fowler J, et al. Resilience, reflection and residual stress in ovarian cancer survivorship: a Gynecologic Oncology Group study. Psychooncology. 2002;11:142–153. doi: 10.1002/pon.567. [DOI] [PubMed] [Google Scholar]


